Abstract
Disrupted microtubule dynamics in neuronal synapses has been suggested as an underlying cause for several devastating neurological diseases, including Hereditary Spastic Paraplegia (HSP) and Fragile X Syndrome (FXS). However, previous studies have been restricted to indirect assays of synaptic microtubules, i.e. immunocytochemistry of microtubule-associated proteins and post-translationally modified tubulins characteristic of microtubules with different stabilities. Very little is known about synaptic microtubule dynamics in vivo, or how microtubule dynamics may be disrupted in disease states. In this study, we develop methods to analyze microtubule dynamics directly in living synaptic boutons in situ. We use fluorescence recovery after photobleaching (FRAP) of transgenic green fluorescent protein (GFP) tagged tubulin at the well-characterized Drosophila neuromuscular junction (NMJ) synapse. FRAP measurements of tubulin-GFP demonstrate biphasic recovery kinetics. Treatment with taxol to stabilize microtubules and promote microtubule assembly reduces both recovery phases. Treatment with vinblastine to disassemble microtubules increases the fast recovery phase and decreases the slow recovery phase. These data indicate that the fast recovery phase is generated by rapid diffusion of tubulin subunits and the slow phase is generated by the relatively slow turnover of microtubules. This study demonstrates that tubulin-GFP fluorescence recovery after photobleaching can be used to assay microtubule dynamics directly in living synapses.
INTRODUCTION
Microtubules provide the primary cytoskeletal scaffold in all eukaryotic cells, with critical roles in mitosis, intracellular transport, cell motility, morphology maintenance and other vital cellular activities. Most microtubule functions are dependent on their inherent dynamic instability, in which the soluble pool of tubulin subunits is under constant interchange with the polymers. Microtubules are abundant throughout neurons and are known to be critical for the generation and maintenance of neuronal processes, including dendrites and axons, transport along these exceptionally elongated, polarized processes, and the development and maintenance of synaptic transmission in both pre- and postsynaptic compartments (Barth et al., 1997; Terada and Hirokawa, 2000; Kneussel 2005; Marques 2005). Not surprisingly, therefore, disrupted neuronal microtubule dynamics has been suggested to be the underlying dysfunction of several human neurological diseases, including Fragile X Syndrome (FXS) and Hereditary Spastic Paraplegia (HSP) (Zhang et al., 2001; Huot et al., 2001; McDermott et al., 2003; Lu et al., 2004; Sherwood et al., 2004; Trotta et al., 2004; Zhang and Broadie, 2005; Orso et al., 2005). To study these disease states, we have developed FXS and HSP models in the Drosophila genetic system (Zhang et al., 2001; Trotta et al., 2004; Zhang and Broadie, 2005). However, our studies, like all others (Huot et al., 2001; McDermott et al., 2003; Lu et al., 2004; Sherwood et al., 2004; Orso et al., 2005), have been hampered by the available, very indirect assays of microtubule states in neuronal synapses. Published studies are restricted to inferences based on immunocytochemistry of tubulin isoforms and microtubule associated proteins in static images of fixed tissue. This technical approach has severely limited our understanding of microtubule dynamics in vivo and prevented insight into how microtubule dynamics may be altered in neurological diseases.
Fluorescent speckle microscopy (FSM) and fluorescence recovery after photobleaching (FRAP) are the two main techniques that have been widely used to study in vivo protein dynamics directly (Danuser and Waterman-Storer, 2006; Reits and Neefjes, 2001). In FSM, a relatively small amount of fluorescent subunit is expressed in a large pool of endogenous unlabeled subunits (Waterman-Storer and Salmon, 1997). A non-uniform “fluorescent speckle” pattern is created by random assembly of fluorescent subunits into the endogenous network. This small fraction of labeled subunits significantly reduces out-of-focus fluorescence and greatly improves visibility of fluorescently labeled structures and their dynamics in living cells (Waterman-Storer and Salmon, 1999). In FRAP, fluorescent molecules are permanently bleached in a small region of a living cell and then the fluorescence recovery in this region is monitored. This technique has been used widely to determine the diffusion constant of biomolecules in membranes or various intracellular compartments (Chen et al., 2006; Carrero et al., 2003). Recent FRAP experiments have investigated direct protein activities, such as interaction with binding partners, with the fluorescence recovery curve analyzed to differentiate fast and slow components that correspond to diffusion and binding events, or different binding states (Tardy et al., 1995; Kimura et al., 2002; Dundr et al., 2002; Carrero et al., 2003; Carrero et al., 2004a; Carrero et al., 2004b; Sprague et al., 2004; McDonald et al., 2006). One recent study combined the FRAP technique with model convolution methods and measured a gradient in microtubule dynamics in yeast spindles at ~65-nm spatial intervals (Pearson et al., 2006). In this study, it was demonstrated that tubulin turnover was greatest near kinetochores and lowest near spindle poles. These advances in FRAP technology suggest that it should be a superior technical approach to investigate microtubule dynamics in living neurons, and specifically to assay microtubule stability locally within the neuronal synapse.
