Abstract
Acoustic exposure to high intensity and/or prolonged noise causes temporary or permanent threshold shifts in auditory perception, reflected by reversible or irreversible damage in the cochlea. Aminoglycoside antibiotics, used for treating or preventing life-threatening bacterial infections, also induce cytotoxicity in the cochlea. Combined noise and aminoglycoside exposure, particularly in neonatal intensive care units, can lead to auditory threshold shifts greater than simple summation of the two insults. The synergistic toxicity of acoustic exposure and aminoglycoside antibiotics is not limited to simultaneous exposures. Prior acoustic insult which does not result in permanent threshold shifts potentiates aminoglycoside ototoxicity. In addition, exposure to sub-damaging doses of aminoglycosides aggravates noise-induced cochlear damage.
The mechanisms by which aminoglycosides cause auditory dysfunction are still being unraveled, but likely include the following: 1) penetration into the endolymphatic fluid of the scala media, 2) permeation of non-selective cation channels on the apical surface of hair cells and 3) generation of toxic reactive oxygen species and interference with other cellular pathways. Here we discuss the synergistic effect of combined noise and aminoglycoside exposure to identify pivotal synergistic events that can potentiate ototoxicity, in addition to a current understanding of aminoglycoside trafficking within the cochlea. Preventing the ototoxic synergy of noise and aminoglycosides is best achieved by using non-ototoxic bactericidal drugs, and by attenuating perceived noise intensity when life-saving aminoglycoside therapy is required.
Aminoglycoside toxicity
Aminoglycosides are antibiotics that are highly effective in treating life-threatening Gram-negative bacterial infections, such as meningitis and bacterial sepsis in infants 1,2. However, aminoglycosides also induce tissue-specific cytotoxicity in the cochlea and kidney. Commonly-used aminoglycosides include amikacin, gentamicin, and tobramycin, and are administered in doses based on body weight and their toxicity is dose-related. Individuals that receive sufficiently high doses of aminoglycosides experience both functional and/or morphological damage in the cochlea 3.
The ototoxicity of aminoglycosides has been extensively researched in small animals such as guinea pigs. Repeated gentamicin doses of 100 mg/kg/day for 10 days, is considered highly ototoxic 4, with both raised hearing thresholds and extensive cochlear hair cell loss 5. Repeated gentamicin administration of 50 mg/kg/day (10-fold higher than the clinical dose used in human adults) for 10 days did not produce overt ototoxicity in guinea pigs 4. However, this does not mean that doses lower than 50 mg/kg/day are non-ototoxic. Many other known ototoxins, such as loop diuretics and noise, can synergistically interact with aminoglycosides and damage the cochlea, when either insult alone appears harmless. Thus, the presence of other known ototoxins always reduces the safe dose of aminoglycosides.
The physiological effects of aminoglycosides include cation channel blockade in a variety of tissues, cation wasting of calcium, sodium potassium magnesium in the urine excreted from the kidneys, extracellular blockade of calcium influx, and even evoke intracellular calcium signaling by activating the calcium sensing receptor 6-13. Aminoglycosides not only block the mechanosensitive transduction channel of inner ear hair cells, but also permeate through it, and other non-selective cation channels 14-17. Once taken up into the cell via ion channel permeation or endocytosis, aminoglycosides induce a wide variety of effects depending on cell type and intracellular conditions: including elevation of intracellular calcium levels 18 and the generation of toxic levels of reactive oxygen species (ROS) 19-21. These effects can initiate several different mechanisms of hair cell death (often simultaneously) that are dependent on the type of aminoglycoside exposure, for example, acute or chronic exposure with low or high dosing regimens. In vivo, aminoglycosides are primarily taken up by basal hair cells, which are also preferentially more susceptible to aminoglycoside-induced cytotoxicity 22-24. The experimental design itself is also critical since apoptotic death pathways are frequently observed during in vitro experiments, while in vivo experiments display necrotic and caspase-independent death pathways more frequently 25-27. Aminoglycoside-induced hair cell death processes may continue for up to 4 weeks after cessation of drug administration.
Acoustic trauma
Acoustic trauma can occur in many environments, including occupational (e.g., industrial, manufacturing, construction, military), or recreational (rock concerts, sports stadia, hunting, headphones). Exposure to damaging levels of sound occurs in two major forms. Firstly, impulse noise from artillery explosions or gunshots that produce high intensity sound that physically damage hair cell stereocilia and produces discrete lesions in the sensory epithelia of the cochlea. Secondly, long-term exposure to lower (but still high) intensity noise generates high levels of reactive oxygen species (ROS), coupled with physiological changes in the blood-labyrinth barrier that result in temporary auditory dysfunction and often permanent hearing loss 28-32.
Noise also induces a variety of cochlear pathologies, ranging from physical disruption of hair cell stereocilia and organ of Corti integrity 29,33 to increased endocytosis, vacuolation, mitochondrial lesions, elevation of intracellular calcium concentrations and the generation of reactive oxygen species 34-36. These phenomena can lead to apoptotic and/or necrotic cell death processes that may continue for up to 30 days after exposure 37-40.
Aminoglycosides and noise
Potentiation of aminoglycoside-induced ototoxicity by concurrent exposure to noise has been investigated since the 1960's when animals receiving aminoglycosides appeared to be more susceptible to noise-induced hearing loss 41 and reported conclusively by Gannon and Tso in 1969 and in subsequent animal studies 42,43. These observations were confirmed by Jack Vernon's group who reported a very prominent synergistic effect of noise and neomycin in the guinea pig 44,45. The well-controlled experiment included conditions of 1) high intensity noise alone (115 dB SPL, 10 hours), 2) 200 mg/kg/day neomycin with low intensity noise (45 dB SPL), and 3) neomycin with high intensity noise. Animals were exposed to neomycin and noise for 7 consecutive days, then allowed to recover for 30 days. The average hearing threshold shifts across tested frequencies were 16 and 17 dB in conditions 1 and 2, while the shift was 62 dB in condition 3 instead of a linear summation of about 20 dB (17 ±3 dB). The histological analysis on hair cell loss presented a very similar synergistic trend. While 17% and 26% outer hair cells (OHCs) were lost in conditions 1 and 2, respectively, in condition 3 the loss of OHCs was close to 100%.
