Abstract
Background
Volatile anesthetics produce immobility primarily by action in the spinal cord; however, anesthetic effects among different neuronal classes located in different spinal regions, and how they relate to immobility, are not understood.
Methods
In decerebrated rats, effects of isoflurane and halothane on movement elicited by electrical microstimulation of the mesencephalic locomotor region (MLR) were assessed in relation to minimum alveolar concentration (MAC). Anesthetic effects on step frequency and isometric limb force were measured. The authors also examined effects of MLR stimulation on responses of nociceptive dorsal horn neurons and limb force responses to tail clamp.
Results
Mean isoflurane requirements to block MLR-elicited stepping were slightly but significantly greater than MAC by 10%. Mean halothane requirements to block MLR-elicited stepping were greater than those for isoflurane and exceeded MAC by 20%. From 0.4 to 1.3 MAC (but not 0.0 to 0.4 MAC), there was a dose-dependent reduction in the frequency and force of hind limb movements elicited by MLR stimulation during both anesthetics. MLR stimulation inhibited noxious stimulus evoked responses of dorsal horn neurons by approximately 80%. Aptly, MLR stimulation produced analgesia that outlasted the midbrain stimulus by at least 15 s, as indicated by an 81% reduction in hind limb force elicited noxious tail clamp.
Conclusions
Because electrical stimulation of the MLR elicits movement independent of dorsal horn activation, the results suggest that the immobilizing properties of isoflurane and halothane are largely independent of action in the dorsal horn. The results suggest that volatile anesthetics produce immobility mainly by action on ventral spinal locomotor networks.
Volatile anesthetics act primarily in the spinal cord to abolish movement in response to noxious stimulation.1–3 However, anesthetic action does not seem to be uniform across different classes of neurons residing in different anatomical locations of the spinal cord. Slightly above and below minimum alveolar concentration (MAC) where noxious stimulus–evoked movement is abolished, isoflurane has little effect on nociceptive dorsal horn neurons4–8 recorded in vivo, whereas ventral horn neurons are depressed.9 Dorsal horn neurons are moderately suppressed by halothane.4,7,10,11 However, halothane depression of dorsal horn neurons was shown to be completely reversed by naloxone, whereas motor output suppression was only partially reversed.10 These data suggest that volatile anesthetic–induced immobility results largely from action in ventral spinal sites.
Nevertheless, at sub-MAC concentrations, volatile anesthetics, including isoflurane, can reduce activity in dorsal horn neurons by 50%12—a perhaps necessary but not sufficient condition for immobility. Moreover, volatile anesthetics could modulate neurotransmitter release from dorsal horn neurons without changing their action potential firing rates. There is also evidence that some subclasses of nociceptive dorsal horn neurons, based on their functional and/or laminar location, are depressed by volatile anesthetics, whereas other neurons are unaffected. 11,13,14 Uncertainty about whether and to what degree a given dorsal horn neuron contributes to movement versus ascending nociceptive transmission per se further complicates current understanding of the mode of volatile anesthetic immobilizing action. Therefore, conclusions regarding the degree of dorsal horn involvement in anesthetic-induced immobility could are difficult to reach.
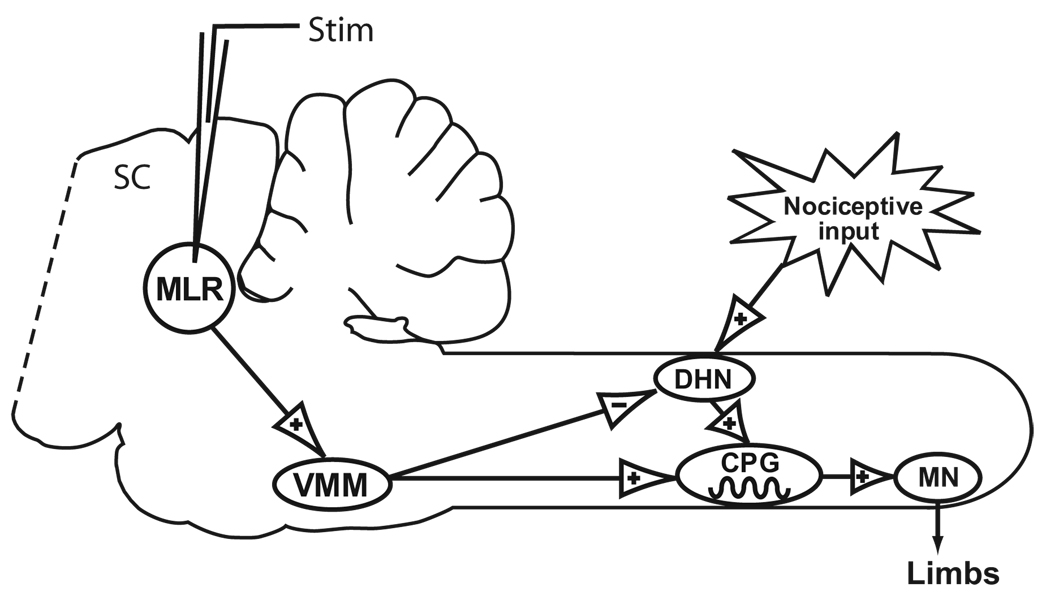
However, it is possible to behaviorally dissect anesthetic immobilizing effects in the dorsal horn from those in the ventral horn using electrical microstimulation of the mesencephalic locomotor region (MLR), corresponding to the cuneiform and pedunculopontine nuclei.15 Electrical or chemical MLR stimulation elicits movement by activating spinal locomotor networks through a descending pathway that is similar across several, if not all, vertebrate classes. Descending locomotor activation from MLR-associated brain regions excites reticulospinal neurons in the ventromedial medulla, which in turn excite spinal lamina VII–VIII locomotor interneurons16 without activating (and in fact inhibiting) dorsal horn neurons,17,18 and producing behavioral analgesia19 (fig. 1). Therefore, activation of locomotor neurons through this descending pathway permits us to test anesthetic effects on movement independent from nociceptive dorsal horn activation.
Fig. 1.