In the current study, we have developed FRAP techniques to analyze microtubule dynamics at the Drosophila neuromuscular junction (NMJ) synapse, which has been used in a large number of previous studies to examine the role of microtubules in synaptic mechanisms (Hummel et al., 2000; Roos et al., 2000; Ruiz-Canada et al., 2004; Ashley et al., 2005). We used FRAP measurements of transgenic tubulin-GFP within living presynaptic boutons in situ to directly assay tubulin diffusion and dynamic turnover within microtubule polymers. Microtubule stability was altered with pharmacological agents known to promote either polymerization or depolymerization, to quantify fast and slow components of tubulin cycling within synapses. These analyses show that both components of the recovery profile can be separably quantified, in particular to study the turnover of the polymerized tubulin pool within microtubules with the movement of diffusing tubulin subunits. Thus, the slow phase of the tubulin-GFP fluorescence recovery after photobleaching can be used to study microtubule dynamics directly. This new technique should prove invaluable in the analyses of the synaptic microtubule cytoskeleton in numerous genetic conditions and disease models.
METHODS AND MATERIALS
Drosophila Stocks
Drosophila melanogaster stocks were cultured on standard medium under standard temperature and light conditions (Ashburner, 1989). The UAS-αtubulin-GFP stock, in which GFP is fused in frame to the N-terminal of α-tubulin, was obtained from the Bloomington Drosophila stock center (Grieder et al., 2000). The neuron-specific elav-Gal4 line was used to drive tubulin-GFP expression specifically in post-mitotic neurons (Brand and Perrimon, 1993).
Fluorescence Microscopy
All Images were collected using a Zeiss LSM 510 META confocal microscope equipped a 30mW Argon laser. Imaging of living tissue was conducted using a 63X/0.95NA Achroplan water immersion objective and fixed tissue imaging was conducted using a 100X/1.4NA Plan Apo oil immersion objective. All experiments were done at the wandering third instar larval NMJ synapse of muscle 13 in segments A3-A6. Time-lapse experiments in living synapses were imaged in standard saline containing 128 mM NaCl, 2 mM KCl, 4 mM MgCl2, 5 mM Trehalose, 65 mM Sucrose, 5 mM HEPES and 1.8mM CaCl2. 1-naphthylacetyl spermine trihydrochloride (100 mM; Sigma, St. Louis, MO), which blocks postsynaptic glutamate receptor activation and muscle contraction (Poskanzer et al., 2006), was used to enable accurate imaging at all time points.
Immunohistochemisty
Animals were dissected dorsally in standard saline on Sylgard-coated coverslips using cyanoacrylate glue (Liquid Suture, Allentown, PA) and then fixed with a mixture of 4% formaldehyde and 0.5% glutaraldehyde at room temperature for 45 minutes. Preparations were then washed in phosphate-buffed saline (PBS) containing 0.5% bovine serum albumin. Subsequently the dissections were incubated with primary antibodies overnight at 4°C and secondary antibodies for two hours at room temperature. α-tubulin antibody was obtained from Sigma (St. Louis, MO) and applied at a dilution of 1:500. Texas-red conjugated horseradish peroxidase (HRP) antibody was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA) and was applied at a dilution of 1:500.
