Abstract
Leptin reduces body weight in ob/ob mice by decreasing food intake and increasing energy expenditure; however, the mechanisms by which it does the latter are not known. Here we report that 30% of the weight loss induced by leptin treatment of ob/ob mice is due to changes in energy expenditure. In assessing leptin's effects on specific tissues, we found that hepatic basal metabolic rate was paradoxically decreased 1.7-fold with leptin treatment, which was the result of a 1.6-fold reduction in mitochondrial volume density and altered substrate oxidation kinetics. The altered kinetics were associated with a decrease in protein levels of 2 mitochondrial respiratory chain components—cytochrome c oxidase subunit VIa and cytochrome c oxidase subunit IV. In addition to reduced hepatic metabolism, there was reduced long chain fatty acid production and a 2.5-fold increase in hepatic lipid export, both of which explain the reduced steatosis in leptin-treated animals. These data help clarify the role of the liver in leptin-mediated weight loss and define the mechanisms by which leptin alters hepatic metabolism and corrects steatosis.
Obesity is a significant global health problem and a leading contributor to morbidity and mortality in the developed and developing world. Obese individuals are at a higher risk for the development of coronary heart disease, diabetes, hypertension, and cancer (1). A fuller understanding of the biological underpinnings of this disorder remains critical for the development of new treatments.
Body weight is maintained at a stable level by a tightly controlled balance between energy intake and expenditure. While food intake is well appreciated as contributing to the regulation weight, it is increasingly clear that differences in energy expenditure, basal metabolic rates, and/or adaptive thermogenesis, are also important variables that contribute to obesity (2–4). For example, low energy expenditure is highly predictive of future weight gain (5, 6). Furthermore, weight loss in humans is met with a compensatory decrease in metabolic rate and increase in appetite, which work in concert to resist further changes in weight (7). Pharmacological agents that augment the increase in resting metabolic rate induced by exercise, and thus counteract the compensatory metabolic changes discussed above, could potentially be valuable tools in our battle against morbid obesity (1, 8, 9).
Leptin is an adipocyte-secreted, negative feedback hormone that acts on the hypothalamus to regulate both food intake and energy expenditure (10, 11). Leptin-deficient ob/ob mice show markedly reduced levels of energy expenditure and become obese even when pair fed compared with littermate controls (12, 13). This and other studies have indicated that leptin's effects on caloric intake in treated ob/ob mice only partially account for leptin-mediated weight loss (12, 14–16). Thus in addition to reducing caloric intake, leptin treatment of ob/ob mice increases total energy expenditure, selectively promotes fat metabolism, and prevents the fall in adaptive thermogenesis that normally occurs with reduced caloric intake (11, 17–23). These observations are consistent with the finding that stimulation of the ventromedial hypothalamus (VMH), a brain center through which leptin exerts some of its catabolic effects, increases metabolism, and decreases the respiratory quotient (RQ), while lesions in this area cause obesity (24). Therefore, a significant portion of leptin-mediated weight loss can be attributed to an increase in energy expenditure, some of which, but apparently not all, being attributable to adaptive thermogenesis.
While leptin's effects on energy expenditure can be easily demonstrated at the level of the whole organism, the tissues responsible for this effect as well as the underlying mechanisms are largely unknown (9). Several studies have suggested that leptin's effects on metabolism are mediated by the liver. This possibility has been suggested based on the liver's integral role in lipid metabolism, its significant energetic demands of approximately 20% of standard metabolic rate (SMR) in the rat (25), and leptin's ability to suppress SCD-1 in liver (26). Skeletal muscle and heart are other highly metabolic organs and have also been suggested to play a role in the increased energy expenditure associated with leptin treatment (27). However leptin's effects on metabolism or mitochondrial function in these tissues have not been directly studied.
In this study, we show that leptin treatment paradoxically decreases basal metabolic rate in the livers of ob/ob mice by reducing mitochondrial volume density and decreasing protein levels of 3 substrate oxidation system components. Consistent with this, we show that the leptin-mediated correction of hepatic steatosis is the result of decreased long chain fatty acid production and increased hepatic lipid export rather than a result of increased energy expenditure. Finally, we identify both heart and skeletal muscle as potential contributors to leptin-mediated changes in energy expenditure, as leptin treatment decreases their respiratory control ratio (RCR), which is defined as coupled respiration divided by uncoupled respiration.
Results
Quantitation of Leptin's Effects on Thermogenesis and Metabolism.
Resting core body temperature in leptin-deficient ob/ob mice is significantly lower (34.7°C) than in wild-type littermates (36.5°C; P < 0.001), and leptin treatment of these mutant mice restores core body temperature to normal/wild-type levels (36.3°C; P < 0.0001; Fig. 1A). This indicates that a proportion of leptin's metabolic effects in ob/ob mice are a result of increased thermogenesis. To assess whether there are additional metabolic effects of leptin treatment, we treated a second group of ob/ob mice with leptin under thermoneutral conditions, thus removing adaptive thermogenesis from brown adipose tissue (BAT) and other tissues as potential contributors to the leptin-induced increase of energy expenditure. ob/ob mice housed at 30°C, the temperature at which metabolic energy is not required to maintain core temperature (28), have core body temperatures that are not significantly different from their littermate controls, and leptin treatment of ob/ob mice housed at this temperature does not significantly alter core body temperature (Fig. 1A). To test whether leptin has additional metabolic effects under these conditions, we compared the effects of leptin treatment to pair feeding ob/ob mice at both 22°C and 30°C.
