Abstract
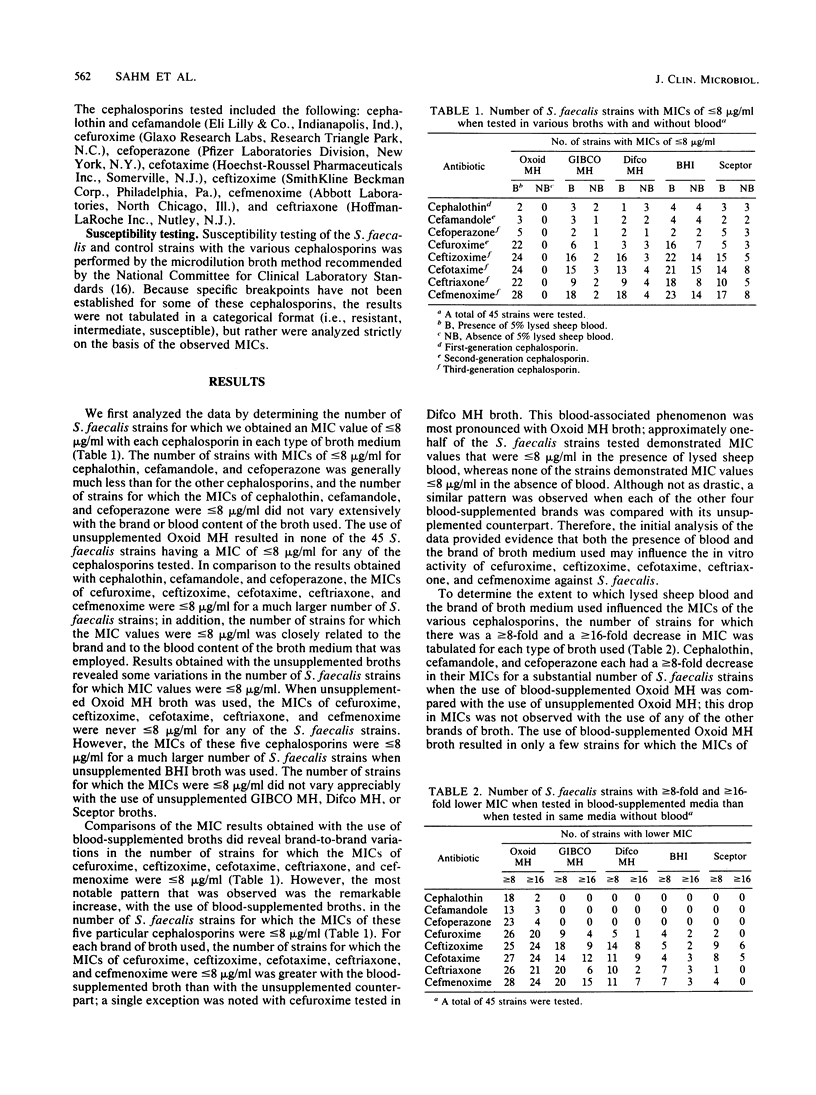
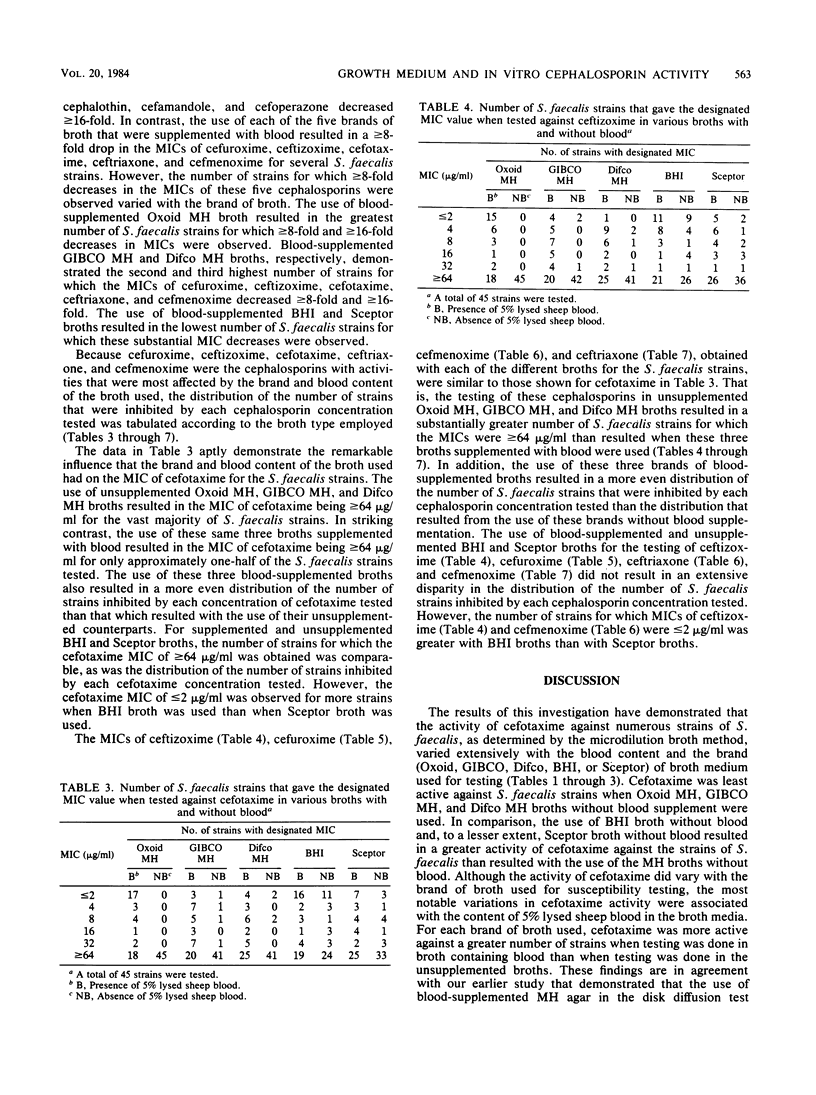
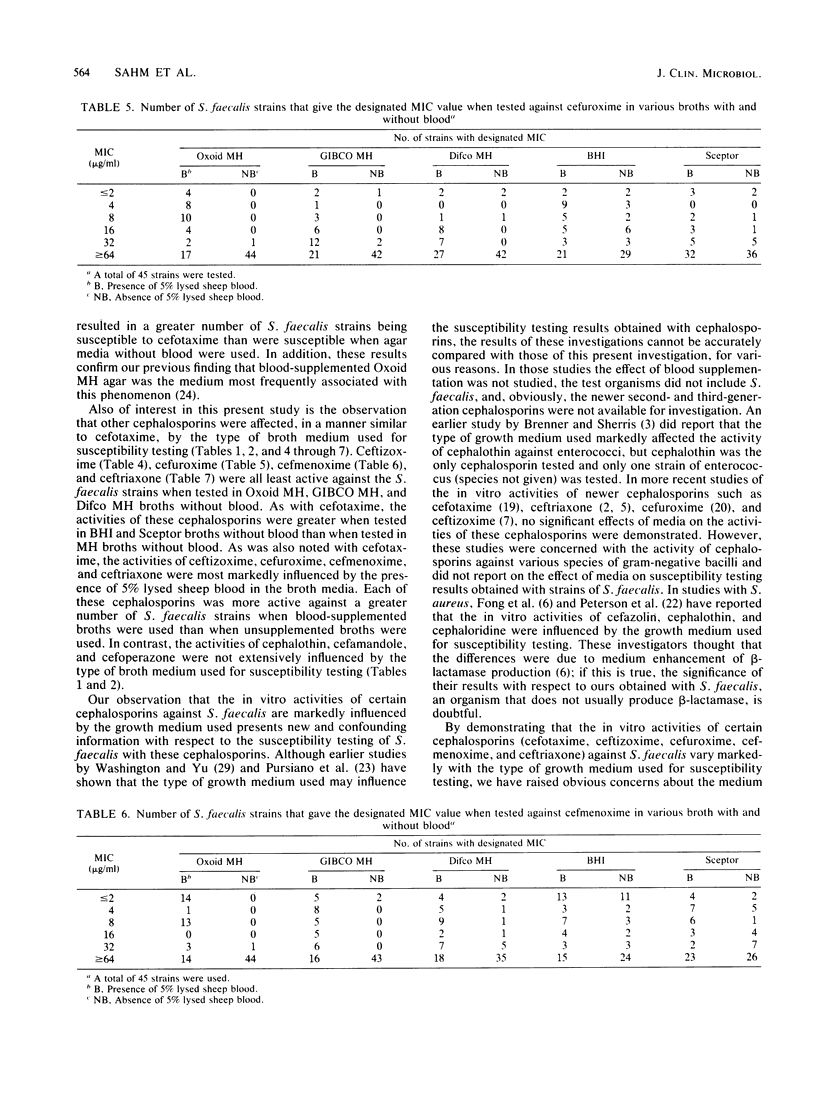
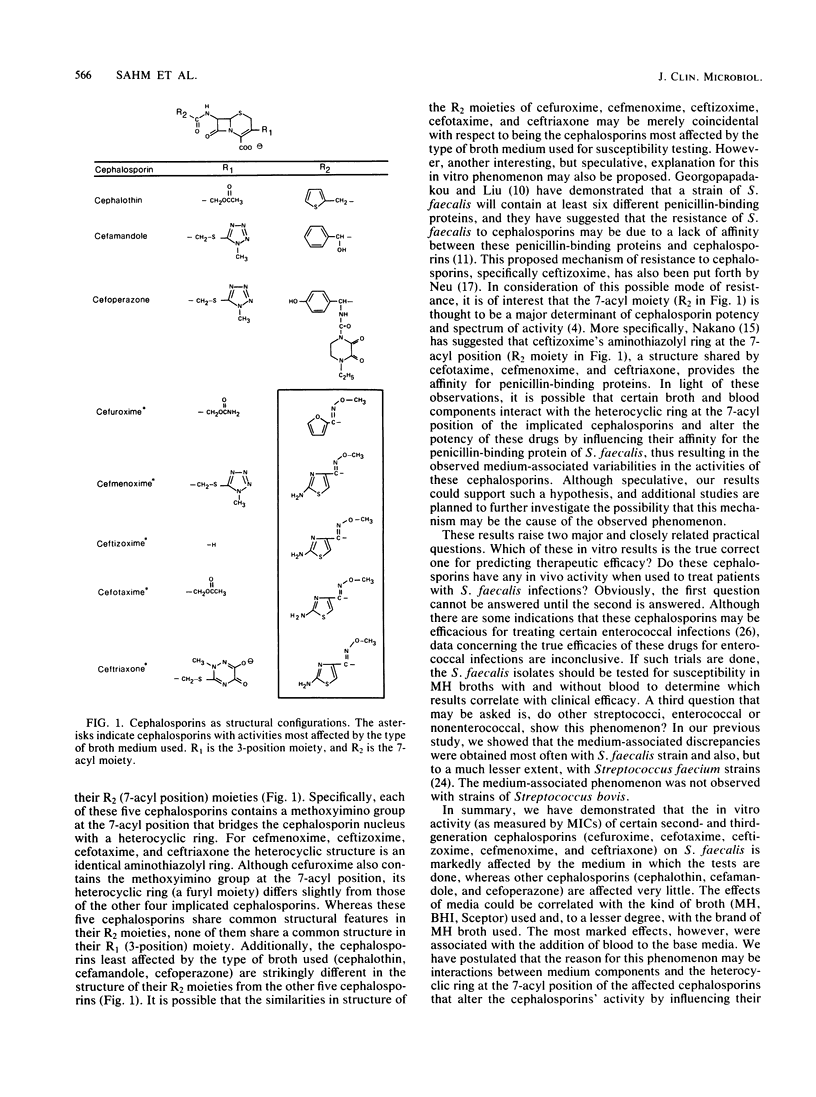
The influence of culture medium of the MICs of eight cephalosporins for 45 strains of Streptococcus faecalis was investigated. The MICs of cephalothin, cefamandole, and cefoperazone were not substantially influenced by the type of culture medium used. In contrast, MICs of cefuroxime, ceftizoxime, cefotaxime, cefmenoxime, and ceftriaxone varied markedly with both the commercial brand and the blood content of the broth used. The use of Mueller-Hinton broths (from Oxoid Ltd., GIBCO Diagnostics, and Difco Laboratories) supplemented with 5% lysed sheep blood frequently resulted in MICs that were greater than or equal to 16 times lower than the MICs obtained with these same broths without blood. Similar, but less marked, patterns were observed when supplemented and unsupplemented brain heart infusion and Sceptor broths were used. The influence of the broth on MICs suggests a complex interaction between some cephalosporins, medium components, and organisms. The cephalosporins that were affected by media share an identical moiety at the 7-acyl position (cefuroxime is slightly different), but this structure is not shared by those cephalosporins that were not affected. This commonality in structure at the 7-acyl position may be partially responsible for the observed results.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonkhai V. I., Cherubin C. E., Shulman M. A., Bancroft U. Comparative in vitro activities of cefmenoxime (SCE-1365) and newer cephalosporin derivatives of clinical utility. Antimicrob Agents Chemother. 