Abstract
Slo3 channels belong to the high conductance Slo K+ channel family. They are activated by voltage and intracellular alkalinization, and have a K+/Na+ permeability ratio (PK/PNa) of only approximately 5. Slo3 channels have only been found in mammalian sperm. Here we show that Slo3 channels expressed in Xenopus oocytes are also stimulated by elevated cAMP levels through PKA dependent phosphorylation. Capacitation, a maturational process required by mammalian sperm to enable them to fertilize eggs, involves intracellular alkalinization and an increase in cAMP. Our mouse sperm patch clamp recordings have revealed a K+ current that is time and voltage dependent, is activated by intracellular alkalinization, has a PK/PNa≥ 5, is weakly blocked by TEA and is very sensitive to Ba2+. This current is also stimulated by cAMP. All of these properties match those displayed by heterologously expressed Slo3 channels, suggesting that the native current we observe in sperm is indeed carried by Slo3 channels.
Keywords: Slo3 channels, sperm, capacitation, potassium currents, cAMP, pH regulation, Slo channels
INTRODUCTION
Fertilization encompasses a dialogue between sperm and egg which requires the regulation of ion transporters and channels. From the large repertoire of ion channels that participate in cell function only two so far appear to be uniquely expressed in sperm. The CatSpers (1-4), a new family of Ca2+ permeable channels [1] and Slo3, a member of the Slo family of K+ channels [2].
K+ channels from the Slo gene family are formed by an α subunit which has a large cytosolic C-terminal proposed to be responsible for ligand regulation. A specific cytosolic ion regulates each of the Slo members: Ca2+ for Slo1, Na+ for both Slo2.1 (Slick) and Slo2.2 (Slack), and H+ for Slo3 reviewed in [3]. Although Slo3 was cloned some years ago from a mouse testis library and heterogeneously expressed [2], it has not been definitively identified in spermatogenic cells or in sperm.
Unlike Slo1 which is conserved in Drosophila [4], C. elegans and mammals, Slo3 channels are only present in mammals where they are apparently expressed exclusively in testis [2]. Remarkably, Slo3 channels display low sequence conservation among different mammalian species; they are highly conductive and are activated by intracellular alkalization and depolarization. However, they display a low selectivity for K+ over Na+ (PK/PNa ~ 5) in comparison with Slo1 channels (PK/PNa >50) [2].
Sperm capacitation and the acrosome reaction, two key events required for sperm to fertilize the oocyte, involve changes in ionic permeability, intracellular pH (pHi) and membrane potential. Capacitation, a maturational process occurring in the female genital tract which requires increased cAMP levels, allows sperm to respond to the egg derived physiological inducer of the acrosome reaction (reviewed in [5]). The unusually low K+ selectivity and pHi dependence of Slo3 could allow this channel multiple roles depending on the extracellular environment that sperm encounter on their journey towards the egg. As the Na+/K+ concentration ratio changes throughout the female reproductive tract, Slo3 could have depolarizing or hyperpolarizing effects on sperm.
Recently Navarro et al. [6] described a pHi-sensitive K+ current in mouse sperm and suggested that it could be carried by Slo3 channels principally because of its pHi sensitivity. However, here we have greatly expanded the analysis of the pHi-sensitive K+ current present in mouse sperm and show that its properties and regulation are similar to heterologously expressed Slo3 currents in Xenopus oocytes. Thus, we show that both putative Slo3 currents observed in sperm, and Slo3 currents recorded in heterologous oocyte expression are similarly activated by cAMP, are blocked by 1 mM Ba2+ and high [TEA] (60 mM), and are activated by alkaline pHi. In addition, we confirm the presence of ENaC and KATP channels in mouse testicular sperm, as had been reported in spermatogenic cells, and consistent with immunolocalization results in mature sperm [7; 8].
METHODS
Materials
Amiloride, 8Br-cyclic AMP (8Br-cAMP), 3-isobutyl-1-methyl xantine (IBMX), choline chloride, tolbutamide, tetraethylamonium (TEA), amonium chloride and N-methyl-D-glucamine (N-Met-Gluc) were from Sigma (St Louis, MO). Barium chloride was from Merck. Borosilicate glass capillaries with an ID 0.8 and OD 1.1 and 100 mm length for patch clamping were from Kimble Chase (Gerresheimer group). Other reagents were from the highest purity commercially available.