Fig. 1.
Components of leptin-mediated weight loss and leptin-mediated correction of hepatic steatosis. (A) ob/ob mice have a lower core body temperature than wild-type mice (measured by rectal thermometer), which is corrected by leptin infusion. Housing the mice at thermoneutral temperatures increases the core body temperature of ob/ob mice and diminishes the significance of body temperature correction by leptin. Ω, P < 0.001; *, P < 0.0001. (B) By leptin treating and pair feeding mice at room and thermoneutral temperatures, we were able to distinguish different components of leptin-mediated weight loss. The difference between each pair-fed and leptin-treated group at a given temperature represents weight loss due to leptin-mediated increases in energy expenditure and basal metabolic rate (P < 0.0001). The difference between the same treatment administered at different temperatures represents weight loss due to ambient temperature dependent effects on satiety (P < 0.0001). (C) Leptin-mediated correction of hepatic steatosis has 1 component that is correlated with ambient temperature dependent induction of satiety by leptin, which is represented by the difference between each pair-fed and leptin-treated group at a given temperature (P < 0.01). It also has another component that is correlated with leptin induced increases in energy expenditure, which is represented by the difference between the same treatment administered at different temperatures (P < 0.01) (D) Plasma levels of triglyceride are increased much faster in 12-day leptin-treated ob/ob mice (■) than in saline-treated littermates (♦) after the injection of 0.5 mg/kg tyloxapol, an inhibitor of VLDL hydrolysis, via the tail vein. The slope of the line indicates the rate of VLDL production, which is increased by 2.48-fold with leptin treatment. *, P < 0.05. Error bars show SEM in all figures.
The ob/ob group treated with leptin at 22°C showed the well-established trajectory of weight loss (12) and overall lost fat mass corresponding to 36.5% of their body weight in 2 weeks (Fig. 1B). Weight loss due to pair feeding represents the effects of leptin on satiety, and leptin-treated animals lost 11% more weight than pair-fed animals at both 22°C and 30°C (P < 0.0001). This indicates that approximately one-third of weight loss under leptin treatment (11/36.5%) is due to nonfeeding effects of the hormone, that is, leptin-mediated increases in energy expenditure. Because the same 11% difference in weight loss between leptin-treated and pair-fed groups is seen at both temperatures, the increase in energy expenditure mediated by leptin is ambient temperature independent. In addition, leptin-treated animals housed at 22°C lost 12% more weight than animals receiving the same treatment at 30°C (P < 0.0001). This suggests that another third of leptin-mediated weight loss (12.0/36.5%) is due to ambient temperature-dependent effects on satiety.
Having quantified the metabolic components of leptin's actions, we next set out to analyze which tissues contribute to the observed increase of energy expenditure. Livers from ob/ob mice show marked steatosis, and 12 days of leptin treatment at ambient temperature corrects this abnormality (Fig. S1A and B). We next measured the liver triglyceride (TG) levels of leptin-treated and pair-fed ob/ob mice housed at 22°C and 30°C. Leptin treatment significantly lowered triglyceride levels by approximately 230μmol/g vs. pair feeding at both 22°C and 30°C (Fig. 1C; P < 0.01), suggesting that leptin-mediated increases in energy expenditure and basal metabolic rate contribute to the improvement in steatosis. Mice housed at 22°C had liver triglyceride levels that were approximately 235μmol/g lower than those housed at 30°C (Fig. 1C; P < 0.01), indicating that leptin's effects on thermoregulation also contributed to the improvement in hepatic steatosis.
We hypothesized that leptin may also be improving hepatic steatosis by regulating fatty acid export by the liver. To address this we quantified hepatic lipid export by injecting tyloxapol, an inhibitor of VLDL hydrolysis, and measured plasma triglyceride levels. The rate of rate of VLDL production was found to be increased by 2.48-fold in ob/ob mice treated with leptin for 12 days compared with those treated with saline (P < 0.05; Fig. 1D). Thus, leptin treatment of ob/ob mice increases fatty acid export by the liver.
Leptin Levels Influence Hepatocyte Metabolism.