1982 Jun;21(6):999–1002. doi: 10.1128/aac.21.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angehrn P., Probst P. J., Reiner R., Then R. L. Ro 13-9904, a long-acting broad-spectrum cephalosporin: in vitro and in vivo studies. Antimicrob Agents Chemother. 1980 Dec;18(6):913–921. doi: 10.1128/aac.18.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner V. C., Sherris J. C. Influence of different media and bloods on results of diffusion antibiotic susceptibility tests. Antimicrob Agents Chemother. 1972 Feb;1(2):116–122. doi: 10.1128/aac.1.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. L. Ceftizoxime and other third-generation cephalosporins: structure-activity relationships. J Antimicrob Chemother. 1982 Nov;10 (Suppl 100):1–10. doi: 10.1093/jac/10.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- Eickhoff T. C., Ehret J. Comparative in vitro studies of Ro 13-9904, a new cephalosporin derivative. Antimicrob Agents Chemother. 1981 Mar;19(3):435–442. doi: 10.1128/aac.19.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong I. W., Engelking E. R., Kirby W. M. Relative inactivation by Staphylococcus aureus of eight cephalosporin antibiotics. Antimicrob Agents Chemother. 1976 Jun;9(6):939–944. doi: 10.1128/aac.9.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. Antibacterial activity of ceftizoxime, a beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1980 Apr;17(4):583–590. doi: 10.1128/aac.17.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Thornsberry C., Jones R. N., Gavan T. L., Gerlach E. H., Sommers H. M. Cefotaxime: in vitro activity and tentative interpretive standards for disk susceptibility testing. Antimicrob Agents Chemother. 