Mouse sperm preparations
Testicles were excised from 3-4 month old CD1 mice after cervical dislocation and suspended on ice-cold dissociation solution containing (in mM): 130 NaCl, 3 KCl, 10 CaCl2, 2 MgCl2, 1 NaHCO3, 0.5 NaH2PO4, 5 Hepes and 10 glucose (pH 7.4). The tunica albuginea was removed and the seminiferous tubules separated using dissecting tweezers. Tubules were dispersed into individual cells and/or synplasts and testicular sperm by mechanical dispersion using Pasteur pipettes. The cells were stored at 4°C until assayed. Subsequently ~300 μl aliquots of cell suspension were dispensed into a recording chamber (1 ml total volume) and subjected to electrophysiological recording.
Expression of Slo3 channels in Xenopus oocytes
Oocytes were harvested from adult female Xenopus laevis as described in [9]. Defolliculated oocytes were injected with 75 ng of cRNA from Slo3 using a Drummond Scientific nanoinjector (Broomall, PA). Injected oocytes were incubated at 18°C in ND96 medium (in mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES pH=7.5 with NaOH. Oocytes were electrophysiologically analyzed 3-5 days after injection.
Whole cell currents from Xenopus oocytes and testicular mouse sperm
Whole cell currents from mock control and injected oocytes were recorded using the two microelectrode voltage clamp technique. Whole-cell ionic currents from testicular sperm were recorded at 22 °C with 4-6 MΩ micropipettes by patch-clamping the sperm cytoplasmic droplet [10]. Currents recorded with an Axopatch 200A amplifier, filtered at 2-5 kHz (4-pole Bessel filter), were digitized at 5-10 kHz using a PC equipped with a DigiData 1200 (Axon). Pulse protocols, data capture and analysis were performed with pCLAMP software (Axon, CNS Molecular Devices, Palo Alto CA) and Origin 6 (Microcal Software, Northampton MA). Cav3 currents were evoked by 300 ms depolarizing steps ranging from -80 mV to 40 mV with 10 mV increments after 1 s hyperpolarization to -100 mV from a holding potential of -70 mV. For Slo3 type currents the holding potential was -40 mV with pulses from -80 mV to 60 mV lasting 300 ms. Capacitative currents were compensated electronically. No leak current compensation was used in the Slo3 type current recordings.
Solutions for sperm Slo3-type current recordings
The external solution contained (in mM): 118 Na-MetSO4, 8 NaCl, 2.5 CaCl2, 2 KSO4, 1 MgCl2, 10 HEPES, 3.3 glucose and pH adjusted to 7.4 with NaOH and osmolarity to 290 mOsmo. The osmolarity of all solutions was adjusted with dextrose. The internal solution contained (in mM): 122 K-MetSO4, 8 KCl, 20 KF, 2.5 CaCl2, 1 MgCl2, 5 EGTA, 10 HEPES; pH was adjusted to 8 with KOH and osmolarity to 270 mOsm.
Solutions for current recordings in Xenopus oocytes
Whole cell currents where recorded in ND96 solution (in mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES pH=7.5 with NaOH. To increase pHi, 20 mM of NaCl were replaced by 20 mM of NH4Cl.
RESULTS
Properties of Slo3 channels heterologously expressed in Xenopus
As reported earlier Slo3 channels expressed in Xenopus oocytes produce whole cell currents that are stimulated by pHi increases induced by adding NH4Cl externally (Fig. 1A). As it was described before [2], unlike Slo1 channels, Slo3 channels are not very sensitive to TEA+, and we show here that high concentrations (60 mM) of TEA+ are required to achieve significant reversible blockade (Fig. 1B). Other members of the Slo channel family, like Slo2 are also not very sensitive to TEA+ but these channels, unlike Slo3, are activated by intracellular Na+ and Cl-. On the other hand, we show for the first time that these Slo3 currents are reversibly blocked by low concentrations of Ba2+ (1 mM) (Fig. 1C).
Figure 1.

