Abstract
The purpose of this archival study was to determine the prevalence and correlates of HIV-related risk behavior among adults with a severe and persistent mental illness (SPMI). Hospital records at a public psychiatric hospital were reviewed to obtain data on demographic and psychiatric characteristics, sexual behavior, and substance use. Data were available from 889 (73%) of 1214 eligible outpatients. Of these 889 patients, 49% were sexually active, 52% used alcohol, and 18% used street drugs during the past year. Eleven percent were at high risk for HIV infection: 7% reported having 3 or more sexual partners, 3% had been infected with an STD, 3% had traded sex, and fewer than 1% had shared injection equipment. HIV-risk status was modeled with logistic regression using diagnosis, type of residence, drug and alcohol use, and demographic variables; five- and six-predictor models were derived for two HIV-risk indicators. A bootstrap simulation supported the reliability of each model. We conclude that approximately one-half of adults living with a SPMI are sexually active, and a minority engages in behaviors that increase risk of HIV infection. Routine screening for HIV risk in psychiatric settings can identify patients who may benefit from risk reduction programs.
The human immunodeficiency virus (HIV) and the resulting acquired immunodeficiency syndrome (AIDS) continue to threaten the public health. The World Health Organization estimates that 30.6 million adults are now infected with HIV (Joint United Nations Programme on HIV/AIDS, 1997). In the US, estimates indicate that 650,000–900,000 persons are infected (Karon et al., 1996). Infection with HIV leads to AIDS, a condition associated with considerable premature morbidity and mortality. Indeed, AIDS has been identified as a leading cause of death among men and women aged 25–44 years (Centers for Disease Control and Prevention, 1996). Besides the human suffering implied by these data, HIV infection results in considerable social cost. Friends and family members, especially young children, must cope with the loss of loved ones. Economically, the cost of medical care is staggering: HIV health care costs from the time of infection to death approximate $95,000 (Gable, Tierce, Simison, Ward, & Motte, 1996). In addition, medical treatments are inconvenient and often associated with side effects, and they provide only palliation but not a cure.
As the epidemic in the United States has progressed, it has become clear that some segments of the population have been more vulnerable to HIV. Infection occurs disproportionately among the urban poor, including young adults who engage in sexual relationships to meet basic survival needs, to allay loneliness, as well as to meet intimacy needs (Rosenberg & Biggar, 1998). Among the most vulnerable are young adults who are living with a severe and persistent mental illness (SPMI). The SPMI descriptor has been used to refer to psychotic disorders (e.g., schizophrenia, schizoaffective disorder, mood disorders) with symptoms that (a) persist over time; (b) are functionally disabling in terms of daily living skills, social interactions, family relations, jobs, or education; and (c) require at least short-term hospitalizations (National Advisory Mental Health Council, 1993). Estimates suggest that approximately 3 million adults in the United States meet these criteria (Auerbach, Wypijewska, & Brodie, 1994).
Adults living with a SPMI appear to be at elevated risk for infection with HIV, with several studies providing alarming estimates. For example, Empfield et al. (1993) used discarded blood samples (i.e., from blood drawn for routine purposes) to complete anonymous testing of 203 homeless patients (146 men, 57 women) admitted to a New York City hospital for extended care. The patients were diagnosed with schizophrenia (51%), schizoaffective disorder (44%), or another psychotic disorder (5%), and all were aged 18 to 59 years. Their results indicated that 6.8% of men and 5.3% of women were infected with HIV. Reviews of seroprevalence studies suggest that 4% to 23% of adults living with a SPMI are infected with HIV (Carey, Weinhardt, & Carey, 1995; Cournos & McKinnon, 1997). These seroprevalence rates far exceed those found in the general population, which are estimated to be approximately 0.32% (McQuillan, Khare, Karon, Schable, & Vlahov, 1997).
Given elevated seroprevalence rates among patients with a SPMI, behavioral interventions designed to reduce the risk of HIV transmission are needed. HIV prevention programs are most likely to be effective if we understand the behavioral epidemiology specific to this population. First, we need to know the prevalence of the specific types of risk behaviors that increase the probability of transmission. Second, we need to know more about the determinants of those risk behaviors. Nearly 30 risk behavior studies have been conducted with these goals in mind. For example, we recruited a sample of 60 adults with a SPMI at an outpatient clinic and surveyed these patients regarding their HIV-related risk behavior (Carey, Carey, Weinhardt, & Gordon, 1997b). We found that 68% had sex in the last year; 13% of men and 30% of women reported two or more male partners; 24% of men also reported two or more female partners; and condom use was inconsistent. Involvement with an injection drug user (10%) or a partner known to be non-monogamous (14%) was substantial; 14% admitted to sex trading and 14% were forced to have sex against their will. Overall, 48% of men and 37% of women reported at least one risk factor. However, participants tended to rate themselves at only slight risk for infection, and indicated only modest levels of self-efficacy regarding their sexual assertiveness and related risk reduction skills.
Comprehensive reviews of the HIV-related risk behavior (Carey, Carey, & Kalichman, 1997a; Kalichman, Carey, & Carey, 1996a) have led us to conclude that HIV-related risk behavior occurs commonly among psychiatric patients, and that persons with a SPMI should receive high priority in the national HIV-prevention strategy, a conclusion shared by the National Institute of Mental Health (NIMH, 1998) and an independent group of clinical researchers (Carey & Cournos, 1997). However, four limitations characterize the literature on which these conclusions were based.
First, most risk behavior studies have relied upon modest samples recruited through therapist referral or patient volunteering. Such samples may not be representative of the larger SPMI population from which they were drawn. Second, one-half of the studies, and nearly all of those that used rigorous recruitment strategies, have been completed in New York City, an AIDS epicenter that is not representative of most US cities (Centers for Disease Control and Prevention, 1997). Third, few studies have attempted to develop a model or profile of risk to guide clinical decision-making. Thus, the information gained from prior work cannot be used optimally to assist risk reduction efforts. Finally, no study has employed validation strategies to determine the replicability of the prediction models that emerged. Thus, risk modeling that has been done most likely capitalizes on chance statistical associations that may not replicate.