Schematic diagram depicting movement pathways from the mesencephalic locomotor region (MLR) and from activation of peripheral nociceptors. In this diagram, + denotes excitatory transmission and − denotes inhibition. Animals were decerebrated (dashed line) at the rostral edge of the superior colliculus (SC). Projections from the MLR in the cuneiform and pedunculopontine nuclei synapse on reticulospinal neurons in the ventromedial medulla (VMM), which in turn project to the spinal cord to activate ventrally located central pattern generating (CPG) networks that produce rhythmic locomotor activity in motoneurons (MN). Activation of the MLR in addition inhibits dorsal horn neurons (DHN), also through projections to the VMM. A noxious stimulus excites DHNs, which in turn activate the CPG to produce rhythmic multilimb movement, which is blocked by minimum alveolar concentrations of anesthetics. By comparing anesthetic requirements to block MLR-elicited movement (that is independent of dorsal horn activation) with those for noxious stimulus–evoked movement (resulting from dorsal horn activation), it is possible to dissect the relative importance of dorsal and ventral spinal cord sites in producing immobility.
In the current study, we assessed the degree of dorsal versus ventral horn involvement in anesthetic immobilizing action by measuring isoflurane- and halothane-induced depression of movement during locomotion elicited by electrical microstimulation of the MLR. We hypothesized that if the dorsal horn indeed plays little or no role in immobility, anesthetic requirements to block MLR-elicited movement should be approximately equal to those needed to block movement that does rely on nociceptive dorsal horn activation (i.e., MAC determined by supramaximal tail clamp).
Materials and Methods
The University of California, Davis Animal Care and Use Committee (Davis, California) approved this study. All experiments were conducted at the University of California, Davis, and animals were given free access to food and water and were maintained on a 12 h–12 h light–dark cycle with lights on at 07:00. Forty-one adult male Sprague-Dawley rats (420–580 g) were used in this study.
Surgery, Setup, and MAC Determination
Anesthesia was induced in an acrylic box with isoflurane (5%) or halothane (4%) and placed on mask delivery until a tracheostomy was made. Animals were then intubated with a 10-gauge catheter and mechanically ventilated with isoflurane or halothane mixed in 100% oxygen. Body temperature was monitored and maintained at 37° ± 1°C with a heating pad. End-tidal carbon dioxide and anesthetic concentration were also monitored continuously with an Ohmeda Rascal II analyzer (Helsinki, Finland). One of the carotid arteries and a jugular vein were cannulated to permit blood pressure recording (model PB-240; Puritan-Bennett Corp., Hazelwood, MO) and fluid administration, respectively. Carotid arterial blood flow to the brain was occluded bilaterally, and dexamethasone (0.8 mg/kg intravenous) was given to prevent excessive bleeding and minimize inflammation from the decerebration procedure (see last paragraph of Surgery, Setup, and MAC Determination). Platinum needles electrodes (Grass-Telefactor, Warwick, RI) were inserted bilaterally into the biceps femoris muscles and sutured in place to record electromyogram activity. For animal groups undergoing limb force measurement, only unilateral placement of electromyogram electrodes was made. For the animal group in which we recorded dorsal horn neuronal activity in paralyzed animals, we performed a laminectomy to expose the lumbar spinal cord.
We determined MAC in each animal before and at least 90 min after a precollicular decerebration. MAC was determined using a tail clamp of supramaximal intensity as previously described.4,20 In brief, animals were anesthetized with either isoflurane or halothane, and the clamp was applied for up to 1 min or until movement was observed. Repetitive limb movements and head turning in response to tail clamp were considered positive movement, whereas gasping and tonic limb movements were considered negative. Depending on the response, the anesthetic concentration was changed in 0.2% increments with an intervening 15- to 20-min equilibration period, and the clamp was reapplied. This procedure was repeated until two anesthetic concentrations were found that just permitted and just prevented movement. The average of these two values was MAC.
The animal was fixed in a stereotaxic frame with an incisor clamp, ear bars, and a hip clamp. Several straps of tape were placed to form a sling that supported the animal from below. During deep anesthesia (2.0% isoflurane or halothane), a craniotomy was made between bregma and lambda, the dura was removed, and the cortex was aspirated to reveal subcortical structures. A complete decerebration (visually verified) was made with a scalpel blade at the rostral edge of the superior colliculus, with the scalpel blade angled 15° with the tip pointed in the rostral direction (dashed line in fig. 1). The empty portion of the skull cavity was packed with gauze and gel foam pledgets. After a 90-min recovery period, the animal’s postdecerebrate MAC was determined.
MLR Stimulation and Data Collection
A fine tungsten electrode (0.5 MΩ AM Systems, Carlsborg, WA) was used for electrical MLR stimulation. Using the center of the intercollicular crux as a zero reference point, we positioned the stimulating electrode 1.8 –2.0 mm lateral to the midline and ±0.5 mm anteroposterior from this point. We searched for the MLR site at 0.0 or 0.4 MAC isoflurane or halothane. Constant-current electrical pulses (0.5-ms pulse duration, 60 Hz) were passed through the electrode using a PSIU6 stimulus isolation unit connected to an S88 stimulator (Grass-Telefactor). Using a stimulus intensity of 60–80 µA, we slowly advanced the electrode into the midbrain to search for the MLR. If a site was found to elicit locomotion, we decreased and increased the stimulus intensity and finely adjusted the position of the electrode to determine the lowest threshold site for four-limb galloping (threshold range, 20–50 µA). If no locomotion or only single-limb stepping was observed, we retracted the electrode and moved it 300–500 µM in the anteroposterior or mediolateral direction until a low-threshold stimulation site was found to produce four-limb galloping. Biceps femoris electromyogram signals were amplified and band-pass filtered 10 Hz to 2 kHz with a Tektronix differential amplifier (model 2601; Beaverton, OR). In animals undergoing limb force measurement, one limb was tied to a force transducer (FT03; Grass-Telefactor) connected to an iWorx bridge amplifier (model ETH-4; Dover, NH). Electromyogram and force transducer signals were fed to a Cambridge Electronic Design, Power 1401 data acquisition system with Spike 2 software (Cambridge, United Kingdom).