Sub-damaging doses of aminoglycosides can aggravate noise-induced cochlear damage. Collins 46 reported synergism between sub-damaging doses of gentamicin and an intense 8-kHz pure tone. Gentamicin was administered at 50 mg/kg/day for 10 days and the tone was played at 116 dB SPL for 1 hour. The combination caused considerable hair cell loss that surpassed simple summation due to each insult alone.
This synergistic effect does not require simultaneous exposure to noise and aminoglycoside. Ryan and Bone investigated the effect of sequential exposure to noise and aminoglycoside 47,48, and reported prominent noise (2-octave wide, 100 dB SPL) potentiation two months later when kanamycin (150 mg/kg/day) was administered. Thus, noise-then-drug paradigm displayed apparent synergism, implying that high level noise exposure has long-term consequences on the physiological condition of the cochlea and its sensory cells. This is particularly relevant for military personnel in combat that subsequently undergo medical evacuation from the battlefield with concomitant prophylactic aminoglycoside treatment.
When kanamycin treatment is followed by noise one month later, no potentiation of hair cell loss or auditory function was observed 48, confirming the original report by Gannon et al. 43 that synergism did not occur if the noise was introduced 30 days after the cessation of kanamycin treatment. However, synergism did occur if the noise was introduced within 20 days after the cessation of drug use 42. Collins (1988) also reported mild drug-then-noise synergism with 10 days in between insults. Hair cell loss caused by combined insults was considerably reduced in the drug-then-noise paradigm compared to that of simultaneous drug-and-noise exposure. Aminoglycosides are cleared from cochlear tissues slowly (with a half life of many days), and cochlear cells may retain aminoglycosides for 6 months or longer 49,50. These observations suggest that synergy between noise and prior administration of aminoglycosides dependent on the clearance of aminoglycosides from the cochlea.
More recently, sensitive distortion product otoacoustic emission (DPOAE) measurements have enabled new opportunity to non-invasively investigate frequency-specific cochlear damage. This measure reveals damage to cochlear function that may not be reflected in other measurements, such as hair cell morphology or hearing threshold shifts. Tan et al. 51 reported that exposure to sub-damaging dose of aminoglycoside antibiotics aggravates noise-induced cochlear damage, using DPOAE measurements with primary tones at 65 dB SPL and 55 dB SPL. In this study, 100 mg/kg/day amikacin was administered intra-muscularly for 10 days caused no significant change in DPOAE amplitude. Exposure to only broadband noise at 105 dB SPL for 12 hours resulted in a temporary decrease of DPOAE amplitude for 4 days. However, when amikacin and broadband noise were presented together (exposed to noise only on day 0), the decreased DPOAE amplitude had not recovered 28 days later. This synergism between aminoglycosides and noise could only be observed by DPOAE measurements, but not by ABR measurements.
Synergism in newborns
Many pediatricians considered aminoglycoside ototoxicity to be less prevalent in neonates than in adults 52,53. In 1981, Bernard demonstrated that pre-term neonates exposed to aminoglycosides experienced hearing loss using ABR measurements 54. In 2007, Rees confirmed that pre-term infants (born <27 weeks) in the neonatal intensive care unit (NICU) who received aminoglycosides 7 days or more while exposed to noise levels produced by mechanical ventilation (>80 dBA for >30 minutes) had a high probability (68%) of developing hearing loss 55. Although we did not find other experiments specifically designed to study noise-and-drug synergism in neonates, the above evidence suggests that synergism between aminoglycosides and noise occurs in infants.
Potential mechanisms for synergistic toxicity induced by noise and aminoglycosides
We have described different classes of synergistic toxicity between noise and aminoglycosides:
Simultaneous exposure to noise and aminoglycosides
Sub-damaging doses of aminoglycosides enhancing noise-induced hearing loss
Prior exposure to noise enhancing subsequent aminoglycoside toxicity
However, it is also necessary to discuss how aminoglycosides are trafficked from the vasculature to cochlear hair cells and whether noise potentiates hair cell uptake of aminoglycosides. Accurate determination of their trafficking route as well as subsequent toxicity will lead to new, and more effective strategies to prevent noise potentiation of aminoglycoside ototoxicity.
Trafficking of aminoglycosides to hair cells
All cells appear to take up aminoglycosides after systemic administration, and most clear the drug. However, kidney proximal tubule cells and cochlear sensory hair cells preferentially retain the drug after systemic administration 56. Aminoglycosides are cationic, hydrophilic molecules that do not permeate lipid membranes easily. Aminoglycosides enter most cells via endocytosis in vivo or by permeating through non-selective cation channels 17,57. Indeed, aminoglycosides enter hair cells through both apical endocytosis and by permeating the mechanosensitive transduction channels at the tips of their stereocilia 14,58. This apical entry route implies that aminoglycosides enter in hair cells from the endolymphatic scala media (Fig. 1). Aminoglycosides could also potentially enter hair cells across their basolateral membranes from perilymph, by either endocytosis or ion channel permeation, although this has not been shown experimentally 35.
Figure 1.
