Cocaine craving in human addicts can be elicited by three major factors: environmental cues associated with drug taking, a stressful life-event or re-exposure to cocaine [26]. Drug craving is modeled in rodents and non-human primates using the reinstatement model of cocaine seeking [13, 19, 39]. The nucleus accumbens plays a major role in mediating the reinstatement of cocaine seeking [27, 36]. The nucleus accumbens receives a major dopaminergic projection from the ventral tegmental area (VTA) and glutamatergic projections from the prefrontal cortex, hippocampus and amygdala [5, 34, 43]. The accumbens is composed of two major subregions, the core and the shell, which have differential afferent and efferent anatomical projections [7, 20, 21, 44]. Functionally, the accumbens shell is considered the more limbic subregion, mediating the acute effects of drug reward [8, 35]. In contrast, the core is more involved in the compulsivity of drug addiction [16] and the effects of drug-associated cues [14, 18, 25]. In terms of cocaine priming-induced reinstatement of drug seeking, dopamine receptors play differential roles in the shell versus the core [2–4]. For example, infusion of dopamine receptor antagonists into the accumbens shell, but not the core, attenuates the reinstatement of cocaine-seeking behavior [2–4]. Similarly, dopamine receptor agonists promote the reinstatement of cocaine seeking when microinjected into the shell, but not core [4, 37]. Collectively, these studies demonstrate that increased dopamine transmission in the nucleus accumbens shell, but not the core, critically mediates the reinstatement of cocaine-seeking behavior.
The glutamatergic projection from the medial prefrontal cortex (mPFC) to the nucleus accumbens is one of the key anatomical substrates underlying cocaine priming-induced reinstatement of drug seeking [27, 37]. There are two major classes of ionotropic glutamate receptors: AMPA/kainate and NMDA. While AMPA receptors in the nucleus accumbens are critically involved in the reinstatement of cocaine-seeking behavior [9, 10, 30, 32, 40], there is evidence that NMDA receptors may also play a role. For example, systemic administration of MK-801, an NMDA receptor channel blocker, robustly reinstated cocaine seeking, without an increase in nonspecific operant responding [12, but see also 6]. In a subsequent study, it was found that intra-accumbal administration of the competitive NMDA receptor antagonist, CPP, had no effect on the reinstatement of cocaine seeking when administered either alone or prior to a systemic priming injection of cocaine [10]. In contrast, intra-accumbal administration of AP-5, a competitive NMDA receptor antagonist, reinstated cocaine-seeking behavior when microinjected into the nucleus accumbens shell subregion [32]. Notably, these studies either did not differentiate between the core and shell subregions of the nucleus accumbens [10] or examined the shell but not the core [32]. Therefore, in the present study we systematically examined the ability of an NMDA receptor antagonist to reinstate cocaine-seeking behavior when administered into either the nucleus accumbens core or shell.
Male Sprague-Dawley rats (Rattus norvegicus) weighing 250–300 g were purchased from Taconic Laboratories (Germantown, NY). Animals were individually housed with food and water available ad libitum. The animal facilities have a 12/12 hr light/dark cycle, with the lights on at 7:00 a.m. All experimental procedures were performed during the light cycle. Behavioral experiments used Med-Associates (East Fairfield, VT) equipment enclosed within ventilated, sound-attenuating chambers. The operant chambers are equipped with two response levers (active and inactive), house light and injection pumps for intravenous drug administration.
Prior to surgery, rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine (Sigma/RBI, St. Louis, MO). An indwelling silastic catheter (CamCaths, Cambridge, UK) was inserted into the right jugular vein (side opposite the heart) and sutured into place. The catheter was connected to a backmount, which was sealed with a plastic obturator when not in use. To maintain patency and prevent infection, catheters were flushed daily with 0.3 ml of the antibiotic Timentin (ticarcillin disodium/potassium clavulanate, 0.93 mg/ml) dissolved in heparinized saline. In all animals, guide cannulae (14 mm, 24 gauge; Small Parts Inc., Roanoke, VA) for microinjection experiments were bilaterally implanted 2 mm dorsal to the nucleus accumbens core and shell and were cemented into place by affixing dental acrylic to stainless steel screws secured in the skull. The coordinates for the placement of guide cannulae, relative to bregma [33], were as follows: nucleus accumbens shell: +1.0 mm A/P, ±1.0 mm M/L, −5.0 mm D/V; nucleus accumbens core: +1.0 mm A/P, ±2.5 mm M/L, −5.0 mm D/V. Obturators (14 mm, 33 gauge) were placed into the guide cannulae to prevent occlusion.