Experimental Design
Motor responses to electrical MLR stimulation were measured during 0.0, 0.4, 0.6, 0.8, and 1.1 MAC. The order of data collection during different MAC fractions was varied from experiment to experiment. At each MAC fraction, responses to MLR electrical stimulation intensities of 1.2× threshold (T), 1.5T, and 2.0T were tested. For 0.0 and 0.4 MAC, movement responses were so vigorous that we limited our stimulation intensities to 1.2T and 1.5T. If positive movement occurred (defined by at least one step cycle in at least one hind limb) at 1.1 MAC or above, the anesthetic concentration was increased by 0.2 MAC until stepping was abolished. At higher anesthetic concentrations, if the animal did not step to the 2.0T stimulus, we increased our stimulation intensity by 1.0T multiples up to 5T for a duration of up to 1 min. In animals undergoing force measurement, we delivered MLR stimulation intensities of 1.2, 1.5, and 2.0, and 3.0T at 0.6, 0.8, and 1.1 MAC. Again, only the 1.2T and 1.5T MLR stimulus intensities were delivered at 0.0 and 0.4 MAC. If the last anesthetic concentration tested was above MAC and no stepping was observed, we repeated one sub-MAC anesthetic concentration to test for recovery. If stepping did not recover or the step threshold for MLR stimulation was increased by more than 25%, we excluded these animals from the study.
We determined isoflurane and halothane MAC needed to block MLR-elicited stepping (MACMLR) by observing limb movement as we did for typical MAC determinations using a tail clamp. We also measured step frequency at each MAC fraction for each anesthetic. In the animal group that had one limb secured to a force transducer, we measured the peak force generated by each MLR stimulus intensity at each isoflurane or halothane MAC fraction. In some animals undergoing hind limb force measurement, we assessed analgesic effects of the MLR stimulus (1.5T) by obtaining a control hind limb force response to tail clamp, then repeating the tail clamp 15 s after the MLR stimulus. Tail clamp responses were obtained again 4 min later to test for recovery.
Electrophysiologic Recording of Dorsal Horn Neurons
We tested the effect of MLR stimulation on the activity of lumbar nociceptive dorsal neurons with receptive fields located on the plantar hind paw. This was accomplished by first searching for and verifying four-limb galloping in response to electrical MLR stimulation (as described in MLR Stimulation and Data Collection). When a low-threshold MLR site was identified, the animal was paralyzed with pancuronium bromide (0.6 mg · kg−1 · h−1), and a tungsten microelectrode (8–10 MΩ FHC, Bowdoinham, ME) was advanced into the lumbar spinal cord to record dorsal horn neuronal activity. Single units were isolated and classified as wide-dynamic-range neurons if they responded to tactile as well as noxious mechanical and thermal stimuli, and nociceptive specific if they responded to noxious mechanical and thermal stimuli but not innocuous tactile stimulation. We tested the effects of MLR stimulation (1.2–2.0T) in the absence and presence of a noxious mechanical (arterial clip) or thermal (hind paw immersed in 52°C hot water) stimulums applied to the neuron’s receptive field. To assess effects of MLR stimulation on neuronal responses, a control noxious stimulus was applied for 15 s. The stimulus was then repeated with MLR stimulation (duration 5 s) turned on in the middle of the dorsal horn neuronal response to the 15-s noxious stimulus (i.e., 5–10 s after the onset of the noxious stimulus). Responses were analyzed by summing the number of action potentials discharged during the 5-s time period when MLR stimulation occurred and comparing this number with the number of action potentials discharged during the equivalent time period of the control response.
Histology
At the end of some experiments, a stimulator (SD-5; Grass-Telefactor) was set to 8 V, and DC anodal current was passed through the stimulating electrode to make an electrolytic lesion near the electrode tip. Rats were killed with potassium chloride, and the hindbrain was extracted and placed in 10% formalin for at least 48 h, followed by 48 h in 30% sucrose. The brain was frozen, cut in 60-µM transverse sections, mounted on slides, stained with cresyl violet, and coverslipped. Sections were examined under light microscopy, and lesion sites were transferred to a representative sagittal template21 based on their rostrocaudal and dorsoventral location.
Statistics
MACMLR values were compared with MAC values (determined by tail clamp) using a two-tailed paired t test. The ratio MACMLR:MAC was compared between isoflurane and halothane groups by a two-tailed, unpaired t test. Maximum step frequencies and hind limb forces were compared across anesthetic concentrations for each anesthetic using analysis of variance with “anesthetic concentration” as a fixed factor and “animal” designated as a random factor. Because we could not obtain responses above the 1.5T MLR stimulus intensity at 0.0 and 0.4 MAC, we separately analyzed 0.0, 0.4, and 0.6 MAC as “sub-MAC” concentrations (maximum responses 1.2–1.5T) and 0.8, 1.1, and 1.3 MAC as “peri-MAC” concentrations (1.5–3.0T). For step frequency data, we conducted post hoc Tukey multicomparisons. For force data, we conducted post hoc Dunnett T3 multi-comparisons that do not assume equal variances, warranted by significant differences found between some groups using an F test. Comparisons between animal groups (isoflurane and halothane) were made using a two-tailed, two-sample t test. We analyzed the analgesic effect of MLR stimulation on dorsal horn neurons and limb force responses to tail clamp using a two-tailed paired t test. A P value less than 0.05 was considered statistically significant. Data analyses were performed using SPSS (Chicago, IL).
Results
Anesthetic Concentrations Needed to Abolish MLR-elicited Locomotion in Relation to MAC
Seventeen animals were studied during isoflurane anesthesia, and 19 separate animals were studied during halothane anesthesia. Before decerebration, the mean MAC value for isoflurane was 1.23 ± 0.10% (SD) and the mean halothane MAC value was 1.04 ± 0.09% (SD). Postdecerebrate MAC values were 1.17 ± 0.07% for isoflurane and 0.99 ± 0.08% for halothane. Decerebration did not significantly change MAC for both isoflurane and halothane groups. However, it was not uncommon that an animal’s MAC value decreased by 0.1% atm after decerebration, and therefore we based our testing on each animal’s decerebrate MAC value. All animals exhibited stepping responses to MLR stimulation up to at least the 3.0T stimulus at 0.8 MAC for both anesthetic groups.
At supra-MAC concentrations, animals that did not respond to the 3T MLR stimulus intensity also did not respond to higher stimulus intensities.
For the isoflurane group, mean MACMLR was slightly but significantly greater than MAC (P < 0.01) by 10%. For the halothane group, MACMLR was significantly greater than MAC by 21% (P < 0.0001). Furthermore, the ratio MACMLR:MAC in the halothane group (1.21 ± 0.1 [SD]) was significantly greater than for the isoflurane group (1.10 ± 0.1 [SD]; P ± 0.016).