The current study addressed these limitations. Thus, using archival hospital records, we attempted a risk census (rather than a convenience sample) at a public psychiatric hospital in a metropolitan area outside of an AIDS epicenter. This study sought to determine the prevalence and correlates of HIV-risk behavior in a population of adults receiving treatment for a SPMI. Archival data regarding demographic and diagnostic characteristics, sexual behavior, and substance use were used to develop the most efficient set of predictors of HIV-risk status. Based upon prior research and theory (Carey et al., 1997a), we expected that higher risk would be evident among patients who were (a) younger, (b) male, (c) single, (d) living in unsupervised residences, (e) diagnosed with Bipolar Disorder, and (f) using alcohol or other drugs. Using logistic regression, we identified predictors of HIV-risk behavior and, using bootstrapping procedures, we estimated the reliability of the models that emerged.
Method
This research relied upon archival data available from medical records at a state psychiatric hospital in Syracuse. With the approval of the hospital Institutional Review Board, we obtained data collected from outpatients aged 18 to 65 years, who spoke English and were listed on the hospital census during January, 1997–March, 1998 (N= 1,214). The mean age of eligible patients (56% male) was 41 years; 73% were European-American, 22% African-American, and 5% Other. Patients’ marital status included never married (59%), separated or divorced (26%), or married (15%); their average education was 12 years and only 7% were employed. Residences included own apartment (52%), a relative or friend’s home (16%), a supervised community residence (12%), or a boarding house/shelter (9%).
Primary psychiatric diagnoses included 29% Schizophrenia, 18% Schizoaffective Disorder, 17% Major Depression or other depressive disorder, 11% Bipolar Disorder, 5% other Psychotic Disorder, 5% Anxiety Disorder, 4% Personality Disorder, and 10% Other. These diagnoses resulted from interviews conducted by hospital psychiatrists at the time of intake using the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994). Although fully structured interviews were not done as a part of the standard clinical care, 144 of the patients included in the current study subsequently participated in a randomized clinical trial (RCT; Carey, Carey, Maisto, & Gleason, 1999), during which Structured Clinical Interviews for the Diagnostic and Statistical Manual of Mental Disorders (SCIDs; First et al., 1995) were completed. Comparison of the SCID diagnoses from the RCT with the intake diagnoses used in this study indicated that the latter were valid (Cramer’s V = .74).
Data used in this study were collected during a brief screening that occurred either at intake, or at the time of a therapy appointment or medication visit. The screen began with general health information (e.g., caffeine use, smoking) and progressed to more sensitive information (i.e., sexual behavior, substance use). Information regarding substance use was obtained from the Alcohol Use Disorders Identification Test (Saunders, Aasland, Babor, De La Fuente, & Grant, 1993) and the short Drug Abuse Screening Test (Skinner, 1982a) whereas data on sexual behavior were collected from an HIV risk screen.
Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993)
The AUDIT is a widely-used 10-item screening measure designed to identify drinkers at risk for alcohol abuse or dependence. A summary score ranging from 0–40 is obtained. AUDIT scores are internally consistent (0.83; Hays, Merz, & Nicholas, 1995) and correlate (rs= 0.62–0.88) with other widely used screening tests (Barry & Fleming, 1993; Hays et al., 1995) and with biochemical measures of drinking (Bohn, Babor, & Kranzler, 1995). A cutoff score of ≥10 identifies persons in treatment for alcohol use disorders at a high level of sensitivity (.99) and specificity (.74) (Bohn et al., 1995). Research with adults with a SPMI suggests that the AUDIT provides a sensitive and specific measure in this population (Maisto, Carey, Carey, Gleason, & Gordon, 1999). Consistent with Bohn et al. (1995), scores of 1–7 were labeled “low risk,” 8–9 “moderate risk,” and 10 or greater “high risk” for this study.
Drug Abuse Screening Test (DAST; Skinner, 1982a)
The DAST-10 is a short version of the 28-item DAST, designed to quantify the degree of drug-related problems experienced in the previous year (Skinner, 1982b). A single summary score reflects the number of drug abuse items endorsed. Prior research indicates that the DAST-10 is internally consistent (alpha = .86), temporally stable (ICC = .71), and able to discriminate between psychiatric outpatients with and without current drug abuse/dependence diagnoses (Cocco & Carey, 1998). Sensitivity and specificity with this population are optimized with a cut score of 2/3 (Maisto et al., 1999). Consistent with these findings, scores of 1–2 were considered “low risk,” 3–5 “moderate risk,” and 6 or greater “high risk” for this study.
HIV risk screen
HIV risk was assessed from responses to 5 items. Participants reported the number of sexual partners, history of sexually transmitted diseases (STDs), number of occasions they had traded sex, engaged in injection drug use (IDU), and shared needles. All items have been used previously with this population (Weinhardt, Carey, Carey, & Verdecias, 1998a). The HIV risk screen yielded two risk markers. First, given the prevalence of HIV infection, the transient relationships, and the low rates of condom use in this population (Carey et al., 1997a), any penetrative sexual activity was considered to be a marker of HIV risk (“Sexually Active”). Second, a more restrictive marker (“High Risk”) involved one or more of the following: 3 or more sexual partners; an STD; sex trading; IDU; or needle-sharing.
Results
Overview of Analyses
The data analyses are presented in three sections. First, we compare screened patients from non-screened to identify possible biasing effects due to availability. Second, we summarize the prevalence of HIV-related risk behaviors, including substance use, and provide univariate descriptions of the HIV risk markers and of their correlates. Third, we characterize the participants who were (or were not) at risk according to each of the HIV risk markers. To do so, we developed and cross-validated logistic regression models to predict risk status (yes vs. no) on both the low (“Sexual Activity”) and high (“High Risk”) HIV risk markers. In this effort, we tested hypothesized relationships but did not confine attention to predictors that have previously been suggested on theoretical or empirical grounds. Our objective was to construct regression models that could, with a small number of variables, predict HIV risk status in the present risk census.
Representativeness of the Screening Data
Table 1 provides demographic and diagnostic information for all hospital outpatients by screening status. Screens were completed with 889 outpatients, representing 73% of the eligible population. Screens were not completed for 325 outpatients (146 were not scheduled for any appointments or were not be located during the study interval; 122 were discharged prior to being screened; 27 refused to be screened; 20 were either physically disable, incarcerated, or institutionalized prior to being screened; and 10 were in acute distress). Screened patients were slightly older, more likely to be diagnosed with Schizophrenia or Schizoaffective Disorder, and less likely to be diagnosed with Major Depression or an Other Disorder.