1980 Jul;18(1):88–93. doi: 10.1128/aac.18.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Jones R. N., Thornsberry C., Barry A. L., Gerlach E. H., Sommers H. M. Cefmenoxime (SCE-1365), a new cephalosporin: in vitro activity, comparison with other antimicrobial agents, beta-lactamase stability, and disk diffusion testing with tentative interpretive criteria. Antimicrob Agents Chemother. 1981 Dec;20(6):747–759. doi: 10.1128/aac.20.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Binding of beta-lactam antibiotics to penicillin-binding proteins of Staphylococcus aureus and Streptococcus faecalis: relation to antibacterial activity. Antimicrob Agents Chemother. 1980 Nov;18(5):834–836. doi: 10.1128/aac.18.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C. Antimicrobial activity of desacetylcefotaxime alone and in combination with cefotaxime: evidence of synergy. Rev Infect Dis. 1982 Sep-Oct;4 (Suppl):S366–S373. doi: 10.1093/clinids/4.supplement_2.s366. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Thornsberry C. Cefotaxime: a review of in vitro antimicrobial properties and spectrum of activity. Rev Infect Dis. 1982 Sep-Oct;4 (Suppl):S300–S315. doi: 10.1093/clinids/4.supplement_2.s300. [DOI] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J. Comparative activities of 13 beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jun;21(6):925–934. doi: 10.1128/aac.21.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H. Structure-activity relationships related to ceftizoxime (FK 749). Med Res Rev. 1981 Summer;1(2):127–157. doi: 10.1002/med.2610010202. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Antibacterial activity of desacetylcefotaxime alone and in combination with cefotaxime. Rev Infect Dis. 1982 Sep-Oct;4 (Suppl):S374–S378. doi: 10.1093/clinids/4.supplement_2.s374. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Aswapokee N., Aswapokee P., Fu K. P. HR 756, a new cephalosporin active against gram-positive and gram-negative aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1979 Feb;15(2):273–281. doi: 10.1128/aac.15.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Factors that affect the in-vitro activity of cephalosporin antibiotics. J Antimicrob Chemother. 1982 Nov;10 (Suppl 100):11–23. doi: 10.1093/jac/10.suppl_c.11. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P. Cefuroxime, a beta-lactamase-resistant cephalosporin with a broad spectrum of gram-positive and -negative activity. Antimicrob Agents Chemother. 1978 Apr;13(4):657–664. doi: 10.1128/aac.13.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Griffiths A., Thornton J. E. Cefuroxime, a new cephalosporin antibiotic: activity in vitro. Antimicrob Agents Chemother. 1976 Mar;9(3):511–519. doi: 10.1128/aac.9.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. R., Gerding D. N., Hall W. H., Schierl E. A. Medium-dependent variation in bactericidal activity of antibiotics against susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Apr;13(4):665–668. doi: 10.1128/aac.13.