Isoflurane and Halothane Effects on Parameters of MLR-elicited Locomotion
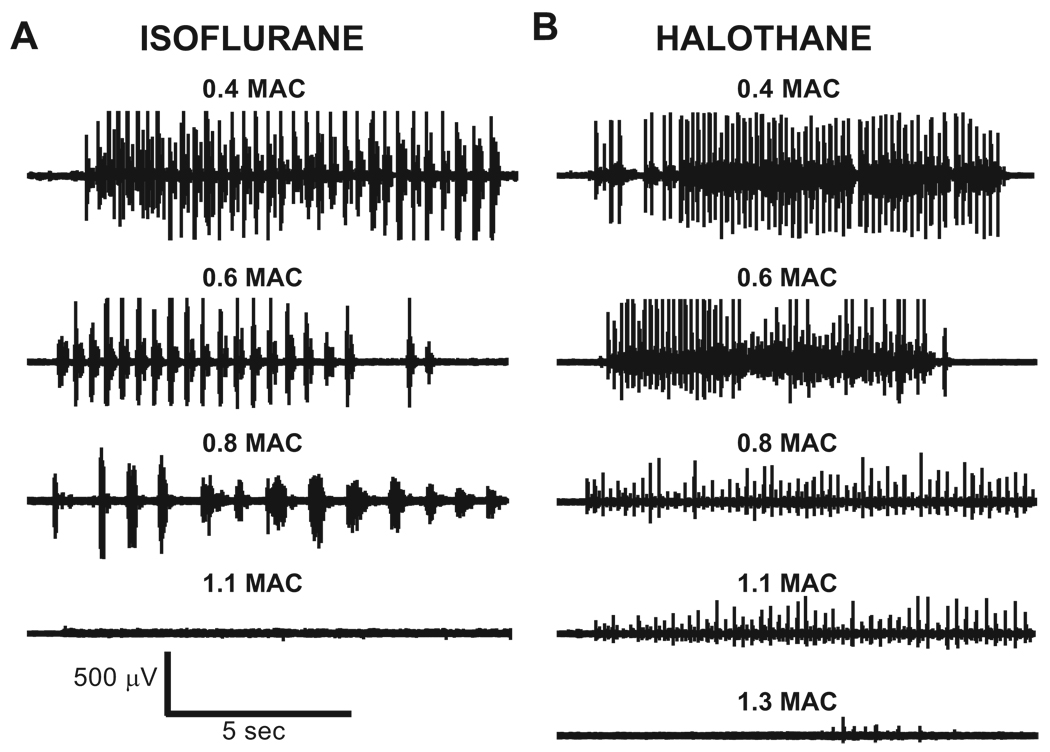
We measured the frequency and force of MLR-elicited stepping in the anesthetic-free condition as well as 0.4–1.3 MAC for halothane and isoflurane. For both isoflurane and halothane, neither the frequency nor the force of stepping was significantly different between 0.0 and 0.4 MAC. However, at 0.6 MAC, there began a significant reduction in the force and the frequency of MLR-elicited stepping during both isoflurane and halothane (P < 0.04 in all cases). These parameters were progressively reduced with further increases in isoflurane and halothane concentration. Individual examples of electromyogram activity during isoflurane and halothane are shown in figure 2, and mean data showing isoflurane and halothane effects on step frequency and force are shown in figure 3.
Fig. 2.
Anesthetic requirements to block mesencephalic locomotor region–elicited locomotion are higher in relation to minimum alveolar concentration (MAC) for halothane than for isoflurane. Individual examples from two animals showing isoflurane (A) and halothane (B) effects on mesencephalic locomotor region–evoked biceps femoris electromyogram activity. During isoflurane anesthesia, mesencephalic locomotor region–evoked stepping was decreased up to 0.8 MAC and finally abolished when isoflurane concentration was increased to 1.1 MAC (bottom left trace). During halothane anesthesia, stepping was also reduced in the 0.4–0.8 MAC range, but this animal still exhibited weak stepping up to 1.3 MAC (bottom right trace) that was finally abolished by 1.5 MAC halothane (data not shown). In each example, locomotor-related electromyogram activity was elicited by 1.5T intensity at 0.4 MAC and by 2.0T for the remaining concentrations.
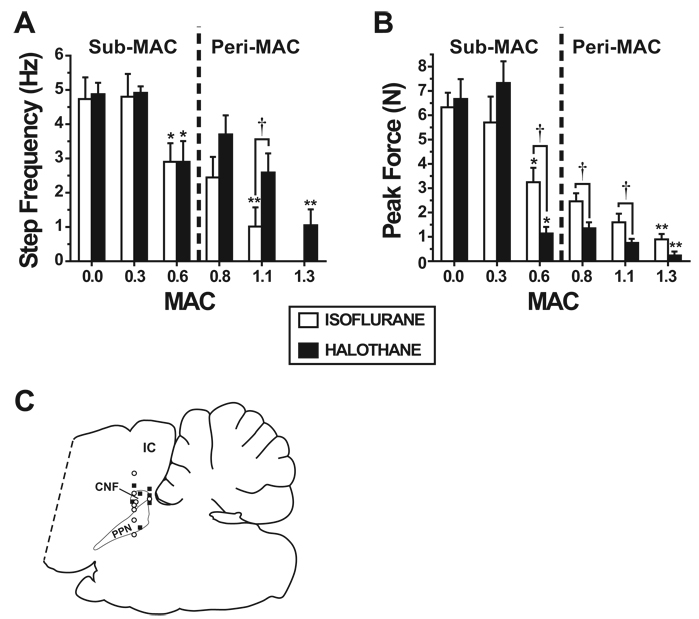
Fig. 3.
The frequency and force of mesencephalic locomotor region–elicited locomotion is greatly reduced or abolished by peri–minimum alveolar concentration (MAC) isoflurane and halothane concentrations. Bar graphs showing mean effects of isoflurane (open bars) and halothane (solid bars) on the frequency (A) and force (B) of movement elicited by electrical mesencephalic locomotor region stimulation at different stimulus intensity multiples of threshold (T) for four-limb galloping. Both isoflurane (n = 9) and halothane (n = 9) dose-dependently reduced the frequency and force of mesencephalic locomotor region–elicited stepping in the 0.4–1.3 MAC range (but not in the 0.0–0.4 MAC range). While stepping occurred during higher mean halothane concentrations compared with isoflurane, the force of limb movement at 0.6–1.1 MAC halothane (n = 9) was significantly less compared with isoflurane (n = 8). Data are shown as mean ± SEM. * Significantly different from responses at 0.0 and 0.4 MAC. ** Significantly different from responses at 0.8 MAC. † Significantly different between isoflurane and halothane groups. (C) A representative sagittal section of the hindbrain21 showing 15 histologically identified stimulation sites from animals used to collect the data shown in A and B. Open circles = stimulation sites in animals anesthetized with isoflurane; solid squares = stimulation sites in animals anesthetized during halothane anesthesia. CNF = cuneiform nucleus; IC = inferior colliculus; PPN = pedunculopontine nucleus.