Table 1.
Demographic and Diagnostic Information by Screening Status
| Screened (n = 889) | Unscreened (n = 325) | t/X2 (df) | p | |
|---|---|---|---|---|
| Age | 41.8 (10.3) | 40.4 (10.7) | 1.94 (1208) | .05 |
| Gender | 1.23 (1) | .27 | ||
| Men | 505 (57%) | 173 (53%) | ||
| Women | 384 (43%) | 152 (47%) | ||
| Ethnicity | 4.65 (2) | .10 | ||
| European-American | 664 (75%) | 227 (70%) | ||
| African-American | 186 (21%) | 75 (23%) | ||
| Other | 39 (4%) | 23 (7%) | ||
| Marital Status | 3.52 (3) | .32 | ||
| Single | 534 (60%) | 177 (54%) | ||
| Married | 84 (9%) | 39 (12%) | ||
| Sep/Div/Widow | 236 (27%) | 95 (29%) | ||
| Unknown | 35 (4%) | 14 (4%) | ||
| Primary Diagnosis | 27.17 (4) | <.0001 | ||
| Schizophrenia | 283 (32%) | 70 (22%) | ||
| Schizoaffective | 177 (20%) | 46 (14%) | ||
| Bipolar | 97 (11%) | 39 (12%) | ||
| Major Depression | 75 (8%) | 45 (14%) | ||
| Other | 257 (29%) | 125 (38%) | ||
| Residence Type | .02 (2) | .99 | ||
| Supervised | 304 (34%) | 110 (34%) | ||
| Unsupervised | 499 (56%) | 184 (57%) | ||
| Unknown | 86 (10%) | 31 (10%) |
Univariate Description of HIV and Substance Use Risk Behavior
Among screened patients, 49% reported that they had been Sexually Active in the past year. Univariate analyses indicated that sexual activity status differed as a function of age, ethnicity, marital status, diagnostic category, and residence type. As detailed in Table 2 (Panel a), Sexually Active patients were more likely to be younger, African-American, married, and diagnosed with a Mood or Other Disorder, and living in an unsupervised setting.
Table 2.
Demographic and Diagnostic Information by (a) Sexually Activity and (b) High Risk Status
| (a) Sexually Active | (b) High Risk | |||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||||
| n=457 (51%) | n=432 (49%) | t/X2 (df) | p | n =788 (89%) | n=100 (11%) | t/X2 (df) | p | |
| Age | 44. 5 (10.1) | 38.9 (9.8) | 8.31 (883) | <.0001 | 42.3 (10.3) | 37.3 (9.7) | 4.65 (882) | <.0001 |
| Gender | 1.00 (1) | .32 | 3.83 (1) | .05 | ||||
| Men | 267 (53%) | 238 (47%) | 349 (91%) | 34 (9%) | ||||
| Women | 190 (49%) | 194 (51%) | 439 (87%) | 66 (13%) | ||||
| Ethnicity | 21.87 (2) | <.0001 | 10.94 (2) | .004 | ||||
| European-American | 373 (56%) | 294 (44%) | 600 (90%) | 66 (10%) | ||||
| African-American | 67 (37%) | 112 (63%) | 147 (82%) | 32 (18%) | ||||
| Other | 17 (40%) | 26 (60%) | 41 (95%) | 2 (5%) | ||||
| Marital Status | 22.80 (3) | <.0001 | 9.88 (3) | .02 | ||||
| Single | 298 (56%) | 236 (44%) | 464 (87%) | 70 (13%) | ||||
| Married | 24 (29%) | 60 (71%) | 77 (92%) | 7 (8%) | ||||
| Sep/Div/Widow | 115 (49%) | 121 (51%) | 219 (93%) | 16 (7%) | ||||
| Unknown | 20 (57%) | 15 (43%) | 28 (80%) | 7 (20%) | ||||
| Primary Diagnosis | 44.18 (4) | <.0001 | 8.31 (4) | .08 | ||||
| Schizophrenia | 186 (66%) | 97 (34%) | 254 (90%) | 29 (10%) | ||||
| Schizoaffective | 95 (54%) | 82 (46%) | 164 (93%) | 13 (7%) | ||||
| Bipolar | 46 (47%) | 51 (53%) | 88 (91%) | 9 (9%) | ||||
| Major Depression | 30 (40%) | 45 (60%) | 66 (88%) | 9 (12%) | ||||
| Other | 100 (39%) | 157 (61%) | 216 (84%) | 40 (16%) | ||||
| Residence Type | 7.10 (2) | .03 | 0.20 (2) | .90 | ||||
| Supervised | 173 (57%) | 131 (43%) | 268 (88%) | 36 (12%) | ||||
| Unsupervised | 237 (47%) | 262 (53%) | 444 (89%) | 54 (11%) | ||||
| Unknown | 47 (55%) | 39 (45%) | 76 (89%) | 10 (11%) | ||||
Eleven percent of the screened patients (n = 100) could be classified as High Risk for HIV. Seven percent reported three or more sexual partners in the past year, 3% reported a STD, 3% reported sex trading, and fewer than 1% reported needle sharing (totals exceed 11% because some patients reported more than one risk indicator). Of the 100 patients identified as High Risk, 80 reported a single risk factor, 15 reported two risk factors, and 5 reported three risk factors. Univariate analyses (summarized in Table 2, Panel b) revealed that patients classified as High Risk for HIV were more likely to be younger, female, African-American, and single (or of unknown marital status). The majority (94%) of patients identified as High Risk were sexually active, with only 6% of High Risk patients being so designated due to injection drug use or needle-sharing exclusively.
Alcohol use during the past year was reported by 52% of the sample, with 36% reporting monthly use, 7% weekly use, and 7% daily use. Among drinkers, 12% reported drinking ten or more drinks on a typical day, 13% reported five to nine drinks, 21% reported three or four drinks, and 54% reported one or two drinks. Twenty-two percent reported drinking six or more drinks at least once during the reporting period. The following risk classification for alcohol abuse was obtained: 37% no risk, 43% low risk, 4% moderate risk, and 15% high risk. Street drug use was reported by 18% of the sample during the past year; risk classifications for drug abuse using DAST scores indicated that 79% were at no risk, 8% low risk, 7% moderate risk, and 6% high risk.