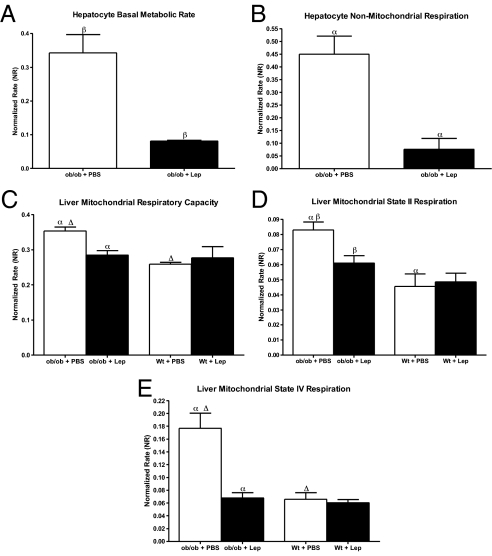
We next analyzed the effects of leptin pretreatment on hepatic metabolism. Oxygen consumption rates were recorded from primary hepatocytes isolated from 1 ob/ob + lep and 1 ob/ob + pbs animal at the same time each morning over 6 consecutive days. Twelve days of leptin treatment of ob/ob mice reduces the basal metabolic rate of primary hepatocytes respiring in a glucose medium to approximately 24% of saline-treated littermate levels (NR of 0.081 vs. 0.343; P < 0.05; Fig. 2A). Nonmitochondrial respiration, as measured after the addition of myxothiazol (see Methods), is also altered dramatically in these hepatocytes, as leptin treatment reduces it to approximately 17% of saline-treated control levels (NR of 0.076 vs. 0.450; P < 0.01; Fig. 2B). A number of processes contribute to nonmitochondrial respiration, and it can thus be somewhat difficult to quantify precisely. Measuring the components of mitochondrial respiration is more straight forward and precise. Also, previous studies show that leptin decreases the levels of SCD-1, which would increase CPT-1 shuttling of fatty acids into the mitochondria and would thus presumably affect mitochondrial respiration (26). In light of these considerations and because we demonstrate that leptin affects mitochondrial and nonmitochondrial respiration to the same extent, we decided to investigate mitochondrial respiration first. In these studies, we corrected for daily variances in recordings by dividing the values from both groups by the total respiration recorded from the PBS-treated ob/ob animals on each day. The measures of cellular metabolism are highly sensitive, and this approach allowed us to correct for to subtle differences in the solutions, electrode sensitivity, cell preparation, etc. Because the same parameters were measured from only 1 ob/ob + lep and 1 ob/ob + pbs animal each day, the normalization method was consistent through the course of the experiment. The data are shown as a “normalized rate (NR).”
Fig. 2.
Leptin decreases hepatocyte basal metabolic rate, nonmitochondrial respiration, and alters hepatic mitochondrial respiration rates. (A) Twelve day leptin treatment of ob/ob mice reduces primary hepatocyte basal metabolic rate (respiration in an isotonic medium containing 10 mM glucose) as compared to saline treatment. (B) Nonmitochondrial respiration (respiration in the presence of myxothiazol) is also decreased with 12 days of leptin treatment. (C) Hepatic mitochondrial respiratory capacity (respiration in the presence of FCCP) is increased in ob/ob mice in relation to their wild-type littermates, and 12 days of leptin treatment brings it back toward wild-type levels. This suggests an increase in the substrate oxidation system during leptin deficiency, and its normalization with leptin treatment. (D and E) State 2 and state 4 rates are both increased in ob/ob mice in relation to wild-type littermates as well, indicating that mitochondrial proton leak rate is increased with leptin deficiency. Twelve days of leptin treatment decreases state 2 and 4 rates in ob/ob mice back to wild-type levels. n = 5 for all points, and each was done in duplicate. α,▵, P < 0.01; β, P < 0.05.
Leptin Inhibits the Hepatic Substrate Oxidation System.
To further dissect the observed changes in hepatocyte basal metabolic rate, we next measured the different components of hepatic mitochondrial respiration including modular kinetic analysis. A series of chemicals were added in a stepwise fashion, to a mitochondrial suspension freshly prepared from livers of ob/ob animals +/− leptin. Rotenone and succinate were added first to measure state 2 respiration, followed by ADP to measure state 3 respiration, oligomycin to measure state 4 respiration, and FCCP to measure total respiratory capacity. Mitochondrial respiratory capacity rates provide insight into the substrate oxidation system, while state 2 and 4 rates give insight into the uncoupled component of respiration (i.e., proton leak).
Recordings from isolated mitochondria revealed that mitochondrial respiratory capacity rates are higher in ob/ob mice than in wild-type mice (NR of 0.353 vs. 0.259; P < 0.001; Fig. 2C). Leptin treatment of these ob/ob mice reduces respiratory capacity to a NR of 0.285 (P < 0.01), which is equivalent to that of wild-type animals. These data are consistent with the possibility that leptin treatment may be decreasing the substrate oxidation system.