Consistent with the MACMLR:MAC data above, step frequency during isoflurane was significantly lower at 1.1 MAC compared with 0.8 MAC (P < 0.03), whereas for halothane this comparison was not statistically significant fig. 3A). Instead, halothane significantly reduced step frequency at 1.3 MAC compared with 0.8 MAC (P < 0.003). Also consistent with the MACMLR:MAC data, step frequency at 1.1 MAC isoflurane was significantly lower compared with that at 1.1 MAC halothane (P < 0.03).
While both isoflurane and halothane significantly reduced hind limb force in response to MLR stimulation between 0.4 and 0.6 MAC, hind limb forces during isoflurane were significantly greater than those during halothane (fig. 3B) at MAC multiples of 0.6 (P < 0.004), 0.8 (P < 0.014), and 1.1 (P < 0.04). Therefore, stepping that occurred during peri-MAC halothane concentrations was extremely weak.
Fifteen midbrain electrical stimulation sites were histologically verified (fig. 3C). Of these sites, eight were from animals anesthetized during isoflurane, and seven were from halothane-anesthetized animals. Sites were within or near the borders of the cuneiform and pedunculopontine nuclei. There was no significant difference in the distribution of MLR stimulation sites between isoflurane- or halothane-anesthetized animals in the rostrocaudal, mediolateral, or dorsoventral planes.
Effects of MLR Stimulation on Dorsal Horn Neurons and Hind Limb Responses to Tail Clamp
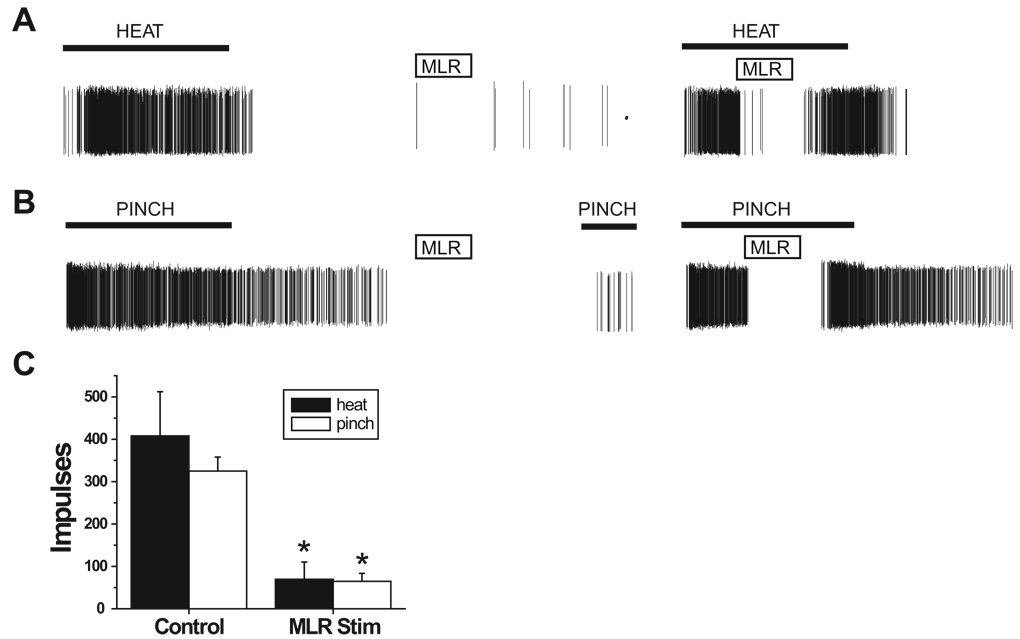
We tested the effects of MLR stimulation on 13 nociceptive dorsal horn neurons in an additional five animals. Neurons were located at laminar depths located throughout the dorsoventral extent of the dorsal horn (range, 95–862 µm), with a mean depth of 535 ± 246 µm (SD). Nine of these neurons were of the wide-dynamic-range type, and 4 were nociceptive specific. MLR stimulation (1.2–2.0T) did not activate any of these neurons and completely inhibited any spontaneous activity. MLR stimulation at 1.2T intensity inhibited noxious heat–evoked responses in all neurons, to a mean of 17% of control (P < 0.0001), and inhibited noxious mechanically evoked responses in all neurons, to a mean of 20% of control (P < 0.003). Usually responses of dorsal horn neurons to noxious stimuli were completely blocked 0.5–2 s after the onset of MLR stimulation. Individual examples of the effect of MLR stimulation on one wide-dynamic-range neuron and one nociceptive-specific neuron are shown in figures 4A and B, respectively. Mean MLR effects on noxious mechanical and thermal responses of dorsal horn neurons are displayed in figure 4C.
Fig. 4.
Mesencephalic locomotor region (MLR) stimulation inhibits nociceptive dorsal horn neurons. (A) Individual example of raw action potentials from a wide-dynamic-range neuron showing a control response to a 15-s noxious thermal stimulus (indicated by thick black horizontal bar) applied to the plantar hind paw (left trace). A 5-s 3T-intensity MLR stimulus inhibited spontaneous activity in this neuron in the absence of noxious stimulation (middle trace). A 5-s 1.2T-intensity MLR stimulus inhibited the response to a second, identical noxious thermal stimulus (right trace). (B) Individual example of raw action potentials from a nociceptive specific type neuron showing a control response to a 15-s noxious mechanical stimulus applied to the plantar hind paw (left trace). A 5-s 3T MLR stimulus did not activate the neuron in the absence of noxious stimulation and reduced the neuron’s response to noxious mechanical stimulation applied 10 s after the termination of the MLR stimulus (middle trace). A 5-s 1.2T-intensity MLR stimulus inhibited the response to noxious mechanical stimulation (right trace). (C) Bar graphs showing mean responses of 13 nociceptive dorsal horn neurons to noxious thermal (solid bars) and noxious mechanical stimuli (open bars) under control conditions (left bars) and during 5-s of 1.2T MLR stimulation. Data are shown as mean ± SE. * Significantly less than control.