Modeling HIV Risk
A three-stage process was used to model HIV risk. In stage 1, a logistic regression model was developed to predict status on each of the two HIV risk markers, selecting from the pool of variables hypothesized to be related to risk: age, gender, type of residence (supervised or not), diagnosis (bipolar vs. others, and schizophrenia vs. others), self-reported alcohol use, DAST risk classification score, race (African-American vs. others) and — for the High Risk marker — marital status (unmarried vs. married). Except for age and DAST risk, each of these factors was defined as an indicator variable. A preliminary model for each risk index was then selected using forward stepwise logistic regression; the criterion for entry into the model was that a predictor must have a coefficient with nominal p-value less than 0.05.
In stage 2, the predictors selected in the first stage were examined for possible interaction effects and for potentially helpful transformations. This resulted in a new pool of predictors, from which a new regression model was developed by dropping candidate predictors from the pool (i.e., by backward elimination); this step used the criterion that each predictor must have a coefficient with nominal p-value less than 0.01 to be retained in the model.
In stage 3, we performed a bootstrap simulation (Davison & Hinkley, 1997) to validate the logistic regression models selected in the second stage. Bootstrapping refers to a non-parametric technique that simulates the effects of repeated sampling of the variables being analyzed from the population of interest by repeatedly sampling from the available sample. This simulation served two purposes: First, the modeling process described earlier is known to yield p-values and confidence intervals for regression coefficients that are too optimistic, just as would be true after using a variable selection technique in ordinary multiple regression. Bootstrapping provides a more conservative estimate of the importance of the predictors in a selected model, by examining the fit of the model when applied to other (simulated) samples from the same population. Second, the same bootstrap simulation can be used to construct a confidence interval for the area under the Receiver Operating Characteristic (ROC) curve. The ROC curve is the graph of sensitivity versus false positive rate over all possible cutpoints on the model’s predicted probability of being at risk, and provides the principal index of predictive utility for our models.
In the bootstrap simulation, each risk index was regressed on the final predictors selected at stage 2, for each of 2000 random bootstrap resamples; in addition, the final prediction equation calculated at stage 2 (using the coefficients from the original sample) was applied to each of the bootstrap resamples, and the area under the resulting ROC curve was calculated. For each risk index, a bias-adjusted confidence interval was then calculated from the 2000 bootstrap resamples of each regression coefficient, as well as of the area under the ROC curve.
The first stage of model building for the Sexually Active marker yielded eight predictors, two of which (Bipolar and Gender) were eliminated during the backward elimination step at stage 2. It was also found at stage 2 that the resulting model could be improved considerably by incorporating race in the form of two distinct coefficients for age, rather than as an (additive) indicator variable. The first 2 stages resulted in a 6-predictor logistic regression model. Table 3 presents the six predictors, estimated regression weights, and area under the ROC curve of the logistic regression model selected at stage 2, along with the corresponding 95% confidence intervals obtained from the bootstrap simulation performed at stage 3. The coefficient for Unsupervised Residence was only marginally significant (p = 0.03); for the other five coefficients, p ≤0.001. The area under the ROC curve of this model was 0.734, and the confidence interval did not include 0.5, the value indicating only a chance relationship between the predictors and criterion.
Table 3.
Logistic Regression Model for the Sexually Active Marker
| Predictor | Coefficient | [Bootstrap 95% C. I.] |
|---|---|---|
| DAST risk summary score | 0.387 | [0.174, 0.614] |
| Age (non-African-American participants) | −0.050 | [−0.066, −0.035] |
| Age (African-American participants) | −0.030 | [−0.047, −0.013] |
| Indicator for Schizophrenia diagnosis | −0.762 | [−1.062, −0.450] |
| Indicator for Unsupervised residence | 0.329 | [0.034, 0.632] |
| Indicator for Alcohol use (≥1 drinks, past year) | 0.528 | [0.235, 0.833] |
|
| ||
| Area under the ROC curve | 0.734 | [0.702, 0.768]□ |
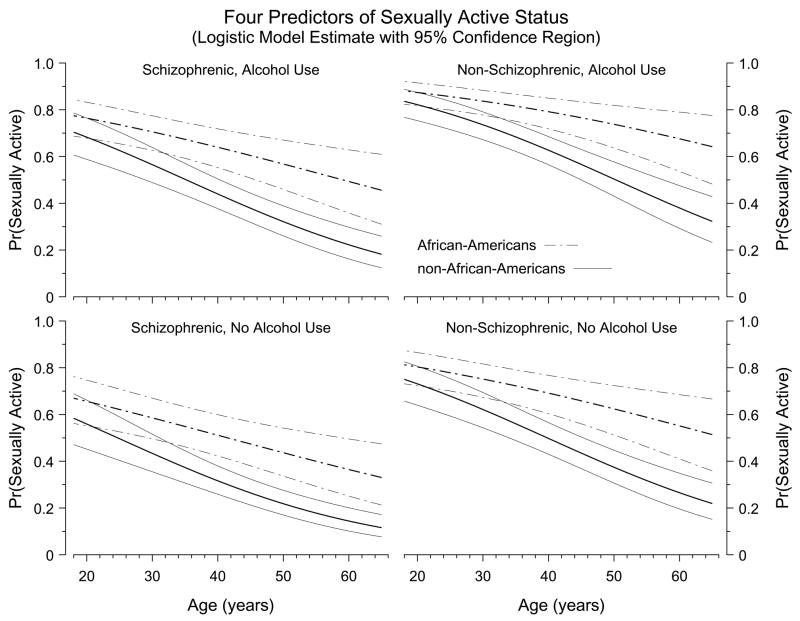
Figure 1 displays the estimated probability of risk for the Sexually Active marker, demonstrating the effects of four of the six predictors in the model; the abscissa of each panel is participant age, the strongest single predictor of the Sexually Active marker. Figure 1 demonstrates that the chance of being sexually active tends to decrease with increasing age. The decrease occurs within each of the subgroups of participants portrayed in Figure 1, but the rate of decline is slower among African-Americans than among non-African-Americans. In addition, there is a marked difference between the schizophrenic and non-schizophrenic groups in the likelihood of being sexually active. The effect of the DAST score (not illustrated in Figure 1) is to increase the overall likelihood of Sexual Activity.
Figure 1.