We next monitored mitochondrial proton leak rate by measuring the resting respiration rates of isolated liver mitochondria. We found that the rate of state 2 respiration was higher in ob/ob mice than in wild-type mice (NR of 0.083 vs. 0.046; P < 0.01; Fig. 2D). Leptin treatment of ob/ob mice significantly reduced state 2 respiration NR to 0.061 (P < 0.05), returning it toward baseline wild-type levels. Consistent with this, state 4 respiration was higher in ob/ob mice than in wild-type mice (NR of 0.177 vs. 0.066; P < 0.01; Fig. 2E), with leptin treatment again returning this measure to wild-type levels (0.068; P < 0.01). These data are consistent with the possibility that leptin treatment is decreasing mitochondrial proton conductance in the liver. However because mitochondrial proton leak rate and substrate oxidation are interdependent, and because both are reduced by leptin treatment, the leptin-mediated suppression of 1 parameter could elicit a secondary reduction in the other (this is elaborated further in the discussion section). To determine which of these parameters was primarily altered by leptin, we used modular kinetic analysis.
Modular kinetic analysis measures mitochondrial respiration as a function of the inner membrane proton-motive force, thus allowing one to study the phosphorylation system, substrate oxidation system, and proton leak individually and independent of each other. Modular kinetic analysis indicated that substrate oxidation system (which includes all complexes of the mitochondrial respiratory chain) is significantly increased in ob/ob mice in comparison to wild-type mice without an evident difference in mitochondrial proton leak or the phosphorylation system (P < 0.0001; Fig. 3C). Moreover, neither the proton leak nor the phosphorylation system changes in ob/ob mice livers after leptin treatment (Fig. 3 A and B). Rather, leptin treatment of ob/ob animals significantly reduces the substrate oxidation system again returning it to wild-type levels (P < 0.0001; Fig. 3C). Overall these results show that leptin modulates mitochondrial function in liver by down-regulating substrate oxidation.
Fig. 3.
Leptin directly modifies hepatic mitochondrial substrate oxidation kinetics. Hepatic mitochondrial leak kinetics (A) and phosphorylation system kinetics (B) do not differ between ob/ob and wild-type mice, and are not affected by 12 days of leptin treatment. (C) The substrate oxidation system, however, is significantly increased in ob/ob mice in comparison to their wild-type littermates (P < 0.0001). Twelve days of leptin treatment of these ob/ob mice significantly reduces the substrate oxidation system back toward wild-type levels (P < 0.0001). (E) Mitochondrial respiratory capacity is increased in hypoleptinemic mice (NC) with respect to saline-treated controls (PBS). Restoration of leptin levels in these mice (FF) brings respiratory capacity back to control levels. (D) Hepatic mitochondrial state 4 respiration, however, is unchanged in both wild-type hypoleptinemic mice (NC) and in those animals with restored levels of leptin (FF) with respect to saline-treated controls (PBS). α,β, P < 0.01. n = 5 for all points, and each was done in duplicate.
Leptin's Effects on Liver Metabolism Are Independent of Its Effects on Hepatic Fat Stores.
Leptin's reduction of the oxidation system in liver mitochondria of ob/ob mice could either be a result of normalizing leptin levels or secondary to its ability to correct hepatic steatosis. To distinguish between these possibilities, we used a leptin withdrawal protocol, in which wild-type mice were first given high doses of leptin (2.5 mg/h) for 8 days to completely deplete their fat reserves (29). Upon the cessation of leptin treatment, there is no endogenous leptin and leptin levels approach 0. At this point if the food intake is restricted to that which the animals ate voluntarily before leptin withdrawal, referred to as normocaloric (NC), leptin levels remain low for an extended period despite the fact that caloric intake is “normal.” This protocol allows one to study the effects of leptin deficiency in wild-type animals independent of leptin's effects on food intake or steatosis. In this case we wished to assess whether hypoleptinemic animals without steatosis manifested the same mitochondrial phenotype as ob/ob mice. After leptin withdrawal, the mice were divided amongst 2 groups: free fed (FF) and normo-caloric (NC). The FF animals were allowed to consume food ad libitum for 7 days and, as previously reported, they rapidly regained their fat mass and restored normal levels of circulating leptin. An additional group of littermates were infused with, and withdrawn from PBS to serve as a further control.
Mitochondrial respiratory capacity was increased in hypoleptinemic NC mice (NR of 0.410) compared with saline treated littermates (NR of 0.335; P < 0.01; Fig. 3E). Restoration of leptin levels in the hypoleptinemic mice brings mitochondrial respiratory capacity back to wild-type levels (NR of 0.339; P < 0.05; Fig. 3E). Consistent with this we found that state 4 respiration rates were not different between hypoleptinemic mice (NC), those with restored levels of leptin (FF), and saline-treated controls (PBS) (Fig. 3D), indicating that mitochondrial proton leak is not changed by differences in circulating levels of leptin. These data indicate that the alterations in the rate of substrate oxidation in ob mice is a result of low circulating levels of leptin and not secondary to hepatic steatosis.
Leptin Levels Influence Mitochondrial Morphology.