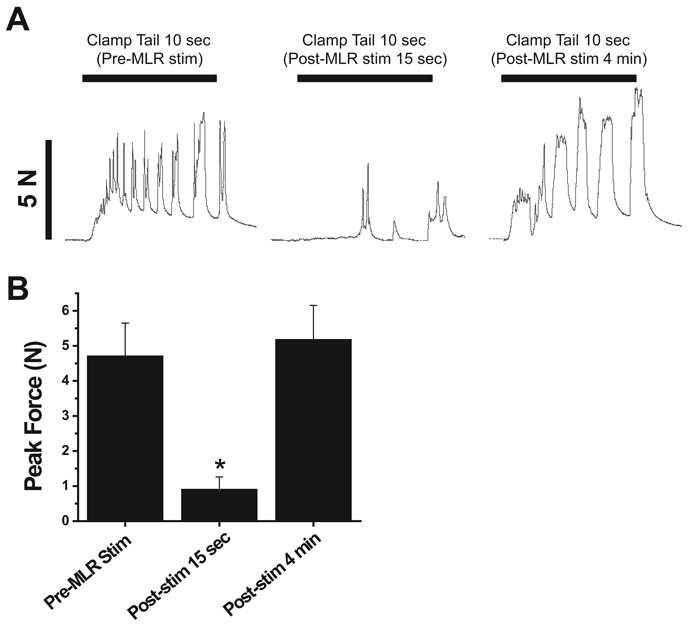
In four animals, we tested whether the MLR stimulus–induced inhibition of dorsal horn neurons would translate to a behavioral analgesia, indicated by a reduction in the force of tail clamp–evoked limb force. Peak hind limb forces elicited by supramaximal intensity tail clamp were significantly reduced by MLR stimulation to 19% of control (P < 0.01) when the tail clamp was applied 15 s after the termination of a 5-s, 1.5T MLR stimulus. Responses to tail clamp completely recovered when tested again 4 min after the MLR stimulus. An individual example showing the effect of MLR stimulation on tail clamp–elicited hind limb force is shown in figure 5A, and mean effects are shown in figure 5B.
Fig. 5.
Mesencephalic locomotor region (MLR) stimulation produces a behavioral analgesia. (A) Individual example showing force tracings in response to a supramaximal intensity tail clamp. The tail clamp elicited robust rhythmic movement of the hind limb (left trace) that was depressed 15 s after a 5-s 1.5T MLR stimulus (middle trace). The hind limb response to tail clamp was recovered when applied again 4 min after the MLR stimulus right trace. (B) Bar graphs showing mean MLR stimulus effects on limb force responses to tail clamp applied before, 15 s after, and 4 min after MLR stimulation. * Significantly reduced compared with control and recovery.
Discussion
The results confirm and extend findings in previous studies suggesting that the primary immobilizing site of volatile anesthetics, namely isoflurane and halothane, is situated ventral to the spinal dorsal horn.4,7,8,10 Because MLR stimulation activates locomotor circuitry while inhibiting nociceptive dorsal horn activity, we were able to behaviorally assess anesthetic immobilizing requirements under conditions that were independent from nociceptive transmission through the dorsal horn. We currently found that locomotion elicited by electrical stimulation of the MLR at low-threshold sites was largely depressed or abolished in the peri-MAC range where multilimb locomotor responses to supramaximal noxious stimuli are abolished22 (fig. 2 and fig 3). Halothane has been documented to have a greater dorsal horn depressant action compared with isoflurane.4,7 We currently found that halothane requirements to block MLR-elicited locomotion were slightly greater than those for isoflurane and 21% greater than MAC, suggesting a possible small dorsal horn contribution to anesthetic induced immobility. Although animals exhibited stepping up to higher average halothane MAC multiples compared with isoflurane (fig. 3A), the force of limb movements during halothane were significantly less than during isoflurane (fig. 3B) and of extremely weak magnitude. Furthermore, during both isoflurane and halothane and regardless of whether or not stepping was abolished, the magnitude of limb movement in the current study was depressed by more than 80% at 1.1 MAC, indicating that the large majority of anesthetic immobilizing action lies within the ventral horn.
Methodologic Validation and Considerations
We validated the use of MLR-elicited movement to distinguish between dorsal and ventral horn effects on several grounds. First, the MACMLR:MAC ratio was proportional to the anesthetics relative dorsal horn depressant action (where halothane > isoflurane4,12). Although by definition a MAC determination is performed with a “supramaximal” noxious stimulus, it is possible that increased MACMLR compared with MAC requirements result from the MLR stimulus being stronger than the tail clamp. However, if this were true, we would expect MLR requirements to be elevated equally above MAC for both anesthetics, but in fact, the MACMLR:MAC ratio for halothane was greater than that for isoflurane. Furthermore, a stimulus intensity of 3T was needed to produce movement in all animals at 0.8 MAC (where all animals moved in response to tail clamp). At higher concentrations, animals that did not move up to the 3T MLR stimulus also did not move at higher stimulus intensities. Moreover, both stimuli elicit similar magnitudes of robust galloping locomotor responses. Therefore, the MLR and tail clamp stimuli seem quite comparable to one another in that they both maximally activate spinal locomotor networks.
It has been previously shown that activation of midbrain regions associated with the MLR inhibits dorsal horn neurons.23 However, most studies recorded dorsal horn neurons receiving muscle afferents,17 or studied descending nociceptive inhibition in deeply anesthetized or paralyzed animals18 without addressing locomotion per se. Therefore, we verified that nociceptive dorsal horn neurons receiving cutaneous nociceptor input were inhibited by the same stimulus parameters we used to elicit locomotion from the MLR. Electrical MLR stimulation powerfully inhibited dorsal horn neurons (fig. 4) and produced behavioral analgesia to tail clamp (fig. 5). It is possible that the role of the dorsal horn in anesthetic-induced immobility could be underestimated if the MLR-elicited inhibition of dorsal horn neurons removed a significant source of excitability to ventral neurons. However, MLR stimulation maximally activated locomotor networks, and by comparison, inhibition of typically very low spontaneous activity in dorsal horn neurons (e.g., figs. 4A and B, middle traces) would be negligible. Furthermore, spontaneous dorsal horn activity is higher during halothane compared with isoflurane.4 Therefore, if inhibition of dorsal horn activity had an appreciable effect on MACMLR, we would expect a lower halothane MACMLR compared with isoflurane, but the opposite was found.