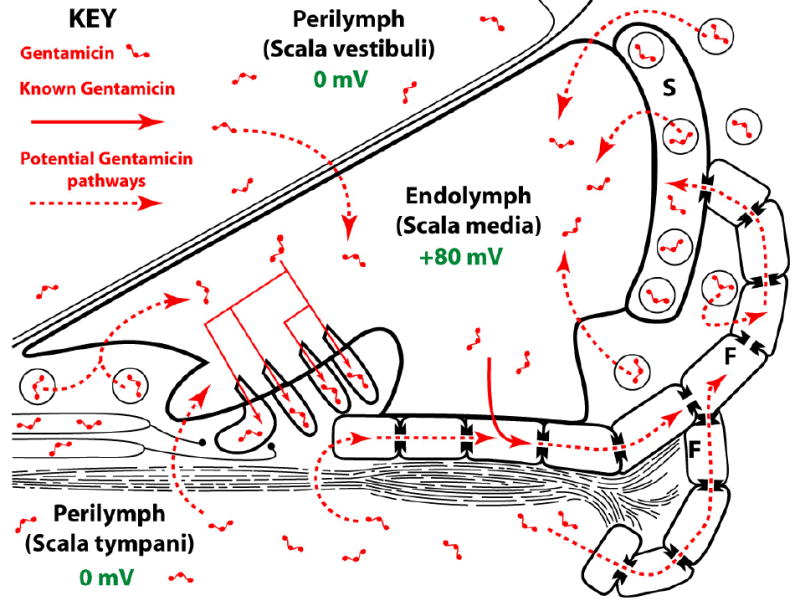
Schematic diagram showing how gentamicin may enter hair cells and supporting cells across their lumenal (endolymphatic) membranes. Aminoglycosides may enter endolymph via transport from lateral wall capillaries, or directly from the perilymphatic compartments. Alternatively, like K+, aminoglycosides may be passaged via supporting cells and fibrocytes (F) to the stria vascularis (S) via gap junctions and transported into marginal cells by as-yet-unidentified transporters and thence into endolymph (see also Fig. 2). Diagram is not to scale.
The trafficking of the cationic, hydrophilic aminoglycosides across the blood-labyrinth barrier into the cochlear fluids during systemic administration has been poorly investigated thus far. Published studies have shown that, marginal cells in the stria vascularis are preferentially loaded with aminoglycosides 59-61. This raises two possibilities: (a) marginal cells are sequestering aminoglycosides from endolymph (thus re-raising the question of how aminoglycosides traffic into endolymph), or (b) that systemically-administered aminoglycosides cross the strial blood-labyrinth barrier, and are trafficked into marginal cells (Fig. 2). In the latter case, aminoglycosides could be cleared from marginal cells directly into endolymph 61-63.
Figure 2.
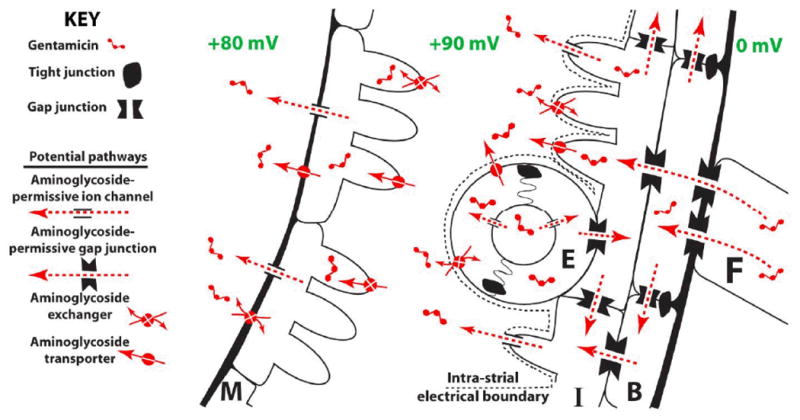
Schematic diagram postulating how gentamicin cross the strial blood-labyrinth barrier into endolymph. Aminoglycosides could clear directly from endothelial cells (E) into the intra-strial space, or passage into intermediate (I) cells via gap junctions from endothelial cells, or basal (B) cells and fibrocytes (F). Once in intermediate cells, aminoglycosides would then be cleared into the intra-strial space. An unidentified mechanism transports aminoglycosides into marginal (M) cells, where aminoglycosides could passively flow down the electrochemical gradient into endolymph, via non-selective cation channels for example. Diagram is not to scale.
Aminoglycosides are also present in perilymph 49,64,65. Following systemic administration, aminoglycosides are presumably trafficked through vascular endothelial cells in the lateral wall, spiral limbus into the perilymphatic fluids. If aminoglycosides enter perilymph first, they could then leak across the perilymph-endolymph tight junction-coupled cells lining the scala media on Reissner's membrane and the basilar membrane. Regardless of how aminoglycosides enter endolymph, they must be trafficked against the electrical gradient, since endolymph has a +80 mV potential compared to perilymph. This positive endocochlear potential is due to the active transport of K+ by the stria vascularis into endolymph to maintain the unique high extracellular potassium concentration (150 mM) that comprises the major depolarizing current into hair cells during sound stimulation. This suggests that active trafficking of aminoglycosides into endolymph occurs, although the mechanism remains unidentified.