There is a positive correlation between the metabolic activity of a tissue and the morphologic features of the mitochondria in that tissue—such as size, volume density, and cristae structure (30). Furthermore, studies of adaptive thermogenesis have shown that oxygen consumption is correlated with cristae length and density (31, 32). Mitochondrial morphology and mass before and after leptin treatment were monitored using electron microscopy. Mitochondria of 12-day leptin treated ob/ob mice (Fig. 4A) were morphologically different from ob/ob controls and exhibited a less dense, “ballooned” appearance compared to those of 12-day pair-fed (Fig. 4B) and 12-day PBS-treated (Fig. 4C) ob/ob mice. The cristae structure was also different in the leptin-infused group compared with the pair-fed and PBS-treated groups. Stereological assessment of these micrographs showed that hepatocytes from leptin-treated ob/ob animals have a decreased mitochondrial volume density of 0.154 μm3/μm3 (Fig. 4D), in comparison to 0.245 and 0.281 μm3/μm3 for saline-treated and pair-fed littermates, respectively (P < 0.05 for both). However, leptin treatment did not alter the average area of individual mitochondria with respect to saline treatment (0.90 vs. 0.97 μm2; Fig. 4E), suggesting that leptin treatment leaves each hepatocyte with fewer mitochondria of approximately the same size. Thus, in addition to the aforementioned changes in mitochondrial function, leptin treatment also results in quantitative differences in mitochondria.
Fig. 4.
Electron microscopic analysis of hepatic mitochondrial structure and size. (A) Mitochondria of 12 day leptin treated ob/ob mice look lighter and ballooned compared to those of 12 day pair-fed (B) and 12 day PBS-treated (C) ob/ob mice. Cristae structure also looks quite different in (A) compared with (B and C). (D) Mitochondrial volume density is significantly lower in leptin-treated ob/ob mice versus pair-fed and PBS-treated ob/ob mice. (E) Leptin treatment of ob/ob mice does not change the area of individual mitochondria, however pair feeding significantly increases it. A total of 168 sections from n = 5 mice in each group were taken and yielded similar results. α,▵, P < 0.01; β,γ = P < 0.05
Proteomic Changes Underlying Leptin's Effects on the Liver.
We next set out to investigate whether the metabolic and morphologic changes that we observed were associated with differences in the levels of specific mitochondrial proteins. Mitochondria from untreated ob/ob, leptin-treated ob/ob, saline-treated wild-type, and leptin-treated wild-type mice were purified over a nycodenz gradient using a standard centrifugation protocol. The protein content of equal numbers of mitochondria was quantitated using the iTRAQ method. This method of protein measurement uses 4 isobaric tags with different reporters, which in tandem mass spectrometry allows one to accurately quantify differences as small as 10% among samples (33). A complete list of the proteins that were detected and their levels is provided (see Table S1 and SI Text). Of note, 3 proteins that are components of the electron transport chain were significantly altered by changes in plasma leptin levels (Table S1). Cytochrome c oxidase subunit VIa protein levels are increased 1.23-fold in ob/ob mice in comparison to wild-type littermates (P < 0.05) and are reduced to 0.81-fold with leptin treatment of the ob/ob mice (P < 0.05). Similarly, mitochondrial NADH dehydrogenase (complex 1) subunit 5 (MT-ND5) protein levels are increased 1.53-fold in ob/ob mice in comparison to wild-type littermates (P < 0.05), and are reduced to 0.77-fold with leptin treatment of the ob/ob mice(P < 0.05). Finally, protein levels of cytochrome c oxidase subunit IV are reduced to 0.83-fold with leptin treatment of ob/ob mice (P < 0.01).
ELOVL5 was the protein with the greatest fold change; it increased by 3.49-fold during leptin deficiency (P < 0.05), and was reduced by 1.89-fold with leptin treatment of ob/ob mice (P < 0.05; Table S1). This enzyme is located in the endoplasmic reticulum and elongates long chain fatty acids. Over-expression of ELOV5 has been shown to cause massive hepatic accumulation of mono-unsaturated fatty acids (34).
Discussion
The anorexigenic effects of leptin can be attributed in part to its action on 2 distinct populations of neurons in the arcuate nucleus, NPY/AgRP cells and POMC/CART cells, which signal to second-order hypothalamic targets in the melanocortin circuit (24, 35, 36). The nature of the CNS pathways regulating leptin's effects on metabolism and thermongenesis are less well understood. In an effort to more fully delineate the cellular mechanisms underlying leptin's effect on energy balance, we first directed our attention to the liver due to its integral role in lipid metabolism, its significant energetic demands (25), and leptin's ability to suppress stearoyl-CoA desaturase-1 (SCD-1) in this tissue (26)—which may even contribute to some of the aforementioned leptin-mediated changes in metabolism. For these reasons, we originally postulated that leptin would increase the metabolic rate of the liver. Measurements of states 2 and 4 and FCCP respiration rates initially suggested that circulating leptin levels might alter both mitochondrial substrate oxidation and leak (Fig. 2). However, these rates are interdependent, as a leptin-mediated decrease in substrate oxidation could lead to a reduced mitochondrial protonmotive force, which could then diminish the driving force for proton leak. Conversely, a leptin-mediated decrease in proton leak would cause a buildup of the mitochondrial protonmotive force, which would increase the resistance against which the substrate oxidation system has to pump. Modular kinetic analysis measures each rate as a function of the mitochondrial proton-motive force (inner membrane potential), and thus allows one to quantitate them independently (37–39). Employing these studies we unequivocally showed that the substrate oxidation system is increased in ob/ob mice in relation to their wild-type littermates, and that 12 days of leptin treatment of these mice returns substrate oxidation toward wild-type levels by changing its kinetic properties (Fig. 3). Proton leak kinetics are not changed, indicating that the observed decrease in hepatic state 4 respiration was secondary to the reduction in substrate oxidation.