Detergent Extraction
To determine the distribution of tubulin-GFP between subunit and polymer pools, animals were dissected dorsally in calcium-free standard saline. Dissected preparations were permeabilized with 0.5% saponin in a microtubule-stabilizing buffer composed of 10 μM paclitaxel (Sigma, St. Louis, MO), 0.1% DMSO, 60 mM NaPIPES, 25 mM NaHEPES, 10 mM NaEGTA, 2 mM MgCl2 (pH 6.9) and protease inhibitor cocktail (Sigma, St. Louis, MO) (Wang and Brown, 2002). The fluorescence intensity was quantified in selected NMJ boutons before and after permeabilization, as described previously (Brown et al., 1992).
Fluorescence Recovery After Photobleaching (FRAP)
A selected type 1b muscle 13 NMJ bouton was photobleached in a 1 μm-square (9×9 pixel region with the setting as described below) using the Zeiss confocal microscope by scanning the region for ten iterations at 100% laser power of the 488 nm line. The laser power was then reduced to 2% for monitoring the recovery of the fluorescence signal over time. 256×256 pixel frames were captured at zoom 5 with a pinhole setting of 3.01 Airy units (thickness of section=3.8um) using 8-bit detector mode. The scan speed was set 12 (1.6μsec/pixel) for fast acquisition to minimize acquisition photobleaching. To maximize the duration of observation without significant photobleaching, the time interval between scans was increased from 500ms to 4s after the first 30 seconds of recovery. Fluorescence recovery curves were generated from between 15 and 20 individual boutons recorded from three separate sets of experiments.
Drug Treatments
For taxol experiments, dissected animals were treated with 50 μM paclitaxel (Sigma, St. Louis, MO) in standard saline for half an hour prior to imaging. For vinblastine experiments, larvae were treated with 1 μM vinblastine sulfate (Sigma, St. Louis, MO) in standard saline for an hour prior to imaging. When vinblastine was used at higher concentration (≥ 5 μM), tubulin-GFP aggregates form in NMJ boutons. This was expected based on the known molecular mechanism of vinblastine (Panda et al., 1996; Gigant et al., 2005).
Image Analysis
Images from the LSM 510 META were directly exported as 16-bit TIFF files. All images in a FRAP series were aligned using MetaMorph (Universal Imaging Corporation, Dawnington, PA) to correct for any movement of bleached regions during imaging. Image brightness not only originates from fluorescence of the tubulin-GFP, but also from detector readout noise, autofluorescence of the imaging medium and coverslip, and reflected light. For correction during image analyses, the average background value (measurement outside of the NMJ bouton area) was subtracted from the average pixel value in the bleaching region throughout the FRAP series using MetaMorph. To correct the acquisition photobleaching in the FRAP series, the fluorescence measurement at each time-point was divided by a function representing the acquisition photobleaching: f(n) = exp(-n/N) (n = image number). N represents the rate of photobleaching, which was determined by measuring the total fluorescence intensity of fluorescence loss in unbleached boutons from corresponding control experiments. In these control experiments, unbleached boutons were imaged exactly the same way as those in the FRAP experiments except that there was no photobleaching in the region of interest. Laser intensity fluctuations during the imaging are apparently negligible and the fluorescence intensity was not corrected for this factor. For quantified analysis and comparison, fluorescence intensity in the photobleached region in the time course was normalized to the one immediately before photobleaching.
RESULTS
To visualize tubulin in living synaptic boutons at the Drosophila neuromuscular junction, we expressed tubulin-GFP in the presynaptic motor nerve terminal using the UAS-GAL4 transgenic system (Brand and Perrimon, 1993). Figure 1 shows a representative NMJ on muscle 13 (segment A4) expressing tubulin-GFP; the arrow points to a typical type Ib synaptic bouton, the focus for the current study. Tubulin-GFP shows a strong fluorescence pattern in all synaptic boutons, with fluorescence observed throughout the bouton (Fig. 1B, top), but often concentrated around the plasma membrane cortex (Fig. 1B, bottom). This latter pattern resembles the microtubule “cortex loop” observed with anti-tubulin immunocytochemistry in fixed tissue (Fig. 1C), which has been documented in numerous recent staining studies of microtubules and microtubule associated proteins (Roos et al., 2000; Packard et al., 2002; Pennetta et al., 2002; Franco et al., 2004; Ruiz-Canada et al., 2004; Sherwood et al., 2004). It is not clear why the tubulin-GFP fluorescence pattern in living synaptic boutons shows a broader expression profile than staining in the fixed tissue, but this likely represents the loss of tubulin subunits during immunocytochemistry processing (see detergent extraction, below). In addition, note that the “cortex loop” pattern of microtubule cytoskeleton architecture is observed in only a small subset of boutons in immunocytochemistry studies. A fairly uniform tubulin expression pattern (as in Fig. 1B, top) is observed in ~60% of boutons, a diffuse cortex pattern (as in Fig. 1B, bottom) in ~30% of boutons and a strong microtubule loop pattern (as in Fig. 1C) in >10% of boutons. This distribution is apparent in published studies of microtubules and microtubule associate protein futsch/MAP1b (Sherwood et al., 2004).