Following a 7-day recovery period from surgery, the rats were placed in operant chambers and trained to lever press for intravenous cocaine infusions (0.25 mg cocaine/59 μl saline/infusion over 5 sec). A 20-sec timeout period during which responses have no scheduled consequences followed each cocaine infusion. Rats initially were trained using a fixed ratio (FR)1 schedule of reinforcement with each daily 2 hour self-administration session initiated by an intravenous injection of cocaine. This injection was performed in order to fill the catheter (i.e. little or none of this noncontingent injection reached the systemic circulation). When the animals achieved stable responding under the FR1 schedule (i.e. less than 15% variation in response rates over two consecutive days and >15 infusions each day) they were switched to an FR5 schedule. During all phases of the experiment, inactive lever responding was also recorded. To avoid overdose, rats were limited to a maximum of 30 cocaine infusions per self-administration session. Following 21 days of self-administration (FR1 + FR5), lever pressing was extinguished by replacing cocaine with saline. Daily 2-hour extinction sessions were conducted until responding was less than 15% of the responses maintained by cocaine self-administration. During the reinstatement phase of the experiment, 3 or 30 μg DL-2-Amino-5-phosphonovaleric acid lithium salt (AP-5) or its saline vehicle was administered into the core or shell of the nucleus accumbens immediately prior to a 2-hour reinstatement session. Doses were chosen based upon a previous reinstatement study from our laboratory, as well studies that assessed locomotor activity and behavioral sensitization [29, 31, 32]. Lower doses, used extensively in learning and memory studies, have not generally been successful in disrupting cocaine-associated behaviors [11, 17, 22, 38, 42]. For the reinstatement sessions, the FR5 schedule was used and satisfaction of the response requirement resulted in a saline infusion. Each reinstatement session was followed by extinction sessions until responding was again less than 15% of the response rate maintained by cocaine self-administration. At various points during the extinction phase, reinstatement of cocaine-seeking behavior was assessed by administering a systemic injection of cocaine (10 mg/kg, i.p.). If a rat failed to reinstate to cocaine (operationally defined as fewer than 40 active lever responses per session), no additional data was collected using that subject.
Obturators were removed from the microinjection guide cannulae and replaced by injection needles (33 gauge; Small Parts Inc.), which extend 2 mm below the end of the guide cannulae into the nucleus accumbens core or shell. Bilateral infusions were made over 120 sec in a volume of 0.5 μl/side. To allow for the compound to diffuse away from the tips of the microinjectors, the microinjectors were left in place for 60 seconds after the infusion and then removed. Whenever possible, each animal served as its own control. That is, each animal received vehicle as well as both doses of AP-5 (up to a maximum of 3 microinjections per brain region per animal). Deviation from this design occurred only when it was unavoidable (i.e. when technical difficulties, such as clogged microinjection cannulae, loss of intravenous catheter patency or an animal no longer reinstated to cocaine, made it impossible to test all doses of a test compound on a specific animal). Treatments were counterbalanced across sessions to avoid any rank-order effects. Similar microinjection procedures (specifically, the use of a 0.5 μl microinjection volume) have been used to identify functional differences between the nucleus accumbens core and shell [2, 3, 15, 23, 28, 37]. DL-2-Amino-5-phosphonovaleric acid lithium salt (AP-5) was purchased from Sigma-Aldrich. Cocaine was a gift from the National Institute on Drug Abuse (NIDA).