To identify proteins that may contribute to the demonstrated reduction in substrate oxidation in the liver, we used the iTRAQ methodology to compare the levels of hepatic mitochondrial proteins in leptin treated vs. control ob/ob and wild-type mice. iTRAQ tandem mass spectrometry uses isobaric tags which, when run through tandem mass spectrometry, allow one to determine as small as 10% differences in protein concentrations between 4 different samples. iTRAQ analysis of pooled samples run in triplicate, each containing 5 livers, identified on average 381 proteins, most of which were mitochondrial and some were from the ER. Proteins that could be identified by at least 2 peptides had a % confidence index of >95% for both the peptide and protein ID and had a greater than 20% change in protein levels with leptin deficiency or treatment with a P < 0.05 were considered to be significant for our analysis.
Among the many significant changes that were observed, we noted that 2 components of the electron transport chain—cytochrome c oxidase subunit VIa and cytochrome c oxidase subunit IV—were significantly increased in abundance during leptin deficiency, and decreased during leptin treatment (Table S1). Because we have shown that the substrate oxidation system is increased during leptin deficiency and decreased with leptin treatment, these changes could be the underlying mechanisms by which leptin affects substrate oxidation kinetics. In addition, NADH dehydrogenase (complex 1) subunit 5 (MT-ND5), was significantly increased in abundance during leptin deficiency and decreased during leptin treatment. Because our mitochondrial measurements used succinate as a substrate and thus bypassed complex 1, these data suggest that leptin's effect on hepatic metabolism may be even more profound than we have demonstrated. This finding is also consistent with studies showing that activation of nutrient-sensing pathways can alter NADH dehydrogenase protein levels (40). Further studies analyzing the effect of over-expression or knockdown of these proteins in ob/ob mice will be necessary to further evaluate their role in leptin-mediated substrate oxidation changes.
These proteomic data could also explain leptin's effects on glucose metabolism. Leptin has potent anti-diabetic effects that are independent of its ability to reduce food intake and body weight. These anti-diabetic effects could thus be mediated in part by the aforementioned changes in protein components of the oxidative phosphorylation pathway as it has been recently shown that mice with a liver-specific decrease in oxidative phosphorylation rates as a result of reduced activity of mitochondrial complex 1 are protected from obesity and diabetes due to improved glucose tolerance, increased insulin sensitivity, and increased glucose uptake (41). We demonstrate here that the same proteins are reduced by leptin treatment, raising the possibility that leptin's anti-diabetic effects are the result of its influence over hepatic mitochondrial complex 1 levels. This too can be assessed in future studies by over-expressing or knocking down the levels of these proteins in the liver.
In addition to improving whole body glucose metabolism, leptin treatment corrects the severe hepatic steatosis exhibited by ob/ob mice (Fig. 1). It is noteworthy that we observed a 1.88-fold change in the levels of ELOV5 after leptin treatment. ELOV5 is long chain fatty acid elongase. ELOVL5 and its over-expression have been shown to cause massive hepatic accumulation of mono-unsaturated fatty acids (34). This would suggest that the enzymatic step catalyzed by ELOV5 is to some extent rate determining for fatty acid synthesis. Thus it is possible that the increased abundance of ELOVL5 in ob/ob mice contributes to the hepatic steatosis observed in these animals, and its decrease with leptin treatment contributes to the correction of hepatic steatosis. This together with the observed increase of hepatic lipid export in ob/ob mice (Fig. 1) after leptin treatment could account for its anti-steatotic effects.
The reduced substrate oxidation and marked increase in VLDL secretion (Fig. 1) by the liver seen after leptin treatment suggest that the hormone causes the liver to reduce its own metabolic activity and shunt lipids to other tissues where they are metabolized. If as the data suggest the liver is a conduit for nutrients that are metabolized elsewhere, where then are these exported hepatic lipid stores being oxidized? Our preliminary results show that leptin treatment decreases coupled respiration in both skeletal muscle and the heart, thus causing a decrease in the RCR of these tissues. Thus the mechanism by which leptin increases energy expenditure is not clear and could include effects on mitochondrial coupling as well as nonmitochondrial respiration, such as metabolic futile cycles in heart and muscle and/or effects in other tissues, such as white adipose tissue (42). Further studies will be necessary to establish the tissues responsible for the leptin-mediated increase in systemic energy expenditure and elucidate the effects of leptin on cellular and mitochondrial metabolism on these and other tissues.