Each panel displays the probability of being Sexually Active during the past year as a function of participant age. The thick lines plot the estimated probability of risk and the thin lines form pointwise 95% confidence bands around that estimate, as derived from the six-predictor logistic regression model; dashed lines show estimates for African-American participants, solid lines for non-African-Americans. The four panels correspond to the possible combinations of two indicator variables in the regression model: schizophrenic diagnosis (Yes/No) and self-reported alcohol use within the past year (Yes/No). Thus, the figure portrays four of the six predictors in the logistic regression model (ROC curve area = 0.722). The predictors not represented in the figure are DAST risk classification score and residence type (supervised vs. unsupervised); adding those two predictors increases the ROC curve area to 0.734.
The stage 1 model for the High Risk marker included the above nine theoretically-motivated predictors, of which five were removed during the backward elimination step at stage 2: Unmarried, Bipolar, Gender, Schizophrenic, and Unsupervised Residence. The predictors remaining were age, DAST risk score, the African-American indicator, and a binge-drinking indicator. Stage 2 also revealed important interactions involving gender and marital status. Adding such interactions to the pool and repeating the backward elimination step then led to a substantially improved 5-predictor model for the High Risk marker. Table 4 shows estimated the estimated coefficient and the definition for each predictor. The coefficient for Male Schizophrenic was only marginally significant (p = 0.04); for each of the remaining coefficients, p ≤ 0.005. The area under the ROC curve of this model was 0.761; a bootstrap 95% confidence interval for this area appears in Table 4, along with a similar interval for each regression coefficient.
Table 4.
Logistic Regression Model for the High Risk Marker
| Predictor | Coefficient | [Bootstrap 95% C. I.] |
|---|---|---|
| DAST risk score | 0.681 | [0.458, 0.866] |
| Indicator for African-American participants | 0.704 | [0.155, 1.181] |
| Indicator for Male with Schizophrenia diagnosis | −0.565 | [−1.162, 0.013] |
| Indicator for Male “Never Married” or “Marital Status Unknown” | 1.420 | [0.784, 2.059]□ |
| Indicator for Female with report of binge drinking (≥6 drinks on a single occasion) | 1.615 | [0.864, 2.371]□ |
|
| ||
| Area under the ROC curve | 0.761 | [0.706, 0.815] |
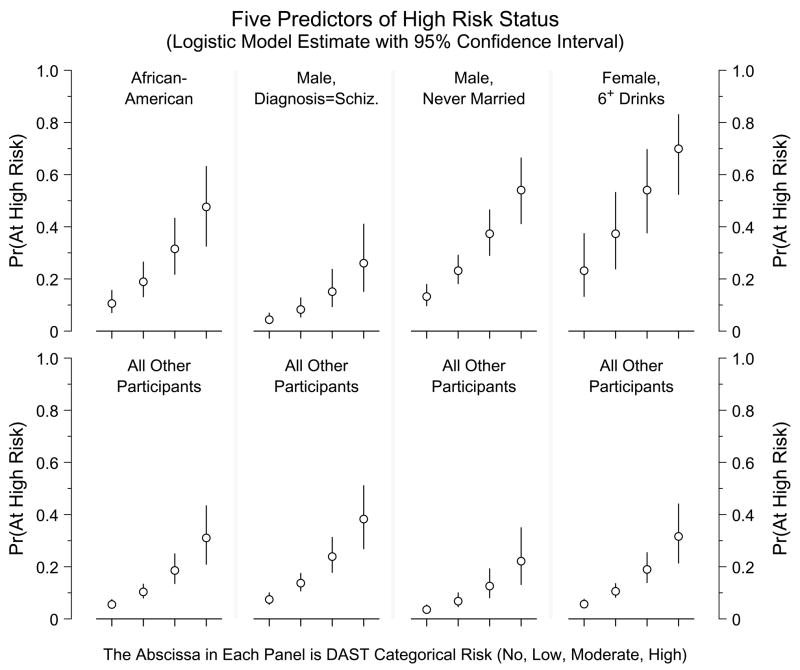
Figure 2 displays the estimated risk probabilities (and their 95% confidence intervals) for the High Risk index, and demonstrates the effect of each predictor in the model; the top panels plot risk for persons positive for the indicator and the bottom panels plot risk for persons negative for that indicator. The abscissa of each panel is the DAST risk classification, the strongest single predictor of the High Risk index. Within each of the portrayed subgroups of participants, the estimated chance of being at high risk increases with the participant’s DAST risk classification. Males who never married and females who reported episodes of binge drinking display particularly high predicted risk probabilities.
Figure 2.
The open circles plot the estimated risk probability for the High Risk marker, as derived from the five-predictor logistic regression model; the attached vertical lines portray pointwise 95% confidence intervals. The abscissa is DAST risk status, one of the five predictors in the model. Each of the other predictors is an indicator variable distinguishing two subsets of participants; the upper panel in each section shows predictions for one subset, the lower panel for the other subset of participants. (Except for the second section, the upper panel represents increased probability of High Risk.) First section: African-Americans vs. all others; second section: males with diagnosis of Schizoprenia vs. all others; third section: males with “Never married” or “Unknown marital status” vs. all others; fourth section: females who reported having had six or more drinks on a single occasion vs. all others. The area under the ROC curve is 0.761 using all five predictors; the strongest single predictor is the DAST classification (ROC area = 0.670).
Discussion
This study used archival data from 73% of the eligible outpatients at a large public psychiatric hospital. This rate of inclusion represents a significant improvement over the use of convenience samples in previous research, and allows for stronger inferences. The results indicated that 49% of screened patients reported that they had been sexually active in the previous year with 11% classified as being at high risk for possible HIV infection. Also in the past year, 52% reported using alcohol, and 18% reported using street drugs. These rates of sexual behavior, HIV-risk taking, and substance use are somewhat lower than the rates found in previous research (Carey et al., 1997a, 1997b; Drake & Wallach, 1989; Kalichman, Kelly, Johnson, & Bulto, 1994; Kelly et al., 1992; Mueser et al., 1990; Test, Wallisch, Allness, & Ripp, 1989); however, these rate differences may reflect methodological variation across studies. That is, previous research has tended to use convenience samples, which may have inadvertently excluded patients at lower risk. Convenience samples tend to be smaller and to recruit participants through patient volunteering or therapist referral. In contrast, this study used archival data obtained during routine clinical care; thus, we included patients who would probably not have been referred by their therapist, nor would they have volunteered for a research study on sexual behavior or substance use because of their (presumed) inactivity or lower risk on these behaviors. If this hypothesis is accurate, it is not surprising that the prevalence estimates that we provide are lower than those reported previously. Clearly, this hypothesis warrants investigation in additional large sample, or population, studies.