After the reinstatement experiments were completed, rats were overdosed with pentobarbital (100 mg/kg, i.p.) and perfused intracardially with 60 ml of 0.9% saline followed by 60 ml of 10% formalin. The brains were removed and stored in 10% formalin. Subsequently, 100 μm coronal sections were taken at the level of the nucleus accumbens with a Vibratome (Technical Products Int., St. Louis, MO, USA). Coronal sections were mounted on gel-coated slides and stained with cresyl violet. An individual blind to the animals’ behavioral responses determined cannulae placements as well as potential drug- or cannula-induced neuronal damage. Light microscopy was used to determine cannulae placements as well as the presence and extent of cell death and associated gliosis.
The mean (±SEM) total active lever responses for 7 representative animals was as follows: last day of cocaine self-administration, 119.86 (±8.09); last day of extinction, 9.14 (±1.18); reinstatement following a systemic priming injection of 10 mg/kg cocaine (10 mg/kg, i.p.), 108.57 (±13.16).
In these experiments a mixed factors design was used in which we aimed to administer each dose of a drug plus the drug vehicle to all subjects in a group during the reinstatement phase. However, technical difficulties (i.e. loss of catheter patency, clogging of guide cannulae, etc.) regularly resulted in missing cells. Therefore, we utilized a statistical model (a mixed model multivariate analysis of variance or MANOVA) that accommodates missing cells in the within-subjects aspect of an experimental design. The AP-5 reinstatement data were analyzed with a 3-way mixed model MANOVA. The factors were brain region, active/inactive lever and drug treatment. The results of this analysis revealed significant main effects of treatment [F(2,42)=16.213, p<0.0001], region [F(1,42)=7.210, p<0.0104] and lever [F(1,42)=48.668, p<0.0001] as well as the following significant interactions: drug × region [F(2,42)=3.791, p<0.0306], lever × treatment [F(2,42)=11.458, p<0.0001], lever × region [F(1,42)=9.589, p<0.0035] and lever × treatment × region [F(2,42)=3.526, p<0.0384].
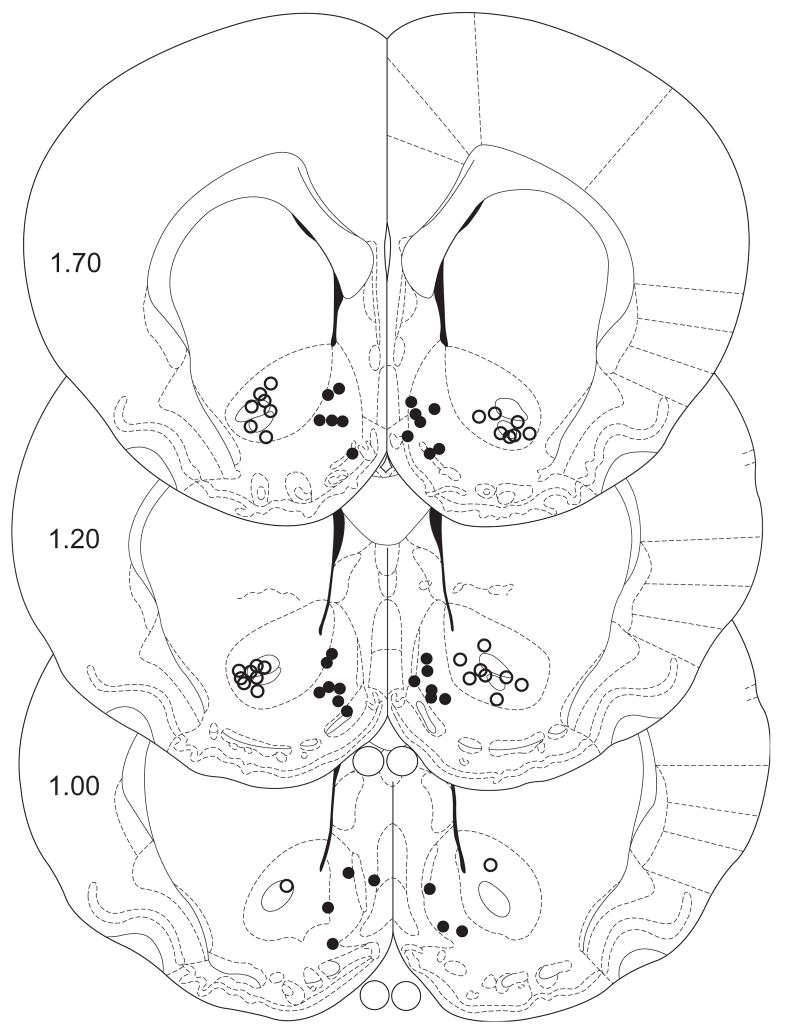
Subsequent pairwise analyses (Fisher’s LSD, p<0.05) of the accumbens shell data (see Figure 1A) revealed significant differences in active lever responding between the following treatments: saline/3 μg AP-5, saline/30 μg AP-5, 3 μg AP-5/30 μg AP-5. There were no significant differences in inactive lever responses among the shell treatments. In terms of the accumbens core data (see Figure 1B), post-hoc analyses (Fisher’s LSD, p<0.05) indicated a significant difference in active lever responding between the saline and 30 μg AP-5 treatments. There were no significant differences in inactive lever responses among the core treatments. The cannulae placements in the accumbens core and shell from these experiments are depicted in Figure 2.