If aminoglycosides use a trans-strial trafficking pathway to enter endolymph, what molecular mechanisms would enable such a pathway? From the strial capillary lumen, aminoglycosides would need to enter, and then clear, endothelial cells (Fig. 2), as passive extravasation of aminoglycoside-laden plasma does not readily occur between adjacent endothelial cells that are also conjoined via tight junctions 61,66,67. Once taken up by endothelial cells, aminoglycosides could also be cleared directly into the intra-strial space, or into the adjacent intermediate cells, mimicking the trafficking of K+ ions through gap junctions. Aminoglycosides would then be cleared from intermediate cells into the intra-strial space, again mimicking K+ trafficking. Alternatively, aminoglycosides could be trafficked by transcytosis (endocytotic transport across the cell) across the endothelial cell directly into the intra-strial space, as shown for other macromolecules 67. Aminoglycosides are then transported by an unidentified (non-endocytotic) mechanism into marginal cells from the intra-strial space 61. Since marginal cells possess an higher cell potential than endolymph, aminoglycosides could then passively flow down the electrochemical gradient into endolymph, for example, through non-selective cation channels, such as the aminoglycoside-permissive TRPV4 channel, on the lumenal surface of marginal cells, 15,68. Having reached the positively-charged endolymph, electro-repulsive forces would electrophoretically drive the cationic aminoglycosides into the negatively-polarized hair cells, where these drugs then exert their cytotoxic effect, disrupting auditory function. This electrophoretic driving force could efficaciously concentrate aminoglycosides in OHCs since their mechanoelectrical transduction channels have an open probability of 0.5 at rest and during sound stimulation 69,70. This same electrophoretic driving force could drive aminoglycosides into the non-sensory cells lining the scala media. Since most cells clear aminoglycosides over time, these aminoglycosides could be cleared into the perilymphatic fluids across their basolateral membranes.
Simultaneous exposure to noise and aminoglycosides
Although there is substantial evidence for noise potentiation of aminoglycoside ototoxicity, the mechanisms by which this occurs remain elusive. A number of mechanisms have been proposed, and these may occur in combination rather than in a mutually exclusive manner.
High intensities of sound can disrupt the permeability of the strial blood-labyrinth barrier and induce a loss of the endocochlear potential, EP 30-32. This could mimic the loss of the EP by loop diuretics that results in rapid loading of the stria vascularis, and endolymph with aminoglycosides, and subsequently causing permanent damage to cochlear morphology and function 65,71,72. Loop diuretic treatment alone only transiently affects auditory function by inhibiting the sodium-potassium-chloride co-transporter at the base of marginal cells in the stria vascularis, with a consequent drop in the endolymphatic potential 73,74. Once loop diuretic inhibition of the sodium-potassium-chloride co-transporter at the base of marginal cells is removed, the EP and auditory function is restored, potentially trapping aminoglycosides within the endolymphatic scala media. This would electrophoretically drive the trapped aminoglycosides into hair cells at concentrations sufficient to induce subsequent hair cell and organ of Corti degeneration.
Lower levels of sound stimulation can increase the EP along with acute, though mild, endolymphatic hydrops 75. This is suggestive of enhanced trafficking of K+ ions into endolymph (with water following to maintain osmolarity). This increased cellular activity may inadvertently increase the trans-strial trafficking of aminoglycosides into endolymph. In addition, an increased EP will increase the electrophoretic driving force of endolymph, and aminoglycosides, into hair cells.
If aminoglycosides enter hair cells primarily from endolymph, then the open probability of the aminoglycoside-permissive mechano-sensitive transduction channel in the hair cell stereocilia may play an important role. In OHCs, the mechanotransduction channel has an open probability of 0.5 during rest or sound stimulation 69,70. Thus, sound stimulation should not significantly increase aminoglycoside uptake into OHCs via the mechanoelectrical transduction channel. However, in inner hair cells, the open probability of the mechanosensitive transduction channel at rest is 0.1, and this increases to 0.5 during sound stimulation 76,77, potentially causing a significant increase in inner hair cell uptake of aminoglycosides and subsequent toxicity.
Although sound stimulation may not significantly increase aminoglycoside entry into OHCs, moderate or loud sound stimulation induces exocytosis of ATP from supporting cells which activate P2X2 channels at the apical membrane of OHCs, a potential shunt mechanism to protect OHCs from noise trauma by reducing the EP 78-80. P2X2 channels are permissive to large organic molecules 81, and if also aminoglycoside-permissive, may inadvertently enable noxious aminoglycoside entry into OHCs during chronic sound stimulation or noise-trauma.
The electrical potential difference across the apical membrane of hair cells is approximately 150 mV (+80 mV in endolymph and -70 mV hair cell receptor potential), the largest cross-membrane electrical potential in the mammalian body. The hair cell glycocalyx is also negatively-charged. Thus, the cationic aminoglycosides would be electrostatically attracted to the apical surface of cochlear hair cells, facilitating endocytotic uptake 35. Stimulation of hair cells also increases apical endocytosis 82 which could facilitate endocytotic uptake of aminoglycosides from endolymph.
After entering hair cells, aminoglycosides will likely aggravate the on-going cellular changes wrought by sound stimulation, or worse, noise trauma. This will include interaction with PIP2 and other aminoglycoside-binding molecules, elevation of intracellular calcium and reactive oxygen species, and the promotion of various cell death processes, overwhelming the nascent pro-survival, anti-oxidant defenses of the hair cell (see 26,34).
Of particular interest is the potential involvement of mitochondria. There are families highly susceptible to aminoglycoside toxicity due to a mutation in their mitochondrial DNA 83-85, suggesting that aminoglycosides likely cross the mitochondrial membrane. Aminoglycosides are also implicated in the generation of reactive oxygen species and the mitochondrial permeability transition pore that releases mitochondrial pro-apoptotic factors into the cytosol 18,86. In addition, high levels of sound intensities also reduce the electrical potential of mitochondria (decreasing ATP production), increase the generation of reactive oxygen species, and release mitochondrial pro-apoptotic factors into the cytosol that initiate active cell death processes 87,88. Determining the contributions of either insult to synergistic ototoxicity will remain elusive until a better understanding of the individual mechanisms of cytotoxicity due to either insult is achieved.