The MLR-elicited movement presumably led to nonnociceptive sensory feedback, well known to influence (but not necessary for) locomotion.24 However, rats did not exhibit movement responses to nonnoxious stimulation at or above 0.6 MAC, making an influence from nonnociceptive dorsal horn activity seem unlikely. Therefore, anesthetic effects on MLR-elicited movement seem to indicate direct depression of circuitry in the ventral spinal cord.
Implications for Sites of Volatile Anesthetic Immobilizing Action
Decerebration did not significantly affect MAC values in isoflurane- or halothane-anesthetized animals, demonstrating that brain structures at least rostral to the superior colliculus are not important to volatile anesthetic–induced immobility, as has been previously demonstrated for isoflurane.25 MLR-elicited locomotion involves the recruitment of reticulospinal neurons in the ventromedial medulla, a supraspinal area that could have been affected by the anesthetics used in the current study. Because isoflurane and halothane immobilizing requirements increase approximately threefold when selectively delivered to the goat brain,1,26 a pronounced direct action in the brainstem leading to motor depression seems unlikely. Isoflurane has pronociceptive and antinociceptive supraspinal effects that influence movement in response to noxious stimuli.27 However, up to isoflurane MAC, supraspinal facilitation seems to counteract a direct and potent spinal depression, because selective isoflurane delivery to the spinal cord in goats or spinal transection in rats reduces isoflurane MAC by 40%.3,28 Therefore, it seems that motor depression seen in the peri-MAC range is primarily of spinal origin.
The current study argues strongly against several possible scenarios that could otherwise implicate the dorsal horn as an important site for volatile anesthetic immobilizing action. It was recently shown that isoflurane’s depressant action on spinal neurons increases with increasing dorsoventral depth in the spinal cord.9 Therefore, a small effect in the dorsal horn and perhaps elsewhere could culminate in a large motor suppression through progressive summation or nonlinear transformations in polysynaptic spinal circuits. Furthermore, isoflurane was shown to depress spinal dorsal horn neuronal responses by approximately 50% from 0.0 to 0.8 MAC, 12 leaving open the possibility that these sub-MAC “analgesic” effects are necessary but not sufficient for immobilization. These possibilities, along with potential presynaptic effects in dorsal horn neurons, do not seem likely to contribute appreciably to immobility. That is, movement elicited independently of dorsal horn activation in the current study was blocked or profoundly suppressed by volatile anesthetic concentrations of 1.2 MAC of less.
Abolition of rhythmic, multilimb, locomotor-type movements in responses to supramaximal intensity noxious stimulation occurs at anesthetic concentrations that bracket MAC.22 In the current study, anesthetic-induced abolition of MLR-elicited locomotion was independent of nociceptive dorsal horn activity (fig. 4). It is therefore possible that much of these immobilizing properties are explained by a preferential action on spinal interneuronal networks responsible for producing rhythmic locomotor activity, termed central pattern generators.29,30 The current methods are limited in that they do not permit us to fully assess to what degree anesthetics affect locomotor networks versus motoneurons directly. However, while anesthetics blocked stepping, high MLR stimulus intensities delivered above MAC often caused weak tonic hind limb flexion, considered negative for MAC determination, but indicating that motor neurons were excitable enough to lift the limbs. Furthermore, stepping was significantly decreased from 0.8 to 1.1 MAC isoflurane, whereas hind limb force was not. A previous study found in spinal cord slices that ventral neurons exhibiting spontaneous bursting properties were depressed by sevoflurane, 31 suggesting potential effects on locomotor networks. We have previously shown evidence of a direct isoflurane action on central pattern generators using bath partitioning for selective anesthetic delivery in the lamprey isolated spinal cord.32 However, anesthetic depression of MLR-elicited movement is not without some presumed motoneuron effect, because volatile anesthetics can directly suppress motoneurons.33
Conclusions
The results suggest that spinal dorsal horn neurons play a minor role in volatile anesthetic–induced immobility but are not a key site for immobility. We therefore propose that anesthetic immobilizing action in the spinal cord is not uniform across neuronal classes and that these agents primarily target ventral spinal sites where locomotor interneurons and motoneurons are situated.34 MLR-elicited movement might be a useful tool in future investigations of the pharmacology of volatile anesthetic immobilizing action, which could obviate nonspecific drug effects in the dorsal horn.
Acknowledgments
Supported by grant Nos. R01 GM 078167 (to Dr. Jinks) and P01 GM47818 from the National Institutes of Health, Bethesda, Maryland, and the Department of Anesthesiology and Pain Medicine, University of California, Davis, California.
Footnotes
Information on purchasing reprints may be found at www.anesthesiology.org or on the masthead page at the beginning of this issue.