Despite minor differences with prior research, the current research documents clearly that HIV-risk taking occurs in a sizable percentage of adults living with a SPMI in community settings. Our results confirm the importance of routine screening of psychiatric outpatients to determine their risk for possible HIV infection. Use of a very brief screen, requiring no more than 10 minutes, may help to identify the small but important subset of persons who are at elevated risk for infection with HIV, substance abuse, and related problems. Brief, psychometrically-sound screening measures are now available for alcohol use (AUDIT; Saunders et al., 1993), drug use (Skinner, 1982a), and sexual behavior (HIV-risk screening instrument; Gerbert, Bronstone, McPhee, Pantilat, & Allerton, 1998). Screening should not take the place of a comprehensive risk assessment, but can help to determine for whom a more detailed assessment might be important.
Using model building analytic strategies, we were also able to develop and validate a profile of those patients most likely to engage in risky practices. Sexual Activity was predicted by younger age (particularly for non-African-Americans), alcohol and drug use, residence in a non-supervised setting, and diagnosis with a disorder other than schizophrenia, whereas High Risk behavior was predicted by drug use, being an African-American, and in special circumstances, gender. Unlike previous studies, both of these models were validated through bootstrapping procedures, enhancing confidence in their reliability.
The emergence of alcohol and drug use as predictors of risk in both models confirms prior findings, theory, and clinical experience, which forecast such an association (Leigh & Stall, 1993). Although causal interpretation of our findings is not warranted, previous experimental research demonstrated the deleterious effects of alcohol use on sexual decision-making and interpersonal skills (Gordon, Carey, & Carey, 1997). Among persons with a SPMI, those who abuse substances tend to be more socially competent but more unstable clinically than non-substance-abusers (Buckley, Thompson, Way, & Meltzer, 1994; Dixon, Haas, Weiden, Sweeney, & Frances, 1991). Settings where alcohol and drugs are used are less likely to be supervised, a characteristic shared by the residence predictor in the model of Sexual Activity. Identification of substance use as a risk factor is noteworthy, especially because it does not reflect IDU exclusively, and warrants increased clinical attention. However, interpretation of the substance use--risky sex association remains complicated because of the “third variable” problem. That is, both types of behavior occur frequently in younger males who have lower education and better premorbid adjustment (Mueser, Bellack, & Blanchard, 1992). Research indicates that a disposition toward sensation seeking may also explain some of the variance in both types of behavior (Kalichman, Heckman, & Kelly, 1996b).
The influence of schizophrenia on HIV-risk taking was also consistent with previous research (Carey et al., 1997a). In the model of Sexual Activity, presence of a schizophrenia diagnosis was associated with less sexual activity. For the High Risk marker, this association held only for men. The lessened risk for persons with schizophrenia may reflect the anhedonia and impoverished social skills often observed with this disorder (Andreasen, Flaum, Swayze, Tyrell, & Arndt, 1990). Contrary to our expectation, Bipolar Disorder was not associated with either Sexual Activity or the High Risk marker.
Gender, age, and ethnicity also forecast risk. Males who never married and those whose marital status could not be determined reported higher risk; it might be speculated that these men lack the social skills needed to establish more stable relationships and resort to higher risk activities to fulfill intimacy needs. Females’ risk was elevated only for those who also reported binge drinking; it is likely that women who drink large quantities at a single occasion may be more vulnerable to sexual coercion (Weinhardt, Bickham, & Carey, 1999).
Ethnic minority status interacted with age (Sexual Activity) and emerged as a main effect (High Risk) to predict risk. In both cases, minority status was associated with greater risk, consistent with prior findings with both the general population (Laumann, Gagnon, Michael, & Michaels, 1994), and persons with a SPMI (Cournos et al., 1991; Cournos, Horwath, Guido, McKinnon, & Hopkins, 1994b). The main effects of age may reflect the effects of aging, a cohort effect, or both. However, previous research in a variety of samples (Laumann et al., 1994), including adults with a SPMI (Cournos et al., 1994a), suggests that the frequency of sexual activity declines over time. Younger persons with a SPMI tend to be more sexually active, and to engage in higher risk practices.
Risk modeling results indicate characteristics that may be associated with higher risk. Risk profiling cannot substitute for careful screening, but can be used to guide the collection of additional data, often through qualitative or more intensive survey studies, that will facilitate the refinement of context-specific, HIV-risk reduction interventions. Although behavior therapists have begun to develop HIV risk reduction programs for adults with a SPMI (e.g., Kelly et al., 1997; Weinhardt et al., 1998a), such programs need to be improved and disseminated more widely. The Office on AIDS at the NIMH has identified this research as a priority area for support (NIMH, 1998).
These prevalence and modeling findings should be interpreted mindful of the study’s limitations. First, the risk behaviors described herein were obtained through self-report. However, self-report remains the current standard regarding the assessment of private behavior, and the procedures used were informed by prior research (Maisto, McKay, & Connors, 1990; Weinhardt, Forsyth, Carey, Jaworski, & Durant, 1998b) to maximize the reliability and validity of the reports obtained. Second, because this was an archival study, psychiatric diagnoses and other data were obtained from medical records, rather than through a structured diagnostic interview. Thus, it was not possible to provide the type of psychometric evidence of reliability and validity that is available for smaller scale investigations. Third, 27% of the hospital census could not be screened. The primary difference between screened and unscreened patients was that the former were more likely to be diagnosed with a schizophrenia-spectrum disorder rather than a mood disorder. This finding, and the data indicating that many of those who were not screened were discharged prior to completing the screen, suggest that screened patients may have been more severely impaired than unscreened patients. Fourth, because this study was a population-wide archival study, we were not able to provide a detailed or comprehensive portrait of risk behavior and its determinants, information that is better obtained by smaller survey and qualitative studies. Data obtained from screening measures often lack specificity, and are interpreted more appropriately as providing an “index of suscipion” that risk behavior may be present. Finally, as with any investigation completed at a single site, these results may not be representative of all adults living with a SPMI. Although there is no reason to suspect that our participants differ in any systematic way from other adults living with a SPMI in other small US cities, it is likely that risk rates will be higher in larger cities or settings were injection drug use is more prevalent.