Aminoglycoside enhancement of noise-induced hearing loss
Sub-damaging doses of aminoglycosides that enhance noise-induced hearing loss are likely to involve many elements of the above proposed mechanisms. Hair cell uptake of aminoglycosides may be enhanced by intense noise levels. Once inside the hair cell, aminoglycosides will induce toxicity mechanisms that will potentiate those described for noise-induced hearing loss. It is likely that the same mechanisms of toxicity are involved, just to different degrees depending on the level of exposure.
Prior noise exposure enhancement of aminoglycoside toxicity
Prior noise exposure potentiates the ototoxic effect of aminoglycosides given weeks later 47,48. This phenomenon does not occur in the opposite direction, i.e, aminoglycoside potentiation of subsequent noise exposure 47,48. This suggests that noise-exposure damages the anti-oxidant capacity of hair cells without initially diminishing auditory function. Alternatively, prior noise exposure may increase the expression of P2X2 channels on the surface of OHCs as a protective measure against noise trauma 89. P2X2 channels are another class of non-selective cation channels that are permeable to FM1-43, and channels that are permeable to FM1-43 also tend to be aminoglycoside-permissive. Thus, when subsequent aminoglycoside treatment occurs, the increased expression of P2X2 may inadvertently enhance hair cell uptake of aminoglycosides. This enhanced aminoglycoside sensitivity would exists for as long as P2X2 channels remain upregulated on the OHC surface. Other unidentified mechanisms of prior noise exposure enhancing aminoglycoside toxicity may also exist.
Preventing synergistic toxicity induced by noise and aminoglycosides
Preventing synergistic toxicity induced by noise and aminoglycosides is likely to involve a combination of aminoglycoside uptake inhibitors (once identified) working at the blood-labyrinth barrier to reduce cochlear uptake of aminoglycosides, combined with a one or several anti-oxidants known to ameliorate either aminoglycoside or noise-induced hearing loss 29,90-92. More immediately, it is important to remove sources of sound stimulation and toxic noise prior to and during critical, life-saving systemic aminoglycoside administration for treating bacterial sepsis, meningitis, and during prophylaxis. Since this is not possible in many cases (e.g., blast injuries), it is increasingly important to better understand how aminoglycosides cross the blood-labyrinth barrier to gain access to hair cells, and the cytotoxic mechanisms they induce within hair cells that potentiate noise-induced changes within hair cells into cytotoxic phenomena.
Acknowledgments
Supported by NIDCD grant DC 04555.
Literature cited
- 1.Pillers DM, Schleiss MR. Gentamicin in the Clinical Setting. The Volta Review. 2005;105:205–210. [Google Scholar]
- 2.Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139(12):97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 3.Johnsson LG, Hawkins JE, Jr, Kingsley TC, Black FO, Matz GJ. Aminoglycoside-induced cochlear pathology in man. Acta Otolaryngol Suppl. 1981;383:1–19. [PubMed] [Google Scholar]
- 4.Collins PW, Twine JM. The ototoxic effects of different doses of gentamicin on the cochlea of pigmented guinea pigs. Br J Audiol. 1985;19(4):257–264. doi: 10.3109/03005368509078981. [DOI] [PubMed] [Google Scholar]
- 5.Aran JM. Evaluation of the ototoxicity of aminoglycosides Comparative study of dibekacin, gentamicin and tobramycin. Nouv Presse Med. 1982;11(46):3426–3431. [PubMed] [Google Scholar]
- 6.Vital Brazil O, Prado-Franceschi J. The nature of neuromuscular block produced by neomycin and gentamicin. Arch Int Pharmacodyn Ther. 1969;179(1):78–85. [PubMed] [Google Scholar]
- 7.Corrado AP, de Morais IP, Prado WA. Aminoglycoside antibiotics as a tool for the study of the biological role of calcium ions. Historical overview. Acta Physiol Pharmacol Latinoam. 1989;39(4):419–430. [PubMed] [Google Scholar]
- 8.Pichler M, Wang Z, Grabner-Weiss C, et al. Block of P/Q-type calcium channels by therapeutic concentrations of aminoglycoside antibiotics. Biochemistry. 1996;35(46):14659–14664. doi: 10.1021/bi961657t. [DOI] [PubMed] [Google Scholar]
- 9.Dulon D, Zajic G, Aran JM, Schacht J. Aminoglycoside antibiotics impair calcium entry but not viability and motility in isolated cochlear outer hair cells. J Neurosci Res. 1989;24(2):338–346. doi: 10.1002/jnr.490240226. [DOI] [PubMed] [Google Scholar]
- 10.Kang HS, Kerstan D, Dai L, Ritchie G, Quamme GA. Aminoglycosides inhibit hormone-stimulated Mg2+ uptake in mouse distal convoluted tubule cells. Can J Physiol Pharmacol. 2000;78(8):595–602. [PubMed] [Google Scholar]
- 11.Quamme GA. Renal handling of magnesium: drug and hormone interactions. Magnesium. 1986;5(56):248–272. [PubMed] [Google Scholar]
- 12.Kidwell DT, McKeown JW, Grider JS, et al. Acute effects of gentamicin on thick ascending limb function in the rat. Eur J Pharmacol. 1994;270(1):97–103. doi: 10.1016/0926-6917(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 13.Ward DT, McLarnon SJ, Riccardi D. Aminoglycosides Increase Intracellular Calcium Levels and ERK Activity in Proximal Tubular OK Cells Expressing the Extracellular Calcium-Sensing Receptor. J Am Soc Nephrol. 2002;13(6):1481–1489. doi: 10.1097/01.asn.0000015623.73739.b8. [DOI] [PubMed] [Google Scholar]