Figure 1. Comparison between transgenic tubulin-GFP expression and native tubulin immunocytochemistry in presynaptic boutons of the Drosophila NMJ.
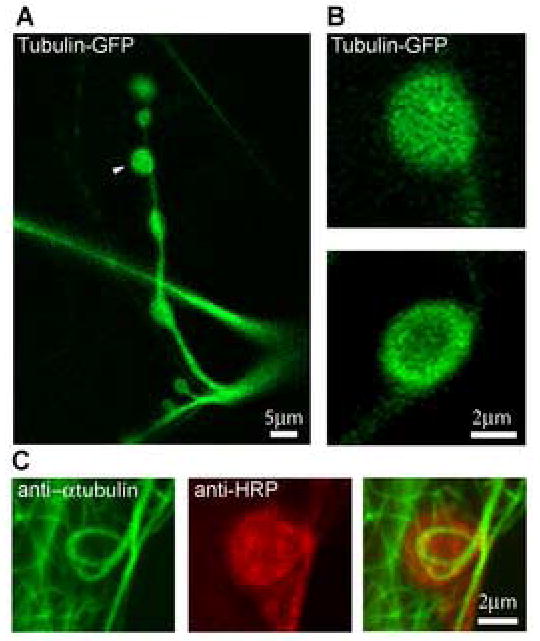
(A) Representative NMJ on muscle 13 (segment A4) expressing UAS-tubulin-GFP driven by elav-GAL4. The terminal is shown at low magnification and the arrow points to a typical type Ib synaptic bouton. Scale bar = 5 μm. (B) Representative tubulin-GFP fluorescence patterns in individual presynaptic boutons at high magnification. Scale bar = 2 μm. (C) Double staining of the fixed NMJ with antibodies specific for α-tubulin (green) and HRP, a presynaptic membrane marker (red). Scale bar = 2 μm.
To estimate the efficiency of tubulin-GFP incorporation into synaptic microtubules, we permeabilized in situ NMJ boutons with 0.5% saponin under conditions that preserve the integrity of microtubule networks (Wang and Brown, 2002). Individual synaptic boutons were imaged immediately before and after permeabilization (Fig. 2A), and the tubulin-GFP intensity quantified. The difference of fluorescence intensity at these two times is explained by the diffusion loss of soluble tubulin-GFP during permeabilization (Brown et al., 1992). The remaining fluorescence should be due to tubulin-GFP stably incorporated in synaptic microtubules. Using this approach, 69% of tubulin-GFP was estimated to be in the microtubule polymer pool and 31% in the diffusible subunit pool (n=50, s.d.=10%; Fig. 2B). This conclusion is consistent with earlier studies of the native tubulin distribution in the squid giant axon, which demonstrated that 22%-36% of the tubulin is in the form of free subunits (Morris and Lasek, 1984), and studies on rat sympathetic neurons, which similarly demonstrated that 70-75% of tubulin is incorporated in microtubule polymers (Black et al., 1986; Wang and Brown, 2002). These data indicate that tubulin-GFP incorporates into endogenous microtubules efficiently, similar to native tubulin, and therefore that tubulin-GFP could be an effective probe for tubulin dynamics in vivo.
Figure 2. Characterization of tubulin-GFP distribution between soluble subunit and polymer pools in the presynaptic bouton under different drug conditions.