Materials and Methods
Leptin Administration.
Male C57BL6/J, C57BL6/J ob/+, and C57BL6/J ob/ob mice (8–10 weeks old) were purchased from The Jackson Laboratory and Charles River and housed in individual cages in a 22°C temperature-controlled room or placed in a small animal intensive care system (Plas Labs) for thermoneutral studies at 30°C. Body temperature was measured by using a rectal probe precision thermometer (VWR International). All mice were fed a standard laboratory chow diet (PicoLab Rodent Diet 5053), which contained 13% of total calories from fat. Leptin-treated animals were fed ad libitum, while pair-fed animals were implanted with a PBS pump and fed the same amount of food that their leptin-treated counterparts ate on each day of treatment. This was accomplished by starting pair-feeding treatments 1 day after the leptin treatments, and giving pair-fed animals the average amount of food consumed by the leptin-treated group the day before. Alzet miniosmotic pumps with a flow rate of 0.5 μL/hr were filled aseptically with either sterile PBS or leptin (400 ng/μL for ob/ob mice; 1 μg/μL for wild-type mice; 5 μg/μL for wild-type NC and wild-type FF mice). The pumps were incubated in a sterile 0.9% NaCl solution for at least 6 hours, and implanted s.c. using isoflurane as an anesthetic. For the withdrawal studies, all pumps were surgically removed from treated and control animals after 8 days, and mice were killed on the 15th day (29). For all other studies, mice were killed after 12 days of either saline or leptin infusion (26).
Measurement of Mitochondrial Respiration.
A Clarke-type oxygen electrode (Dual Digital Model 20, Rank Brothers) fitted with a circulating water bath (Model 3016H, Fisher Scientific) was used to measure mitochondrial respiration rate. Mitochondria were suspended in either KHEP [120 μM KCl, 5 mM KH2PO4, 3 mM Hepes, 1 mM EGTA, 0.3% defatted BSA, rotenone (5 μM), pH 7.4] or MRS [70 mM sucrose, 43 mM KCl, 3.6 mM MgCl2, 7.2 mM KH2PO4, 36 mM Tris-HCl, 0.3% defatted BSA, rotenone (5 μM), pH 7.4] at a concentration of 0.5 mg/mL and maintained at 37°C throughout the recordings. All figures shown are from the recordings using MRS. The oxygen solubility of the medium was considered to be 406 nmol/mL. Succinate (4 mM), ADP (1M), oligomycin (1 μg/mL), and FCCP (0.3 μM) were added to the recording chamber in a stepwise fashion to measure State 2, State 3, State 4, and maximal respiratory capacity rates, respectively. Data were handled and analyzed using a PowerLab 4/20 data recorder and Chart Pro Software (both from AD Instruments).
Liver Triglyceride Levels.
Total lipids were extracted from liver as described in previously established protocols (43) Quantification of fatty acids was done through liquid chromatography in accordance to previously published methods (44, 45). For the quantification of fatty acids, pentadecanoic acid (Sigma-Aldrich) was added as an internal standard.
Statistics.
All statistics were calculated using the paired or unpaired Student's t test as appropriate, with the exception of statistics for iTRAQ protein identification, which are detailed in the iTRAQ methods section. Values on graphs are expressed ± SEM.
Isolation of Mitochondria, Modular Kinetic Analysis, iTRAQ Labeling and Analysis, Mitochondrial Volume Density and Area, Isolation of Primary Hepatocytes, Measurement of Basal Metabolic Rate, VLDL Secretion Assay.
See SI Text.
Supplementary Material
Acknowledgments.
We thank Susan Korres for assistance in preparing this manuscript.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903723106/DCSupplemental.