- 14.Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567(Pt 2):505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karasawa T, Wang Q, Fu Y, Cohen DM, Steyger PS. TRPV4 enhances cellular uptake of aminoglycoside antibiotics. J Cell Sci. 2008 doi: 10.1242/jcs.023705. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroese AB, Das A, Hudspeth AJ. Blockage of the transduction channels of hair cells in the bullfrog's sacculus by aminoglycoside antibiotics. Hear Res. 1989;37(3):203–217. doi: 10.1016/0378-5955(89)90023-3. [DOI] [PubMed] [Google Scholar]
- 17.Myrdal SE, Steyger PS. TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hear Res. 2005;204(12):170–182. doi: 10.1016/j.heares.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose K, Westrum LE, Stone JS, Zirpel L, Rubel EW. Dynamic studies of ototoxicity in mature avian auditory epithelium. Ann N Y Acad Sci. 1999;884:389–409. doi: 10.1111/j.1749-6632.1999.tb08657.x. [DOI] [PubMed] [Google Scholar]
- 19.Hirose K, Hockenbery DM, Rubel EW. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear Res. 1997;104(12):1–14. doi: 10.1016/s0378-5955(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 20.Lautermann J, McLaren J, Schacht J. Glutathione protection against gentamicin ototoxicity depends on nutritional status. Hear Res. 1995;86(12):15–24. doi: 10.1016/0378-5955(95)00049-a. [DOI] [PubMed] [Google Scholar]
- 21.Clerici WJ, DiMartino DL, Prasad MR. Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear Res. 1995;84(12):30–40. doi: 10.1016/0378-5955(95)00010-2. [DOI] [PubMed] [Google Scholar]
- 22.Hiel H, Schamel A, Erre JP, et al. Cellular and subcellular localization of tritiated gentamicin in the guinea pig cochlea following combined treatment with ethacrynic acid. Hear Res. 1992;57(2):157–165. doi: 10.1016/0378-5955(92)90148-g. [DOI] [PubMed] [Google Scholar]
- 23.Harrison RV, Evans EF. The effects of hair cell loss (restricted to outer hair cells) on the threshold and tuning properties of cochlear fibres in the guinea pig. In: Portmann M, Aran JM, editors. Inner ear biology. Paris: INSERM; 1977. pp. 105–24. [Google Scholar]
- 24.Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978;41(2):365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- 25.Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000;5(1):3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- 26.Rybak LP, Talaska AE, Schacht J. Drug-induced hearing loss. In: Schacht J, Popper AN, Fay RR, editors. Auditory trauma, protection, and repair. New York: Springer; 2008. pp. 219–256. [Google Scholar]
- 27.Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death and Differentiation. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlemiller KK, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiology and Neuro-otology. 1999;45:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- 29.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27(1):1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki M, Yamasoba T, Ishibashi T, Miller JM, Kaga K. Effect of noise exposure on blood-labyrinth barrier in guinea pigs. Hear Res. 2002;164(12):12–18. doi: 10.1016/s0378-5955(01)00397-5. [DOI] [PubMed] [Google Scholar]
- 31.Goldwyn BG, Quirk WS. Calcium channel blockade reduces noise-induced vascular permeability in cochlear stria vascularis. Laryngoscope. 1997;107(8):1112–1116. doi: 10.1097/00005537-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Hukee MJ, Duvall AJ., 3rd Cochlear vessel permeability to horseradish peroxidase in the normal and acoustically traumatized chinchilla: a reevaluation. Ann Otol Rhinol Laryngol. 1985;94(3):297–303. [PubMed] [Google Scholar]
- 33.Ahmad M, Bohne BA, Harding GW. An in vivo tracer study of noise-induced damage to the reticular lamina. Hear Res. 2003;175(12):82–100. doi: 10.1016/s0378-5955(02)00713-x. [DOI] [PubMed] [Google Scholar]
- 34.Henderson D, Hu B, Bielefeld EC. Patterns and mechanisms of noise-induced cochlear pathology. In: Schacht J, Popper AN, Fay RR, editors. Auditory trauma, protection, and repair. New York: Springer; 2008. pp. 195–217. [Google Scholar]
- 35.Lim DJ. Effects of noise and ototoxic drugs at the cellular level in the cochlea: a review. Am J Otolaryngol. 1986;7(2):73–99. doi: 10.1016/s0196-0709(86)80037-0. [DOI] [PubMed] [Google Scholar]
- 36.Fridberger A, Flock A, Ulfendahl M, Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc Natl Acad Sci U S A. 1998;95(12):7127–7132. doi: 10.1073/pnas.95.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamernik RP, Turrentine G, Roberto M, Salvi R, Henderson D. Anatomical correlates of impulse noise-induced mechanical damage in the cochlea. Hear Res. 1984;13(3):229–247. doi: 10.1016/0378-5955(84)90077-7. [DOI] [PubMed] [Google Scholar]
- 38.Bohne BA, Harding GW, Nordmann AS, et al. Survival-fixation of the cochlea: a technique for following time-dependent degeneration and repair in noise-exposed chinchillas. Hear Res. 1999;134(12):163–178. doi: 10.1016/s0378-5955(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 39.Hu BH, Henderson D, Nicotera TM. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res. 2002;166(12):62–71. doi: 10.1016/s0378-5955(02)00286-1. [DOI] [PubMed] [Google Scholar]