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. R., Gerding D. N., Hall W. H., Schierl E. A. Medium-dependent variation in bactericidal activity of antibiotics against susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Apr;13(4):665–668. doi: 10.1128/aac.13.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursiano T. A., Misiek M., Leitner F., Price K. E. Effect of assay medium on the antibacterial activity of certain penicillins and cephalosporins. Antimicrob Agents Chemother. 1973 Jan;3(1):33–39. doi: 10.1128/aac.3.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Baker C. N., Jones R. N., Thornsberry C. Medium-dependent zone size discrepancies associated with susceptibility testing of group D streptococci against various cephalosporins. J Clin Microbiol. 1983 Oct;18(4):858–865. doi: 10.1128/jcm.18.4.858-865.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Jones R. N., Barry A. L., Fuchs P. C. Antimicrobial susceptibility tests with cefotaxime and correlation with clinical bacteriologic response. Rev Infect Dis. 1982 Sep-Oct;4 (Suppl):S316–S324. doi: 10.1093/clinids/4.supplement_2.s316. [DOI] [PubMed] [Google Scholar]
- Verbist L., Verhaegen J. In vitro activity of Ro 13-9904, a new beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Feb;19(2):222–225. doi: 10.1128/aac.19.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., Yu P. K. Regression curve analysis of cephalosporin activity. Appl Microbiol. 1970 Apr;19(4):589–593. doi: 10.1128/am.19.4.589-593.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Frogge J. A. Hydrolysis of 3-acetoxymethyl cephalosporins by lysed whole blood. Antimicrob Agents Chemother. 1980 Jan;17(1):99–100. doi: 10.1128/aac.17.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]