A. mSlo3 current amplitude increases with intracelullar alkalinization. Current traces recorded from oocytes injected with mSlo3 cRNA. Whole cell currents were obtained from a holding potential (Vh) of -90 mV in 10 mV steps from -80 to +80 mV. Left panel, control currents and right panel in the presence of 20 mM NH4Cl. B. mSlo3 currents are inhibited by high concentrations of TEA (60 mM). Whole cell currents in control conditions (upper traces), in the presence of TEACl (60 mM) (middle traces) and during drug wash out (lower traces). Right: Corresponding I-V curves. Note that the blockade by TEACl is almost fully reversible. Currents traces were obtained from a Vh = -70 mV with depolarizing voltage steps from -90 to +70 mV in 10 mV steps. C. mSlo3 currents are inhibited by 1 mM Ba2+. Whole cell currents and their corresponding I-V relationships in oocytes expressing mSlo3 channels showing mM Ba2+ sensitivity. Control current traces (top), plus 1 mM BaCl (middle) and wash out (lower traces). Right: I-V currents in the different recording conditions. The pulse protocol used was as in fig 1A. Current blockade was partially reversible. D. PKA activation increases mSlo3 current amplitude. Upper left: mSlo3 currents from channels expressed in Xenopus oocytes. Currents obtained from a Vh = -80 mV applying 10 mV steps from -110 to +90 mV, during perfusion of the bath with saline containing (ND96) alone (control) or with the addition of IBMX (1 mM) and forskolin (100 μM). Upper right: I-V plots in control conditions vs perfusion with IBMX +forskolin. Bottom left: Control mSlo3 currents. Bottom middle: during perfusion with IBMX and forskolin after egg incubation with 10 μM H-89, a specific PKA inhibitor. Currents were obtained using the same protocol as in A. Bottom right: I-V plots obtained after incubation with H-89. Note that the effect of PKA is reduced after incubation with H-89.
We also newly found that heterologously expressed mSlo3 currents are modulated by cAMP. Increasing intracellular cAMP levels by treating oocytes simultaneously with a phosphodiesterase inhibitor (IBMX) [11] and forskolin [12], a general agonist of membrane adenylate cyclases, stimulated mSlo3 currents (Fig. 1D). This cAMP regulation appears to involve phosphorylation as it is inhibited by incubation of the eggs for 2hs with 10 μM of H89, an inhibitor of cAMP dependent kinases (PKA) (Fig. 1D).
Voltage clamping testicular mouse sperm
Recently a new technique was developed by Kirichock et al, [10], that allows recording whole cell currents from epidydimal sperm. It consists of patch clamping the sperm cytoplasmic droplet, a remnant of the precursor germ cell cytoplasm located along the flagellum of mature sperm. The cytoplasmic droplet membrane is loosely attached to the stiff structural elements of the sperm flagellum, allowing reproducible whole cell recordings. In this configuration the pipette has electrical and diffusion access to all sperm compartments [10].
Testicular mouse sperm, which are less mature than epidydimal sperm, have a larger cytoplasmic droplet extending from the head-flagella connection to the flagellar principal piece [13]. This morphological feature should facilitate a good voltage clamp. To exploit this feature we compared the properties of voltage dependent (Cav) currents from testicular sperm (Supplementary Fig. 1) with those from spermatogenic cells [14; 15]. Mouse testicular sperm displayed mibefradil sensitive currents with the typical crisscrossing pattern of T-type (Cav3) currents and their IV relation [16]. Mibefradil (10 μM), a specific Cav3 channel blocker, inhibited > 90 % of the inward component of the sperm ion currents (dashed line Supp. Fig. 1C). Nifedipine, a dihidropyridine known to inhibit Cav3 channels in spermatogenic cells [14; 15], reduced the inward current by 70 % (dotted line Suppl. Fig. 1C). The voltage dependence of activation and inactivation of these Cav channels is shown in Suppl. Fig. 1D. The similarity between the voltage dependent characteristics of the currents recorded in mouse spermatogenic cells and in testicular sperm indicates that a reasonable voltage-clamp is achieved in the testicular sperm (Supp. Fig. 1E).
K+ currents sensitive to both pHi and cAMP are present in mouse sperm, possibly Slo3 channels?
Slo3 channels were reported to exclusively express in mouse spermatogenic cells and sperm but were not functionally characterized in these cells [2]. Recently Navarro et al. [6] reported K+ currents that are pHi dependent and strongly influence the membrane potential of mouse epididymal sperm. They suggested these sperm K+ currents could be due to Slo3 channels, though they did not fully match the known Slo3 characteristics.