- 40.Bohne BA, Harding GW, Lee SC. Death pathways in noise-damaged outer hair cells. Hear Res. 2007;223(12):61–70. doi: 10.1016/j.heares.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Darrouzet J, Limasobrinhoe DE. the Internal Ear, Kanamycin and Acoustic Trauma. Experimental Study. Revista brasileira de cirurgia. 1963;46:120–134. [PubMed] [Google Scholar]
- 42.Gannon RP, Tso SS, Chung DY. Interaction of kanamycin and noise exposure. J Laryngol Otol. 1979;93(4):341–347. doi: 10.1017/s0022215100087119. [DOI] [PubMed] [Google Scholar]
- 43.Gannon RP, Tso SS. The occult effect of Kanamycin on the cochlea. Excerpta Medica. 1969;189:98. [Google Scholar]
- 44.Vernon J, Brown J, Meikle M, Brummett RE. The potentiation of noise-induced hearing loss by neomycin. Otolaryngology. 1978;86(1):ORL-123–124. doi: 10.1177/019459987808600129. [DOI] [PubMed] [Google Scholar]
- 45.Brown JJ, Brummett RE, Meikle MB, Vernon J. Combined effects of noise and neomycin Cochlear changes in the guinea pig. Acta Otolaryngol (Stockh) 1978;86(56):394–400. doi: 10.3109/00016487809107518. [DOI] [PubMed] [Google Scholar]
- 46.Collins PW. Synergistic interactions of gentamicin and pure tones causing cochlear hair cell loss in pigmented guinea pigs. Hear Res. 1988;36(23):249–259. doi: 10.1016/0378-5955(88)90066-4. [DOI] [PubMed] [Google Scholar]
- 47.Ryan AF, Bone RC. Potentiation of kanamycin ototoxicity by a history of noise exposure. Otolaryngology. 1978;86(1):ORL-125–128. doi: 10.1177/019459987808600130. [DOI] [PubMed] [Google Scholar]
- 48.Ryan AF, Bone RC. Non-simultaneous Interaction of exposure to noise and kanamycin intoxication in the chinchilla. Am J Otolaryngol. 1982;3(4):264–272. doi: 10.1016/s0196-0709(82)80065-3. [DOI] [PubMed] [Google Scholar]
- 49.Tran Ba Huy P, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. J Clin Invest. 1986;77(5):1492–1500. doi: 10.1172/JCI112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dulon D, Hiel H, Aurousseau C, Erre JP, Aran JM. Pharmacokinetics of gentamicin in the sensory hair cells of the organ of Corti: rapid uptake and long term persistence. C R Acad Sci III. 1993;316(7):682–687. [PubMed] [Google Scholar]
- 51.Tan CT, Hsu CJ, Lee SY, Liu SH, Lin-Shiau SY. Potentiation of noise-induced hearing loss by amikacin in guinea pigs. Hear Res. 2001;161(12):72–80. doi: 10.1016/s0378-5955(01)00359-8. [DOI] [PubMed] [Google Scholar]
- 52.McCracken GH, Nelson JD. Antimicrobial therapy for newborns : practical application of pharmacology to clinical usage. New York; London: Grune and Stratton; 1977. p. x.p. 177. [Google Scholar]
- 53.McCracken GH., Jr Aminoglycoside toxicity in infants and children. Am J Med. 1986;80(6B):172–178. doi: 10.1016/0002-9343(86)90497-3. [DOI] [PubMed] [Google Scholar]
- 54.Bernard PA. Freedom from ototoxicity in aminoglycoside treated neonates: a mistaken notion. Laryngoscope. 1981;91(12):1985–1994. doi: 10.1288/00005537-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Rees KC. Public Health. Baltimore: The Johns Hopkins University; 2007. The combined effect of noise and aminoglycoside antibiotic exposure on the auditory system of the pre-term infant. [Google Scholar]
- 56.Dai CF, Mangiardi D, Cotanche DA, Steyger PS. Uptake of fluorescent gentamicin by vertebrate sensory cells in vivo. Hear Res. 2006;213(12):64–78. doi: 10.1016/j.heares.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myrdal SE, Johnson KC, Steyger PS. Cytoplasmic and intra-nuclear binding of gentamicin does not require endocytosis. Hear Res. 2005;204(12):156–169. doi: 10.1016/j.heares.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashino E, Shero M. Endocytosis of aminoglycoside antibiotics in sensory hair cells. Brain Res. 1995;704(1):135–140. doi: 10.1016/0006-8993(95)01198-6. [DOI] [PubMed] [Google Scholar]
- 59.Roehm P, Hoffer M, Balaban CD. Gentamicin uptake in the chinchilla inner ear. Hear Res. 2007;230(12):43–52. doi: 10.1016/j.heares.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q, Dai CF, Steyger PS. Gentamicin Trafficking in the Cochlea. ARO Midwinter Meeting Abstracts. 2008;31:725. [Google Scholar]
- 61.Dai CF, Steyger PS. A systemic gentamicin pathway across the stria vascularis. Hear Res. 2008;235(12):114–124. doi: 10.1016/j.heares.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steyger PS. Cellular uptake of aminoglycosides. The Volta Review. 2005;105:299–324. [Google Scholar]
- 63.Wang Q, Balaban C, Salt AN, Steyger PS. Aminoglycosides preferentially load the blood-endolymph barrier over the blood-perilymph barrier: implications for drug induced ototoxicity. 13th Annual Blood-brain Barrier Consortium Meeting.2007. [Google Scholar]
- 64.Tran Ba Huy P, Manuel C, Meulemans A, Sterkers O, Amiel C. Pharmacokinetics of gentamicin in perilymph and endolymph of the rat as determined by radioimmunoassay. J Infect Dis. 1981;143(3):476–486. doi: 10.1093/infdis/143.3.476. [DOI] [PubMed] [Google Scholar]
- 65.Tran Ba Huy P, Manuel C, Meulemans A, et al. Ethacrynic acid facilitates gentamicin entry into endolymph of the rat. Hear Res. 1983;11(2):191–202. doi: 10.1016/0378-5955(83)90078-3. [DOI] [PubMed] [Google Scholar]
- 66.Vass Z, Steyger PS, Hordichok AJ, et al. Capsaicin stimulation of the cochlea and electric stimulation of the trigeminal ganglion mediate vascular permeability in cochlear and vertebro-basilar arteries: a potential cause of inner ear dysfunction in headache. Neuroscience. 2001;103(1):189–201. doi: 10.1016/s0306-4522(00)00521-2. [DOI] [PubMed] [Google Scholar]