Acknowledgments
This study was supported by a grant from the NIMH to the first author (R01-MH54929). The authors thank Kristin Barnes, Connie Basta, Susan Bland, Brian Borsari, Christopher Correia, Lauren Durant, Don Fredericks, Julie Fuller, JulieAnn Hartley, Deborah Kahkejian, Jaejin Kim, Pat Lewis, William Licurse, Jeanette Mattson, Teal Pedlow, David Peppel, Eileen Ryan, and Lance Weinhardt for their assistance with the Health Improvement Project. Correspondence concerning this article should be sent to Michael P. Carey, Center for Health and Behavior, 430 Huntington Hall, Syracuse University, Syracuse, NY 13244-2340.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: Author; 1994. [Google Scholar]
- Andreasen NC, Flaum M, Swayze VW, Tyrell G, Arndt S. Positive and negative symptoms in schizophrenia: A critical reappraisal. Archives of General Psychiatry. 1990;47:615–621. doi: 10.1001/archpsyc.1990.01810190015002. [DOI] [PubMed] [Google Scholar]
- Auerbach JD, Wypijewska C, Brodie HKH. AIDS and behavior: An integrated approach. Washington, DC: National Academy Press; 1994. [Google Scholar]
- Barry KL, Fleming MF. The Alcohol Use Disorders Screening Test (AUDIT) and the SMAST-13 predictive validity in a rural primary care sample. Alcohol and Alcoholism. 1993;28:33–42. [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Screening Test (AUDIT): Validation of a screening instrument for use in medical settings. Journal of Studies on Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Buckley P, Thompson P, Way L, Meltzer HY. Substance abuse among patients with treatment-resistant schizophrenia: Characteristics and implications for clozapine therapy. American Journal of Psychiatry. 1994;151:385–389. doi: 10.1176/ajp.151.3.385. [DOI] [PubMed] [Google Scholar]
- Carey MP, Carey KB, Kalichman SC. Risk for human immunodeficiency virus (HIV) infection among adults with a severe mental disorder. Clinical Psychology Review. 1997a;17:271–291. doi: 10.1016/s0272-7358(97)00019-6. [DOI] [PubMed] [Google Scholar]
- Carey MP, Carey KB, Maisto SA, Gleason JR. Reducing HIV infection among the seriously mentally ill. Research in progress 1999 [Google Scholar]
- Carey MP, Carey KB, Weinhardt LS, Gordon CM. Behavioral risk for HIV infection among adults with a severe and persistent mental illness: Patterns and psychological antecedents. Community Mental Health Journal. 1997b;33:133–142. doi: 10.1023/a:1022423417304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Cournos F. HIV and AIDS among the severely and persistently mentally ill: Introduction to the special series. Clinical Psychology Review. 1997;17:241–245. doi: 10.1016/s0272-7358(97)00016-0. [DOI] [PubMed] [Google Scholar]
- Carey MP, Weinhardt LS, Carey KB. Prevalence of infection with HIV among the seriously mentally ill: Review of research and implications for practice. Professional Psychology: Research and Practice. 1995;26:262–268. [Google Scholar]
- Centers for Disease Control and Prevention. Mortality patterns -- United States, 1993. Morbidity and Mortality Weekly Report. 1996;45:161–164. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. Vol. 9. Atlanta, GA: Public Health Service; 1997. [Google Scholar]
- Cocco KM, Carey KB. Psychometric properties of the Drug Abuse Screening Test in psychiatric outpatients. Psychological Assessment. 1998;10:408–414. [Google Scholar]
- Cournos F, Empfield M, Horwath E, McKinnon K, Meyer I, Schrage H, Currie C, Agosin B. HIV seroprevalence among patients admitted to two psychiatric hospitals. American Journal of Psychiatry. 1991;148:1225–1230. doi: 10.1176/ajp.148.9.1225. [DOI] [PubMed] [Google Scholar]
- Cournos F, Guido JR, Coomaraswamy S, Meyer-Bahlburg H, Sugden R, Horwath E. Sexual activity and risk of HIV infection among patients with schizophrenia. American Journal of Psychiatry. 1994a;151:228–232. doi: 10.1176/ajp.151.2.228. [DOI] [PubMed] [Google Scholar]
- Cournos F, Horwath E, Guido JR, McKinnon K, Hopkins N. HIV-1 infection at two public psychiatric hospitals in New York City. AIDS Care. 1994b;6:443–452. doi: 10.1080/09540129408258659. [DOI] [PubMed] [Google Scholar]
- Cournos F, McKinnon K. HIV seroprevalence among people with severe mental illness in the United States: A critical review. Clinical Psychology Review. 1997;17:259–270. doi: 10.1016/s0272-7358(97)00018-4. [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV. Bootstrap methods and their application. New York: Cambridge University Press; 1997. [Google Scholar]
- Dixon L, Haas G, Weiden PJ, Sweeney J, Frances AJ. Drug abuse in schizophrenic patients: Clinical correlates and reasons for use. American Journal of Psychiatry. 1991;148:224–230. doi: 10.1176/ajp.148.2.224. [DOI] [PubMed] [Google Scholar]
- Drake RE, Wallach MA. Substance abuse among the chronically ill. Hospital and Community Psychiatry. 1989;40:1041–1046. doi: 10.1176/ps.40.10.1041. [DOI] [PubMed] [Google Scholar]
- Empfield M, Cournos F, Meyer I, McKinnon K, Horwath E, Silver M, Schrage H, Herman R. HIV seroprevalence among homeless patients admitted to a psychiatric inpatient unit. American Journal of Psychiatry. 1993;150:47–52. doi: 10.1176/ajp.150.1.47. [DOI] [PubMed] [Google Scholar]
- First MG, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-- Patient version (SCID-I/P, Version 2.0) New York: New York State Psychiatric Institute, Biometrics Department; 1995. [Google Scholar]
- Gable CB, Tierce JC, Simison D, Ward D, Motte K. Costs of HIV+/AIDS at CD4+ counts disease stages based on treatment protocols. Journal of Acquired Immunodefiency Syndrome and Human Retrovirology. 1996;12:413–420. doi: 10.1097/00042560-199608010-00013. [DOI] [PubMed] [Google Scholar]