References
- 1.Friedman J. Modern science vs the stigma of obesity. Nat Med. 2004;10:563–569. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard C, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 3.Ravussin E. Metabolic differences and the development of obesity. Metabolism. 1995;44:12–14. doi: 10.1016/0026-0495(95)90312-7. [DOI] [PubMed] [Google Scholar]
- 4.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 5.Ravussin E, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Eng J Med. 1988;318:467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 6.Roberts SB, Savage J, Coward WA, Chew B, Lucas A. Energy expenditure and intake in infants born to lean and overweight mothers. N Eng J Med. 1988;318:461–466. doi: 10.1056/NEJM198802253180801. [DOI] [PubMed] [Google Scholar]
- 7.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Eng J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 8.Harper J, Dickinson K, Brand M. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev. 2001;2:255–265. doi: 10.1046/j.1467-789x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 9.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 11.Halaas JL, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 13.Flier JS. Obesity wars: Molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 14.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 15.Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc Natl Acad Sci USA. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins S, et al. Role of leptin in fat regulation. Nature. 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 17.Hwa JJ, et al. Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol Regul Integr Comp Physiol. 1997;41:R1204–R1209. doi: 10.1152/ajpregu.1997.272.4.R1204. [DOI] [PubMed] [Google Scholar]
- 18.Scarpace P, Matheny M, Pollock B, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273:E226–E230. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- 19.Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 20.van Dijk G, et al. Metabolic, gastrointestinal, and CNS neuropeptide effects of brain leptin administration in the rat. Am J Physiol. 1999;276:R1425–1433. doi: 10.1152/ajpregu.1999.276.5.R1425. [DOI] [PubMed] [Google Scholar]
- 21.Mistry A, Swick A, Romsos D. Leptin rapidly lowers food intake and elecates metabolic rates in lean and ob/ob mice. J Nutr. 1997;127:2065–2072. doi: 10.1093/jn/127.10.2065. [DOI] [PubMed] [Google Scholar]
- 22.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute intravenous and intracerebroventricular leptin infusion increases glucose uptake and glucose turnover by an insulin independent mechanism. Nature. 1997;389:374–377. [Google Scholar]
- 23.Doring H, Schwarzer B, Nuesslein-Hildesheim B, Schmidt I. Leptin selectively increases energy expenditure of food-restricted lean mice. Int J Obes. 1998;22:83–88. doi: 10.1038/sj.ijo.0800547. [DOI] [PubMed] [Google Scholar]
- 24.Elmquist JE, Elias CF, Saper CB. From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 25.Porter R, Brand M. Body mass dependence of H+ leak in mitochondria and its relevance to metabolic rate. Nature. 1993;362:628–630. doi: 10.1038/362628a0. [DOI] [PubMed] [Google Scholar]
- 26.Cohen P, et al. Role for stearoyl-CoA desaturase-1 in leptin mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 27.Minokoshi Y, et al. Leptin stimulates fatty acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. 2002. [DOI] [PubMed] [Google Scholar]
- 28.Gordon C. Temperature Regulation in Laboratory Rodents. New York: Cambridge Univ Press; 1993. pp. 62–66. [Google Scholar]
- 29.Montez J, et al. Acute leptin dificiency, leptin resistance, and the physiologic response to leptin withdrawal. Proc Natl Acad Sci. 2005;102:2537–2542. doi: 10.1073/pnas.0409530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghadially F. Ultrastructural Pathology of the Cell and Matrix. 3rd Ed. London: Butterworths; 1988. [Google Scholar]
- 31.Martin G, et al. Metabolism and morphology of brown adipose tissue from Brahman and Angus newborn calves. J Anim Sci. 1999;77:388–399. doi: 10.2527/1999.772388x. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Cuenca S, et al. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem. 2002;277:42958–42963. doi: 10.1074/jbc.M207229200. [DOI] [PubMed] [Google Scholar]
- 33.Keshamouni V, et al. Differential protein expression profiling by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing epithelial-mesenchymal transition reveals a migratory/invasive phenotype. J Proteome Res. 2006;5:1143–1154. doi: 10.1021/pr050455t. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, et al. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J Lipid Res. 2006;47:2028–2041. doi: 10.1194/jlr.M600177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elias C, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz M, Woods S, Porte DJ, Seeley R, Baskin D. Central nervous system control of food intake. Nature. 2000;404:661–667. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 37.Hafner R, Brown G, Brand M. Analysis of the control of respiration rate, phosphorylation rate, proton leak rate and protonmotive force in isolated mitochondria using the ‘top-down’ approach of metabolic control theory. Eur J Biochem. 1990;188:313–319. doi: 10.1111/j.1432-1033.1990.tb15405.x. [DOI] [PubMed] [Google Scholar]
- 38.Brand M, Chien L, Rolfe F. Control of oxidative phosphorylation in liver mitochondria and hepatocytes. Biochem Soc Trans. 1993;3:757–762. doi: 10.1042/bst0210757. [DOI] [PubMed] [Google Scholar]
- 39.Porter R, Brand M. Causes of differences in respiration rate of hepatocytes from mammals of different body mass. Am J Physiol. 1995;269:R1213–R1224. doi: 10.1152/ajpregu.1995.269.5.R1213. [DOI] [PubMed] [Google Scholar]
- 40.Obivi S, et al. Identification of biochemical link between energy intake and energy expenditure. J Clin Invest. 2002;109:1599–1605. doi: 10.1172/JCI15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pospisilik J, et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131:476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, Lee Y, Unger R. Novel form of lipolysis induced by letpin. J Biol Chem. 1999;274:17541–17544. doi: 10.1074/jbc.274.25.17541. [DOI] [PubMed] [Google Scholar]
- 43.Bligh E, Dyer W. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 44.Miyazaki M, Kim H-J, Man WC, Ntambi JM. Oleoyl-CoA is the major de novo product of stearoyl-CoA desaturase 1 gene isoform and substrate for the biosynthesis of the harderian gland 1-alkyl-2,3-diacylglycerol. J Biol Chem. 2001;276:39455–39461. doi: 10.1074/jbc.M106442200. [DOI] [PubMed] [Google Scholar]
- 45.Asilmaz E, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113:414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.