- 67.Sakagami M, Matsunaga T, Hashimoto PH. Fine structure and permeability of capillaries in the stria vascularis and spiral ligament of the inner ear of the guinea pig. Cell Tissue Res. 1982;226(3):511–522. doi: 10.1007/BF00214780. [DOI] [PubMed] [Google Scholar]
- 68.Wangemann P, Schacht J. Homeostatic mechanisms in the cochlea. In: Dallos P, Popper AN, Fay RR, editors. The cochlea. New York: Springer-Verlag; 1996. pp. 130–185. [Google Scholar]
- 69.Cody AR, Russell IJ. The response of hair cells in the basal turn of the guinea-pig cochlea to tones. J Physiol (Lond) 1987;383:551–569. doi: 10.1113/jphysiol.1987.sp016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Legan PK, Lukashkina VA, Goodyear RJ, et al. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28(1):273–285. doi: 10.1016/s0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 71.Mathog RH, Capps MJ. Ototoxic interactions of ethacrynic acid and streptomycin. Ann Otol Rhinol Laryngol. 1977;86(2 pt 1):158–163. doi: 10.1177/000348947708600204. [DOI] [PubMed] [Google Scholar]
- 72.Nakai Y. Combined effect of 3′, 4′-dideoxykanamycin B and potent diuretics on the cochlea. (A scanning and transmission electron microscopic evaluation) Laryngoscope. 1977;87(9 Pt 1):1548–1558. doi: 10.1288/00005537-197709000-00015. [DOI] [PubMed] [Google Scholar]
- 73.Matz GJ. The ototoxic effects of ethacrynic acid in man and animals. Laryngoscope. 1976;86(8):1065–1086. doi: 10.1288/00005537-197608000-00001. [DOI] [PubMed] [Google Scholar]
- 74.Nin F, Hibino H, Doi K, et al. The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc Natl Acad Sci U S A. 2008;105(5):1751–1756. doi: 10.1073/pnas.0711463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salt AN. Acute endolymphatic hydrops generated by exposure of the ear to nontraumatic low-frequency tones. J Assoc Res Otolaryngol. 2004;5(2):203–214. doi: 10.1007/s10162-003-4032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jia S, Dallos P, He DZ. Mechanoelectric transduction of adult inner hair cells. J Neurosci. 2007;27(5):1006–1014. doi: 10.1523/JNEUROSCI.5452-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Russell IJ, Sellick PM. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol (Lond) 1983;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. 2001;121(1):10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- 79.Thorne PR, Munoz DJ, Housley GD. Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the Guinea pig. J Assoc Res Otolaryngol. 2004;5(1):58–65. doi: 10.1007/s10162-003-4003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Housley GD, Kanjhan R, Raybould NP, et al. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19(19):8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meyers JR, MacDonald RB, Duggan A, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23(10):4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griesinger CB, Richards CD, Ashmore JF. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature. 2005;435(7039):212–215. doi: 10.1038/nature03567. [DOI] [PubMed] [Google Scholar]
- 83.Fischel-Ghodsian N. Mitochondrial mutations and hearing loss: paradigm for mitochondrial genetics. Am J Hum Genet. 1998;62(1):15–19. doi: 10.1086/301695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischel-Ghodsian N, Prezant TR, Chaltraw WE, et al. Mitochondrial gene mutation is a significant predisposing factor in aminoglycoside ototoxicity. Am J Otolaryngol. 1997;18(3):173–178. doi: 10.1016/s0196-0709(97)90078-8. [DOI] [PubMed] [Google Scholar]
- 85.Pandya A, Xia X, Radnaabazar J, et al. Mutation in the mitochondrial 12S rRNA gene in two families from Mongolia with matrilineal aminoglycoside ototoxicity. J Med Genet. 1997;34(2):169–172. doi: 10.1136/jmg.34.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dehne N, Rauen U, de Groot H, Lautermann J. Involvement of the mitochondrial permeability transition in gentamicin ototoxicity. Hear Res. 2002;169(12):47–55. doi: 10.1016/s0378-5955(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 87.Shi X, Han W, Yamamoto H, Omelchenko I, Nuttall A. Nitric oxide and mitochondrial status in noise-induced hearing loss. Free radical research. 2007;41(12):1313–1325. doi: 10.1080/10715760701687117. [DOI] [PubMed] [Google Scholar]
- 88.Han W, Shi X, Nuttall AL. AIF and endoG translocation in noise exposure induced hair cell death. Hear Res. 2006;211(12):85–95. doi: 10.1016/j.heares.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Wang JC, Raybould NP, Luo L, et al. Noise induces up-regulation of P2X2 receptor subunit of ATP-gated ion channels in the rat cochlea. Neuroreport. 2003;14(6):817–823. doi: 10.1097/00001756-200305060-00008. [DOI] [PubMed] [Google Scholar]
- 90.Sha SH, Schacht J. Salicylate attenuates gentamicin-induced ototoxicity. Lab Invest. 1999;79(7):807–813. [PubMed] [Google Scholar]
- 91.Sha SH, Qiu JH, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354(17):1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- 92.Wu WJ, Sha SH, McLaren JD, et al. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158(12):165–178. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]