- Gerbert B, Bronstone A, McPhee S, Pantilat S, Allerton M. Development and testing of an HIV-risk screening instrument for use in health care settings. American Journal of Preventive Medicine. 1998;15:103–113. doi: 10.1016/s0749-3797(98)00025-7. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Carey MP, Carey KB. Effects of a drinking event on behavioral skills and condom attitudes in men: Implications for HIV risk from a controlled experiment. Health Psychology. 1997;16:490–495. doi: 10.1037//0278-6133.16.5.490. [DOI] [PubMed] [Google Scholar]
- Hays RD, Merz JF, Nicholas R. Response burden, reliability, and validity of the CAGE, Short MAST, and AUDIT alcohol screening measures. Behavior Research Methods, Instruments, & Computers. 1995;27:277–280. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS. Report on the Global HIV/AIDS Epidemic. Geneva: Author; 1997. [Google Scholar]
- Kalichman SC, Carey MP, Carey KB. Human immunodeficiency virus (HIV) risk among the seriously mentally ill. Clinical Psychology: Science and Practice. 1996a;3:130–143. [Google Scholar]
- Kalichman SC, Heckman T, Kelly JA. Sensation seeking as an explanation for the association between substance use and HIV-related risky sexual behavior. Archives of Sexual Behavior. 1996b;25:141–154. doi: 10.1007/BF02437933. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Kelly JA, Johnson JR, Bulto M. Factors associated with risk for HIV infection among chronic mentally ill adults. American Journal of Psychiatry. 1994;151:221–227. doi: 10.1176/ajp.151.2.221. [DOI] [PubMed] [Google Scholar]
- Karon JM, Rosenberg PS, McQuillan G, Khare M, Gwinn M, Petersen LR. Prevalence of HIV infection in the United States, 1984 to 1992. Journal of the American Medical Association. 1996;276:126–131. [PubMed] [Google Scholar]
- Kelly JA, McAuliffe TL, Sikkema KJ, Murphy DA, Somlai AM, Mulry G, Miller JG, Stevenson LY, Fernandez MI. Reduction in risk behavior among adults with severe mental illness who learned to advocate for HIV prevention. Psychiatric Services. 1997;48:1283–1288. doi: 10.1176/ps.48.10.1283. [DOI] [PubMed] [Google Scholar]
- Kelly JA, Murphy DA, Bahr GR, Brasfield TL, Davis DR, Hauth AC, Morgan MlG, Stevenson LY, Eilers MK. AIDS/HIV risk behavior among the chronic mentally ill. American Journal of Psychiatry. 1992;149:886–889. doi: 10.1176/ajp.149.7.886. [DOI] [PubMed] [Google Scholar]
- Laumann EO, Gagnon JH, Michael RT, Michaels S. The social organization of sexuality: Sexual practices in the United States. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- Leigh BC, Stall R. Substance use and risky sexual behavior for exposure to HIV: Issues in methodology, interpretation, and prevention. American Psychologist. 1993;48:1035–1045. doi: 10.1037//0003-066x.48.10.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Carey MP, Carey KB, Gleason JR, Gordon CM. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. 1999 doi: 10.1037//1040-3590.12.2.186. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Maisto SA, McKay JR, Connors GJ. Validity of drug abusers’ self-reports of drug use and related behaviors. Behavioral Assessment. 1990;12:117–134. [Google Scholar]
- McQuillan GM, Khare M, Karon JM, Schable CA, Vlahov D. Update on the seroepidemiology of human immunodeficiency virus in the United States household population: NHANES III, 1988–1994. Journal of Acquired Immunodefiency Syndrome and Human Retrovirology. 1997;14:255–360. doi: 10.1097/00042560-199704010-00008. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Bellack AS, Blanchard JJ. Comorbidity of schizophrenia and substance abuse: Implications for treatment. Journal of Consulting and Clinical Psychology. 1992;60:845–856. doi: 10.1037//0022-006x.60.6.845. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Yarnold PR, Levinson DF, Singh H, Bellack AS, Kee K, Morrison RL, Yadalam KG. Prevalence of substance abuse in schizophrenia: Demographic and clinical correlates. Schizophrenia Bulletin. 1990;16:31–55. doi: 10.1093/schbul/16.1.31. [DOI] [PubMed] [Google Scholar]
- National Advisory Mental Health Council. Health care reform for Americans with severe mental illnesses: Report of the National Advisory Mental Health Council. American Journal of Psychiatry. 1993;150:1447–1465. doi: 10.1176/ajp.150.10.1447. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. HIV/AIDS and the severely mentally ill. 1998 (Program Announcment available at x http://www.nih.gov/grants/guide/pa-files/PA-98-080.html).
- Rosenberg PS, Biggar RJ. Trends in HIV incidence among young adults in the United States. Journal of the American Medical Association. 1998;1998:1894–1899. doi: 10.1001/jama.279.23.1894. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Screening Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The Drug Abuse Screening Test. Addictive Behaviors. 1982a;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The Drug Abuse Screening Test (DAST): Guidelines for administration and scoring. Toronto: Addiction Research Foundation; 1982b. [Google Scholar]
- Test MA, Wallisch LS, Allness DJ, Ripp K. Substance use in young adults with schizophrenia. Schizophrenia Bulletin. 1989;15:465–475. doi: 10.1093/schbul/15.3.465. [DOI] [PubMed] [Google Scholar]
- Weinhardt LS, Bickham N, Carey MP. Sexual coercion among women living with a severe and persistent mental illness: Implications for clinical practice. Aggression and Violent Behavior: A Review Journal. 1999;4:307–317. [Google Scholar]
- Weinhardt LS, Carey MP, Carey KB, Verdecias RN. Increasing assertiveness skills to reduce HIV risk among women living with a severe and persistent mental illness. Journal of Consulting and Clinical Psychology. 1998a;66:680–684. doi: 10.1037//0022-006x.66.4.680. [DOI] [PubMed] [Google Scholar]
- Weinhardt LS, Forsyth AD, Carey MP, Jaworski BC, Durant LE. Reliability and validity of self-report measures of HIV-related sexual behavior: Progress since 1990 and recommendations for research and practice. Archives of Sexual Behavior. 1998b;27:155–180. doi: 10.1023/a:1018682530519. [DOI] [PMC free article] [PubMed] [Google Scholar]