We corroborated the expression of the corresponding protein in mouse sperm (Supplementary Fig. 2). To explore if Slo3 channels are functional in testicular sperm, we recorded whole cell currents sealing in their large cytoplasmic droplet. Fig. 2 shows the total currents recorded in these cells under normal ionic conditions with the exception that most Cl- was substituted by MeSO4- from both external and internal solutions to basically eliminate this anion’s contribution to the currents (see Methods and legend). Repetitive 300 ms voltage steps from -80 mV to +60 mV, from a holding potential of -40 mV, revealed rapidly activating and very mildly inactivating currents (control, upper A and B traces). Since we have recorded KATP and ENaC type currents in mouse spermatogenic cells [7; 8], we used saturating concentrations of their corresponding inhibitors (tolbutamide 500 μM, and amiloride 2 μM respectively) to eliminate these currents. The lower traces in Figs. 2 A and B show that indeed, these antagonists decreased the currents. Under physiological ionic conditions KATP channels would mainly conduct outward K+ currents at positive potentials and ENaCs would conduct Na+ inward currents at negative potentials. The IV curves shown in Figs. 2C and D document that amiloride inhibited the total current at more negative potentials (40 % at -80 mV) while tolbutamide inhibited at positive potentials (~20 % at +60 mV). As these results (summarized Fig. 3F) are consistent with the presence of KATP and ENaC channels in testicular sperm, the following experiments were performed in the presence of tolbutamide and amiloride to eliminate these currents.
Figure 2. KATP and ENaCs antagonists diminish testicular whole sperm currents.
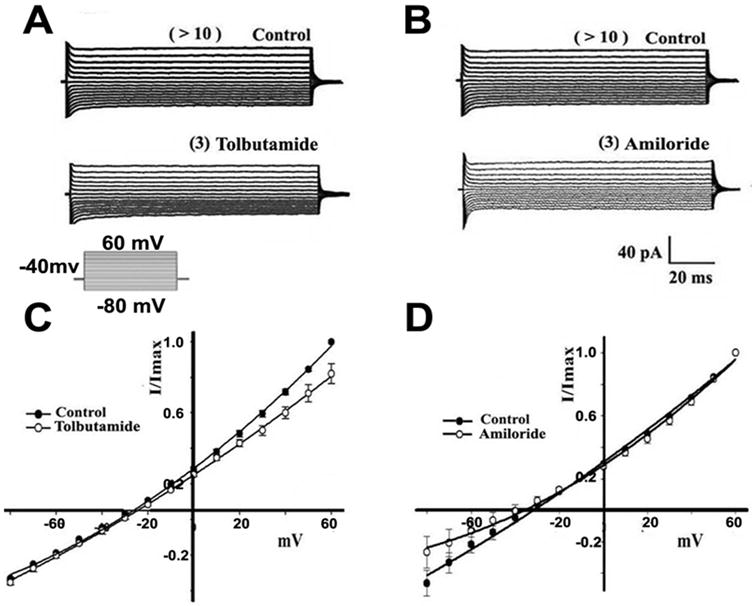
Rapidly activating and very mildly inactivating average currents from control sperm were revealed using voltage steps (300 ms) from -80 mV to +60 mV, from a Vh = -40 mV. A. Tolbutamide (500 μM), a KATP antagonist, inhibited 15 % of the current at positive potentials. B. Amiloride (2 μM), an EnaC blocker, decreased the control current by ~ 13 % at negative voltages. C. and D. show I-V relationships from experiments in A and B.
Figure 3. Intracellular alkalinization and 8Br-cAMP increase whole sperm currents.

A. Average control currents recorded at 60 mV (control) and their activation by elevating pHi with 30 mM NH4Cl (top) or elevating [cAMP]i with 400 μM 8Br-cAMP (bottom). B. 8Br-cAMP had no effect on sperm currents when added at pHi 7.0. C. Average currents obtained as in Fig. 3 stimulated with 8Br-cAMP plus NH4Cl at pHi 8.0 (top) were partially blocked by 60 mM TEA (bottom). D. The TEA sensitive currents obtained by subtracting the 8Br-cAMP plus NH4Cl activated currents from those obtained in the presence of TEA (60 mM). E I-V relationships from average 8Br-cAMP plus NH4Cl stimulated currents (●), those obtained after treatment with 60 mM TEA (▼) and their difference (●)-(▼) current when are present. F. Summary of all agents used to dissect the Slo3 type currents.
Considering that heterologously expressed Slo3 channels are stimulated by high pHi and cAMP we determined if this was the case for the testicular currents remaining after eliminating KATP and ENaC channels. Fig. 3A illustrates the current stimulation caused by either increasing pHi after adding 30 mM NH4 (top) (~30%) or by adding 400 μM cAMP permeable analog, 8Br-cAMP (bottom) (~100%). The current reported by Navarro et al. [6] was not sensitive to cAMP, however Slo3 heterologously expressed is stimulated when elevating cAMP by adding IBMX and forskolin (Fig. 1D). This discrepancy could be due to a pHi dependence for Slo3 phosphorylation, as they used a pHi of 7.0 vs 7.4 used here. As shown in the IV curve displayed in Fig. 3B, the 8Br-cAMP stimulation does not occur when pHi is lowered from 7.4 (A) to 7.0.