References
- 1.Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244–1249. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Rampil IJ. Anesthetic potency is not altered after hypothermic spinal cord transection in rats. Anesthesiology. 1994;80:606–610. doi: 10.1097/00000542-199403000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Jinks SL, Dominguez CL, Antognini J. Drastic decrease in isoflurane minimum alveolar concentration and limb movement forces following thoracic spinal cooling and chronic spinal transection in rats. Anesthesiology. 2005;102:624–632. doi: 10.1097/00000542-200503000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Jinks SL, Martin JT, Carstens E, Jung SW, Antognini JF. Peri-MAC depression of a nociceptive withdrawal reflex is accompanied by reduced dorsal horn activity with halothane but not isoflurane. Anesthesiology. 2003;98:1128–1138. doi: 10.1097/00000542-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Antognini JF, Carstens E. Increasing isoflurane from 0.9 to 1.1 minimum alveolar concentration minimally affects dorsal horn cell responses to noxious stimulation. Anesthesiology. 1999;90:208–214. doi: 10.1097/00000542-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Jinks SL, Antognini JF, Carstens E. Isoflurane depresses diffuse noxious inhibitory controls in rats between 0.8 and 1.2 minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:111–116. doi: 10.1213/01.ane.0000066259.39584.f7. [DOI] [PubMed] [Google Scholar]
- 7.Cuellar JM, Dutton RC, Antognini JF, Carstens E. Differential effects of halothane and isoflurane on lumbar dorsal horn neuronal windup and excitability. Br J Anaesth. 2005;94:617–625. doi: 10.1093/bja/aei107. [DOI] [PubMed] [Google Scholar]
- 8.Jinks S, Antognini JF, Carstens E, Buzin V, Simons C. Isoflurane can indirectly depress lumbar dorsal horn activity in the goat via action within the brain. Br J Anaesth. 1999;82:244–249. doi: 10.1093/bja/82.2.244. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Yao A, Atherley R, Carstens E, Jinks SL, Antognini JF. Neurons in the ventral spinal cord are more depressed by isoflurane, halothane, and propofol than are neurons in the dorsal spinal cord. Anesth Analg. 2007;105:1020–1026. doi: 10.1213/01.ane.0000280483.17854.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You HJ, Colpaert FC, Arendt-Nielsen L. Nociceptive spinal withdrawal reflexes but not spinal dorsal horn wide-dynamic range neuron activities are specifically inhibited by halothane anaesthesia in spinalized rats. Eur J Neurosci. 2005;22:354–360. doi: 10.1111/j.1460-9568.2005.04234.x. [DOI] [PubMed] [Google Scholar]
- 11.Kitahata LM, Ghazi-Saidi K, Yamashita M, Kosaka Y, Bonikos C, Taub A. The depressant effect of halothane and sodium thiopental on the spontaneous and evoked activity of dorsal horn cells: Lamina specificity, time course and dose dependence. J Pharmacol Exp Ther. 1975;195:515–521. [PubMed] [Google Scholar]
- 12.Mitsuyo T, Dutton RC, Antognini JF, Carstens E. The differential effects of halothane and isoflurane on windup of dorsal horn neurons selected in unanesthetized decerebrated rats. Anesth Analg. 2006;103:753–760. doi: 10.1213/01.ane.0000230605.22930.52. [DOI] [PubMed] [Google Scholar]
- 13.Barter LS, Carstens EE, Jinks SL, Antognini JF. Halothane and isoflurane depress dorsal horn nociceptive specific but not wide dynamic range neurons (abstract) Anesthesiology. 2007;107:A1915. [Google Scholar]
- 14.Hagihira S, Taenaka N, Yoshiya I. Inhalation anesthetics suppress the expression of c-Fos protein evoked by noxious somatic stimulation in the deeper layer of the spinal cord in the rat. Brain Res. 1997;751:124–130. doi: 10.1016/s0006-8993(96)01398-4. [DOI] [PubMed] [Google Scholar]
- 15.Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984;323:385–389. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- 16.Whelan PJ. Control of locomotion in the decerebrate cat. Prog Neurobiol. 1996;49:481–515. doi: 10.1016/0301-0082(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 17.Degtyarenko AM, Kaufman MP. Fictive locomotion and scratching inhibit dorsal horn neurons receiving thin fiber afferent input. Am J Physiol Regul Integr Comp Physiol. 2000;279:R394–R403. doi: 10.1152/ajpregu.2000.279.2.R394. [DOI] [PubMed] [Google Scholar]
- 18.Carstens E, Klumpp D, Zimmermann M. Differential inhibitory effects of medial and lateral midbrain stimulation on spinal neuronal discharges to noxious skin heating in the cat. J Neurophysiol. 1980;43:332–342. doi: 10.1152/jn.1980.43.2.332. [DOI] [PubMed] [Google Scholar]
- 19.Sandkuhler J, Gebhart GF. Characterization of inhibition of a spinal nociceptive reflex by stimulation medially and laterally in the midbrain and medulla in the pentobarbital-anesthetized rat. Brain Res. 1984;305:67–76. doi: 10.1016/0006-8993(84)91120-x. [DOI] [PubMed] [Google Scholar]
- 20.Quasha AL, Eger EI, Tinker JH. Determination and applications of MAC. Anesthesiology. 1980;53:315–334. doi: 10.1097/00000542-198010000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edition. New York: Academic Press; 1998. p. 83. [Google Scholar]
- 22.Antognini JF, Wang XW, Carstens E. Quantitative and qualitative effects of isoflurane on movement occurring after noxious stimulation. Anesthesiology. 1999;91:1064–1071. doi: 10.1097/00000542-199910000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Willis WDJ. Anatomy and physiology of descending control of nociceptive responses of dorsal horn neurons: Comprehensive review. Prog Brain Res. 1988;77:1–29. doi: 10.1016/s0079-6123(08)62776-4. [DOI] [PubMed] [Google Scholar]
- 24.Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- 25.Rampil IJ, Mason P, Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993;78:707–712. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Antognini JF, Carstens E, Atherley R. Does the immobilizing effect of thiopental in brain exceed that of halothane? Anesthesiology. 2002;96:980–986. doi: 10.1097/00000542-200204000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Kingery WS, Agashe GS, Guo TZ, Sawamura S, Frances DM, David CJ, Kobilka BK, Maze M. Isoflurane and nociception: Spinal α2A adrenoceptors mediate antinociception while supraspinal α1 adrenoceptors mediate pronociception. Anesthesiology. 2002;96:367–374. doi: 10.1097/00000542-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Borges M, Antognini JF. Does the brain influence somatic responses to noxious stimuli during isoflurane anesthesia? Anesthesiology. 1994;81:1511–1515. doi: 10.1097/00000542-199412000-00027. [DOI] [PubMed] [Google Scholar]
- 29.Alford S, Schwartz E, Viana di Prisco G. The pharmacology of vertebrate spinal central pattern generators. Neuroscientist. 2003;9:217–228. doi: 10.1177/1073858403009003014. [DOI] [PubMed] [Google Scholar]
- 30.Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol. 2001;11:R986–R996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- 31.Grasshoff C, Antkowiak B. Propofol and sevoflurane depress spinal neurons in vitro via different molecular targets. Anesthesiology. 2004;101:1167–1176. doi: 10.1097/00000542-200411000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Jinks SL, Atherley RJ, Dominguez CL, Sigvardt KA, Antognini JF. Isoflurane disrupts central pattern generator activity and coordination in the lamprey isolated spinal cord. Anesthesiology. 2005;103:567–575. doi: 10.1097/00000542-200509000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Cheng G, Kendig JJ. Enflurane directly depresses glutamate AMPA and NMDA currents in mouse spinal cord motor neurons independent of actions on GABAA or glycine receptors. Anesthesiology. 2000;93:1075–1084. doi: 10.1097/00000542-200010000-00032. [DOI] [PubMed] [Google Scholar]
- 34.Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: A lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]