Figure 1.
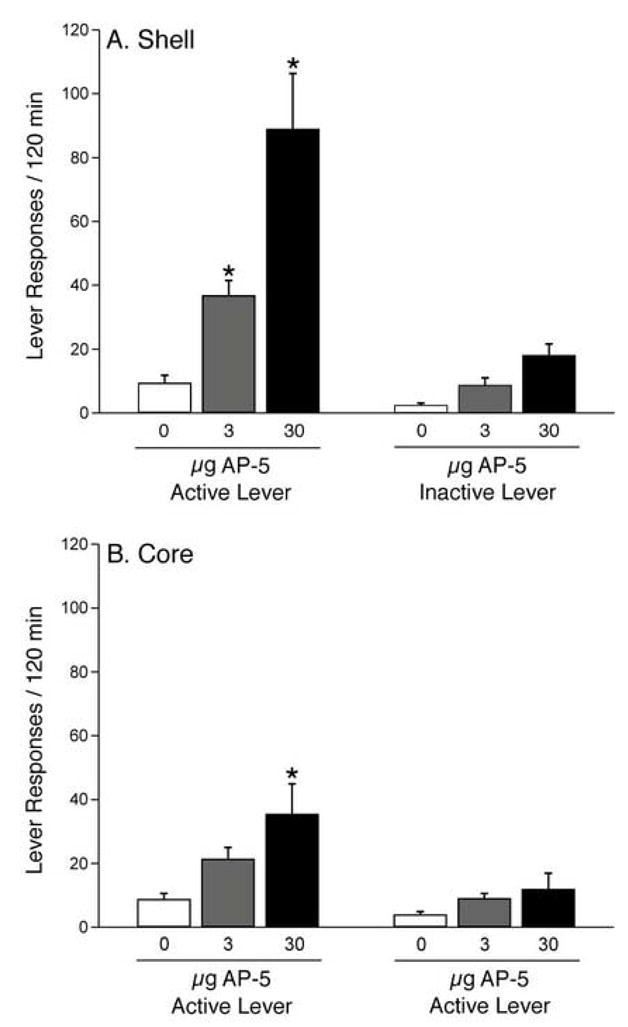
When microinjected into the nucleus accumbens shell, the NMDA receptor antagonist AP-5 dose-dependently reinstated cocaine-seeking behavior (see Figure 1A). Following microinjection of both doses of AP-5 (3 and 30 μg), there was a significant increase in active lever responding compared to that following microinjection of saline vehicle. There was no significant effect on inactive lever responding. The number of rats was as follows: saline, n=6; 3 μg AP-5, n=10; 30 μg AP-5, n=9. As shown in Figure 1B, intra-accumbens core administration of 30 μg AP-5 reinstated cocaine-seeking behavior. When microinjected into the nucleus accumbens core, AP-5 did not affect inactive lever responding. The number of rats was as follows: saline, n=8; 3 μg AP-5, n=8; 30 μg AP-5, n=7. The asterisks represent significant differences from the respective saline control groups (Fisher’s LSD, p<0.05).