To further characterize the currents possibly ascribed to Slo3, recordings were performed stimulating with both NH4Cl and 8Br-cAMP. Fig. 3C shows currents displaying a slow activating component at positive potentials. This later component is insensitive to low TEA+ concentrations (not shown), but as with heterologously expressed Slo3, is blocked by 60 mM TEA+. Part D of this figure illustrates the currents that are blocked by 60 mM TEA+ whose kinetics resemble those of heterologously expressed Slo3. The IV curves of the currents in the presence of NH4Cl+8Br-cAMP, their blockade by 60 mM TEA+ and the difference is shown in part E. A summary of these experiments is presented in the last panel of this figure (F).
As shown in Fig. 1B, heterologously expressed Slo3 is strongly blocked by 1 mM Ba2+. Fig. 4 shows testicular sperm currents stimulated by NH4Cl+8Br-cAMP are sensitive to this same Ba2+ concentration (A-D). The characteristics of their IV curve are similar to those of Slo3 (compare Fig. 1 and 4E). Panel F of this figure compares the K+ dependence of Erev of the testicular sperm currents before and after 8Br-cAMP stimulation with a theoretical curve obtained using the selectivity characteristics of heterologously expressed Slo3. Stimulation with 8Br-cAMP displaces the Erev vs external K+ curve towards that of Slo3, as expected if this would be the main channel stimulated. As anticipated, the match is not perfect since other cationic channels such as TRPs are also present in sperm [5].
Figure 4. 8Br-cAMP and NH4Cl stimulated current is affected by barium.

A-B Control and stimulated current. C. Current after adding 1 mM of Ba2+. The change in the activation kinetic of the current should be the Slo3 contribution to whole current. D-E The Ba2+ blocked currents and their IV relationships are showed. This component is the 8Br-cAMP and NH4Cl stimulated current minus Ba2+ remnant current. F. Comparative graph showing the theoretical Erev change for a channel with a K+/Na+ selectivity = 3, also showing the Erev change from control and 8Br-cAMP activate current when the [K+]o was change
DISCUSSION
In this paper we are able to correlate the key functional properties of heterologously expressed mSlo3 currents (pHi dependence, blockade by TEA and Ba2+, and activation by cAMP) with those of a native K+ current recorded in testicular sperm. First we show that mouse sperm posses a delayed rectifier K+ current that is activated by both voltage and intracellular alkalinization. Secondly we demonstrate that these currents lack high sensitivity to inhibition by TEA, requiring up to 60 mM TEA to achieve significant block. In contrast, both the heterologously expressed Slo3 channels and the native currents are inhibited by low Ba2+ concentrations (1 mM). Unfortunately no specific blockers are available for Slo3 channels that can be tested on both heterologously expressed and putatively native Slo3 channels. Thirdly, we also provide evidence that, like heterologously expressed Slo3 channels, the K+ current recorded in sperm is activated by cAMP probably through PKA dependent phosphorylation. Our findings reveal a component of the whole cell current in mouse sperm that displays several hallmarks of the heterologously expressed Slo3 currents, suggesting the likelihood that these are, indeed, native Slo3 currents.
In a recent paper Navarro et al. [6], found a pHi sensitive current which they named IKsper located in the principal piece of mouse sperm. Although similar to the current we observe, there are several reported differences. In contrast to our observations in both heterologous expression and in native sperm, the currents reported by Navarro et al. [6] do not show any time dependent activation. This could be explained by the fact that most of their recordings were obtained in symmetrical K+ concentrations where the instantaneous leak currents seem to be bigger. In contrast, our voltage clamp experiments undertaken under more physiological conditions show a time and voltage-dependent current in mature sperm with striking similarity to Slo3 currents expressed in our heterologous system. Our results also show a more positive Erev (-~33 mV) than the one reported for IKsper at physiological K+ concentrations (-72.5 mV). Thus, our findings are consistent with the prior report that Slo3 channels expressed in Xenopus oocytes are far less selective for K+ over Na+ than most other potassium channels [2]. In the report by Navarro et al, [6], the fact that IKsper was recorded in the absence of KATP blockers could account for some of these differences.
cAMP concentrations and PKA activity change during sperm capacitation and may have an important modulatory effect in ion channel function [17]. In contrast to our findings, Navarro et al. [6] do not find that IKsper is sensitive to modulation by cAMP. As we mention earlier, this discrepancy could be due to a pHi dependence for the phosphorylation of Slo3; Navarro et al. [6] used a pHi of 7.0 in those experiments. As shown in the IV curve in Fig. 3B, 8Br-cAMP stimulation does not occur when pHi is lowered from 7.4 to 7.0.