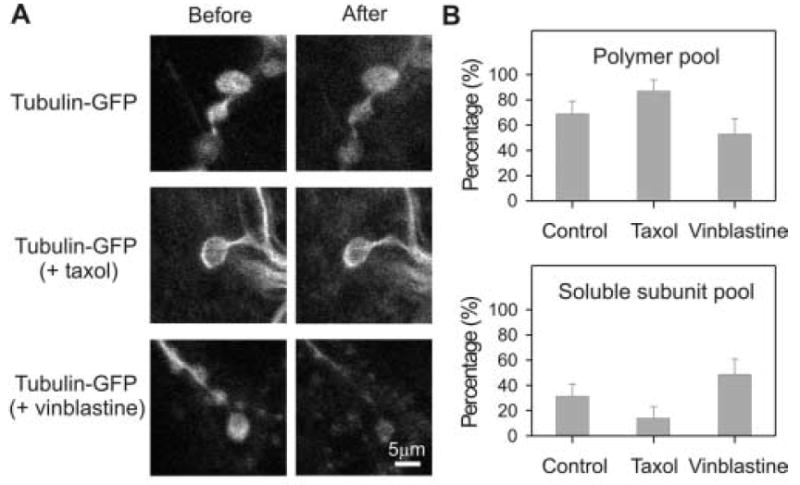
In situ NMJ synapses were permeabilized with 0.5% saponin in the presence of microtubule stabilizing buffer. The fluorescence immediately before permeabilization represents total tubulin-GFP (before) and fluorescence immediately after permeabilization represents tubulin-GFP assembled into microtubule polymers (after). A) Representative images of synaptic boutons without treatment (top), with 50 μM taxol for 30 minutes before permeabilization (middle) and with 1 μM vinblastine for 60 minutes before permeabilization (bottom). All images scaled identically for comparison. Scale bar = 5 μm. B) Histograms showing the quantification of the proportion of tubulin proteins in polymer and soluble subunit pools under the three different conditions.
If our interpretation of the tubulin-GFP extraction data is correct, treatment with drugs that either stabilize or disassemble microtubules would be predicted to alter the ability to extract tubulin-GFP from synaptic boutons. To test this hypothesis, we compared tubulin-GFP extraction from NMJ synaptic boutons in the control condition to terminals treated with either taxol or vinblastine. Taxol stabilizes microtubules at low concentration and promotes microtubule assembly at high concentration (Schiff et al., 1979; Xiao et al., 2006). Vinblastine inhibits microtubule dynamics at low concentration and disassembles microtubules and induces tubulin self-association into spiral aggregates at high concentration (Yu and Baas 1995; Panda et al., 1996; Gigant et al., 2005). We observed qualitative effects supporting these specific drug activities at the Drosophila NMJ (data not shown). The distributions of tubulin-GFP between subunit and polymer pools were then quantified by extracting boutons in microtubule stabilizing buffer, comparing the control condition with both drug treatments (Fig. 2A). After treatment with 50μM taxol for 30 minutes, 87% of tubulin-GFP fluorescence remained in the synaptic bouton after detergent extraction (n=33, s.d.=9%; Fig. 2B). This finding supports the conclusion that taxol stabilizes existing microtubules and also promotes microtubule assembly. After treatment with 1μM vinblastine for 60 minutes, 53% of tubulin-GFP distributes in the polymer pool (n=27, s.d.=12%; Fig. 2B). These data suggest that microtubules have been destabilized within the synaptic bouton. Thus, tubulin-GFP effectively incorporates into synaptic microtubules and the stability of these microtubules can be quantitatively altered with pharmacological treatments.
Previous reports of neuronal axons indicate highly stable microtubules (Schulze and Kirschner, 1986; Kirschner and Mitchison, 1986). For example, the average half-life of axonal microtubules is approximately 2.2 hours in immature rat sympathetic neurons (Li and Black, 1996). It has been suggested that microtubules in the specialized presynaptic bouton compartment are much more dynamic, but this has never been assayed. To study tubulin dynamics directly in living synapses, we performed FRAP analysis of tubulin-GFP in individual boutons of the NMJ synapse. Four factors might contribute to tubulin-GFP fluorescence recovery after photobleaching in the bouton: local protein synthesis, active transport of tubulin proteins, passive diffusion of tubulin subunits and dynamic exchange between free tubulin subunits and microtubules.