Figure 2.
Microinjection placements in the nucleus accumbens shell and core, according to the atlas of Paxinos and Watson [33]. Black dots represent placements into the nucleus accumbens shell; open circles represent placements into the nucleus accumbens core.
Consistent with a previous report [32], the present findings indicate that administration of the competitive NMDA receptor antagonist, AP-5, into the shell of the nucleus accumbens reinstates cocaine-seeking behavior. The current results also indicate that injection of AP-5 directly into the core of the nucleus accumbens reinstates cocaine seeking. However, whereas intra-shell administration of 3 or 30 μg AP-5 reinstated cocaine seeking, only the higher dose produced a significant effect when injected into the core. Moreover, the magnitude of the AP-5 effect was much greater when the drug was microinjected into the shell relative to the core. Since the accumbens core and shell are relatively small and adjacent nuclei, it is possible that the effect of intra-core AP-5 was due to lateral diffusion into the shell. While this explanation cannot be ruled out, it is important to note that the procedures used here were similar or identical to previous studies that demonstrated clear and statistically significant functional differences between the core and shell subregions of the nucleus accumbens [2, 3, 15, 23, 28, 37].
The present results suggest that increased glutamate transmission through accumbal NMDA receptors should inhibit the reinstatement of cocaine seeking. In contrast, previous results indicate that administration of cis-ACDA, an NMDA agonist, into the nucleus accumbens also reinstates cocaine seeking in rats [9]. However, intra-accumbal cis-ACDA also increased responding on the inactive lever, suggesting that the increase in responding on the active lever may be due to a nonspecific increase in motor activity [9]. Taken together, the results of several experiments focused on a potential role of accumbal NMDA receptors in the reinstatement of cocaine seeking have not resulted in clear or straightforward interpretations [10, 36].
Although we have not directly investigated the mechanisms underlying the reinstatement of cocaine seeking promoted by intra-accumbal AP-5, several explanations are plausible. Although most striatal NMDA receptors are post-synaptic, there is evidence of pre-synaptic NMDA receptors on corticostriatal terminals [41]. Interestingly, systemic administration of the NMDA receptor antagonist PCP increases glutamate levels in the nucleus accumbens [1]. Given that increases in glutamate transmission through accumbal AMPA receptors play a critical role in cocaine priming-induced reinstatement of drug seeking [9, 10, 32], it is possible that blocking presynaptic nucleus accumbens NMDA receptors increases glutamate release in the nucleus accumbens, which then promotes the reinstatement of cocaine seeking via activation of post-synaptic AMPA receptors.
Systemic administration of the NMDA receptor antagonist PCP also increases extracellular dopamine levels in the nucleus accumbens [1]. Additionally, intra-PFC infusion of either PCP or MK-801 promotes local dopamine release [24]. Based on these results, it is plausible that intra-accumbal core and shell administration of AP-5 reinstates cocaine seeking by increasing local dopamine release. Previous work focusing on the role of accumbens dopamine in the reinstatement of cocaine seeking indicates that enhanced dopaminergic transmission in the nucleus accumbens shell, but not core, reinstates cocaine-seeking behavior [4, 37]. Interestingly, our results indicate that AP-5 has a much more pronounced effect on the reinstatement of cocaine seeking when administered into the accumbens shell. Thus, it is possible that the effect of administering AP-5 into the shell is mediated through an increase in local dopamine levels. While further studies need to be performed to confirm these hypotheses, the current studies indicate that antagonizing NMDA receptors in the nucleus accumbens core or shell is sufficient to promote the reinstatement of cocaine-seeking behavior.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NIH) to RCP (R01 DA15214 and K02 DA18678). KRF was also supported by a National Research Service Award (NRSA) from the NIH (F30 DA19304), as well as an NIH training grant (T32 GM008541-7). HDS was also supported by an NRSA from the NIH (DA16824). The authors would like to thank Audrey Pierce for administrative assistance and Judy Yee for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–54. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–8. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- 4.Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- 5.Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–47. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 6.Bespalov AY, Zvartau EE, Balster RL, Beardsley PM. Effects of N-methyl-D-aspartate receptor antagonists on reinstatement of cocaine-seeking behavior by priming injections of cocaine or exposures to cocaine-associated cues in rats. Behav Pharmacol. 2000;11:37–44. doi: 10.1097/00008877-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–78. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 8.Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–22. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–67. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- 10.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Leonibus E, Oliverio A, Mele A. A study on the role of the dorsal striatum and the nucleus accumbens in allocentric and egocentric spatial memory consolidation. Learn Mem. 2005;12:491–503. doi: 10.1101/lm.94805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. MK-801 reinstates drug-seeking behaviour in cocaine-trained rats. Neuroreport. 1998;9:637–40. doi: 10.1097/00001756-199803090-00014. [DOI] [PubMed] [Google Scholar]