In summary we found a K+ current in mouse sperm that shows time and voltage dependence, is activated by intracellular alkalinization, has a PK+/PNa+ ≥ 5, is weakly blocked by TEA, and is very sensitive to block by Ba2+. In addition, the current is modulated by cAMP. All of these properties match the characteristics of mSlo3 currents expressed in Xenopus oocytes making it a prime candidate to be the native Slo3 current. More conclusive evidence could be obtained with the knock-out slo3 gene, showing that this current is absent in the transgenic animals. As this pHi activated K+ current could play a key role in setting the resting potential in sperm during capacitation where intracellular alkalinization and cAMP increases occur, establishing its molecular identity is a key issue in sperm physiology. Finally, further studies of this current and its role during fertilization could lead to new drugs useful to control and regulate fertility.
Supplementary Material
Acknowledgments
Supported by: NIH Grants R01 HD038082-07A1 (to PEV and AD), 1R21HD056444-01A1 (to CS) and R01 GM067154-01A1 (to LS) and CONACyT-Mexico, 49113 (to AD), DGAPA/UNAM IN211809 (to AD) and IN204109 (to CT), and the Wellcome Trust (to AD). We thank Elizabeth Mata, Marcela Ramírez and Shirley Ainsworth for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A. 2007;104:1219–23. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem. 1998;273:3509–16. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- 3.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–31. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–5. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- 5.Darszon A, Acevedo JJ, Galindo BE, Hernandez-Gonzalez EO, Nishigaki T, Trevino CL, Wood C, Beltran C. Sperm channel diversity and functional multiplicity. Reproduction. 2006;131:977–88. doi: 10.1530/rep.1.00612. [DOI] [PubMed] [Google Scholar]
- 6.Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci U S A. 2007;104:7688–92. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acevedo JJ, Mendoza-Lujambio I, de la Vega-Beltran JL, Trevino CL, Felix R, Darszon A. KATP channels in mouse spermatogenic cells and sperm, and their role in capacitation. Dev Biol. 2006;289:395–405. doi: 10.1016/j.ydbio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Gonzalez EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, Lopez-Gonzalez I, Demarco I, Wertheimer E, Darszon A, Visconti PE. Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem. 2006;281:5623–33. doi: 10.1074/jbc.M508172200. [DOI] [PubMed] [Google Scholar]
- 9.Yuan A, Dourado M, Butler A, Walton N, Wei A, Salkoff L. SLO-2, a K+ channel with an unusual Cl- dependence. Nat Neurosci. 2000;3:771–9. doi: 10.1038/77670. [DOI] [PubMed] [Google Scholar]
- 10.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–40. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 11.Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56:453–9. [PubMed] [Google Scholar]
- 12.Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol. 2003;23:305–14. doi: 10.1023/A:1023684503883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper TG, Yeung CH. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech. 2003;61:28–38. doi: 10.1002/jemt.10314. [DOI] [PubMed] [Google Scholar]
- 14.Santi CM, Darszon A, Hernandez-Cruz A. A dihydropyridine-sensitive T-type Ca2+ current is the main Ca2+ current carrier in mouse primary spermatocytes. Am J Physiol. 1996;271:C1583–93. doi: 10.1152/ajpcell.1996.271.5.C1583. [DOI] [PubMed] [Google Scholar]
- 15.Arnoult C, Villaz M, Florman HM. Pharmacological properties of the T-type Ca2+ current of mouse spermatogenic cells. Mol Pharmacol. 1998;1104(11):53. [PubMed] [Google Scholar]
- 16.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–61. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 17.Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol. 2002;133(50):53. doi: 10.1016/s0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Gonzalez EO, Trevino CL, Castellano LE, de la Vega-Beltran JL, Ocampo AY, Wertheimer E, Visconti PE, Darszon A. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J Biol Chem. 2007;282:24397–406. doi: 10.1074/jbc.M701603200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