- 13.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 14.Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–73. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–60. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 16.Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–9. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 17.Ferretti V, Sargolini F, Oliverio A, Mele A, Roullet P. Effects of intra-accumbens NMDA and AMPA receptor antagonists on short-term spatial learning in the Morris water maze task. Behav Brain Res. 2007;179:43–49. doi: 10.1016/j.bbr.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–65. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- 19.Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–61. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- 20.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 21.Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–81. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez PJ, Andrzejewski ME, Sadeghian K, Panksepp JB, Kelley AE. AMPA/kainate, NMDA, and dopamine D1 receptor function in the nucleus accumbens core: a context-limited role in the encoding and consolidation of instrumental memory. Learn Mem. 2005;12:285–95. doi: 10.1101/lm.93105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holahan MR, Kalin NH, Kelley AE. Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity. Psychopharmacology (Berl) 1997;130:189–96. doi: 10.1007/s002130050228. [DOI] [PubMed] [Google Scholar]
- 24.Hondo H, Yonezawa Y, Nakahara T, Nakamura K, Hirano M, Uchimura H, Tashiro N. Effect of phencyclidine on dopamine release in the rat prefrontal cortex; an in vivo microdialysis study. Brain Res. 1994;633:337–42. doi: 10.1016/0006-8993(94)91558-x. [DOI] [PubMed] [Google Scholar]
- 25.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–97. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 26.Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- 27.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 28.Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study. Behav Brain Res. 1997;89:107–13. doi: 10.1016/s0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 29.Licata SC, Schmidt HD, Pierce RC. Suppressing calcium/calmodulin-dependent protein kinase II activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine-induced behavioural sensitization in rats. Eur J Neurosci. 2004;19:405–14. doi: 10.1111/j.0953-816x.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 30.Mead AN, Zamanillo D, Becker N, Stephens DN. AMPA-Receptor GluR1 Subunits are Involved in the Control Over Behavior by Cocaine-Paired Cues. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301045. [DOI] [PubMed] [Google Scholar]
- 31.Ouagazzal A, Amalric M. Competitive NMDA receptor antagonists do not produce locomotor hyperactivity by a dopamine-dependent mechanism. Eur J Pharmacol. 1995;294:137–46. doi: 10.1016/0014-2999(95)00518-8. [DOI] [PubMed] [Google Scholar]
- 32.Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–25. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- 34.Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–96. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- 35.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–8. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 Dopamine Receptors in the Shell, but Not the Core, of the Nucleus Accumbens Reinstates Cocaine-Seeking Behavior in the Rat. European Journal of Neuroscience. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- 38.See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–10. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- 39.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–59. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- 41.Tarazi FI, Baldessarini RJ. Regional localization of dopamine and ionotropic glutamate receptor subtypes in striatolimbic brain regions. J Neurosci Res. 1999;55:401–10. doi: 10.1002/(SICI)1097-4547(19990215)55:4<401::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 42.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–70. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright CI, Groenewegen HJ. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J Comp Neurol. 1995;361:383–403. doi: 10.1002/cne.903610304. [DOI] [PubMed] [Google Scholar]
- 44.Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–28. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]