There is little evidence of translation in presynaptic boutons. Nevertheless, the translation of β-tubulin has been demonstrated specifically in the distal axons of rat sympathetic neurons (Eng et al., 1999). However, the amount of the protein synthesized in the axon is very small (≪1%) compared with protein synthesized in the cell body and then transported into the axon (Eng et al., 1999). In addition, FRAP studies were performed here for around ten minutes, which is a short period for significant local protein synthesis. Therefore, we reason that local protein synthesis provides no or negligible contribution to fluorescence recovery. The form in which tubulin proteins are actively transported along the axon has been debated for more than three decades. The “subunit model” proposes that tubulin subunits are transported and locally assembled into polymers, while the “polymer model” proposes that microtubules are assembled in the cell body and then transported along the axon (Baas and Brown, 1997; Hirokawa et al., 1997). Recently, fluorescently labeled tubulin proteins have been observed to move predominantly in the form of filamentous structures (Wang and Brown, 2002), which provides strong support for polymer transport model. It has been proposed that polymers move at fast rate along the axon but the frequency of this movement is very low (Wang and Brown, 2002; Brown, 2003). Based on polymer transport model, if active transport of tubulin polymers is happening into the photobleached region of the bouton during FRAP study, one would expect a sudden change in fluorescence intensity, which would be reflected in a big jump in the fluorescence recovery curve. We observed no such acute changes during the sum total of all our FRAP analyses. This suggests that active tubulin polymer transport is not contributing to the fluorescence recovery in the bouton.
Finally, close inspection of the fluorescence recovery curve shows that FRAP of tubulin-GFP in the bouton has two phases (Fig. 3). The curve can be best fit with two exponential rises with average half time of 3 seconds for the first phase and 1682 seconds for the second phase (Table I). These kinetics indicate that the first phase will be ~90% complete in less than ten seconds and the second phase will be ~90% complete after 1.5 hours (Fig. 3). This is consistent with the fast FRAP phase being due to the rapid diffusion of diffusible tubulin subunits within the bouton and the slow FRAP phase being due to the interchange between tubulin subunits and polymers. Thus, only these two factors detectably contribute to tubulin-GFP FRAP in synaptic boutons.
Figure 3. FRAP of tubulin-GFP under conditions that alter microtubule dynamics.
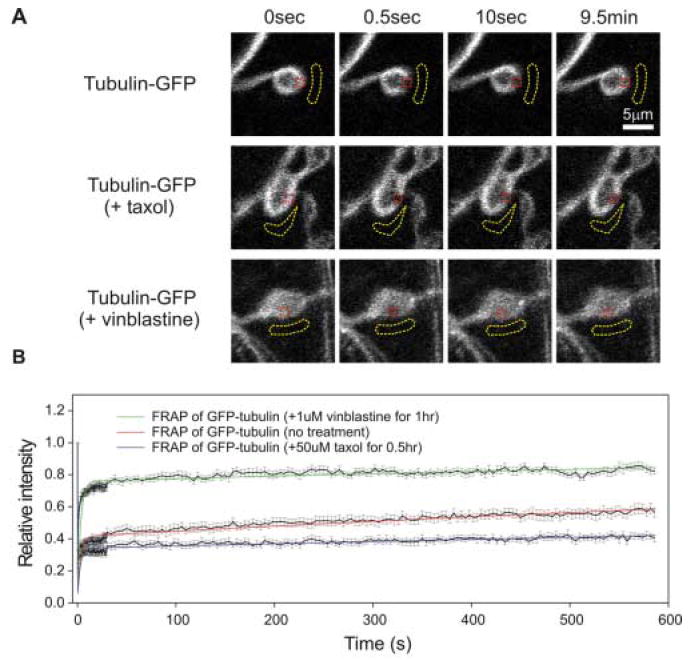
(A) Representative time-course images of tubulin-GFP in a control bouton (top), treated with taxol (middle) and treated with vinblastine (bottom). Red dotted lines indicate the bouton regions that were photobleached and subsequently monitored for fluorescence recovery. The yellow dotted lines indicate the background regions that were used in the fluorescence analysis (see details in Methods and Materials). Each panel in the FRAP series shows before (0 sec), immediately after photobleaching (0.5 sec), ten seconds and nine and half minutes after photobleaching. All images in each condition were scaled identically for comparison. Scale bar = 5 μm. (B) FRAP recover curves of tubulin-GFP under the three conditions. The black solid lines connect the mean value and the bars are s.e.m at each time point. The colored lines are the best two exponential fit to all time points. The equation that was used for the fitting is A = 1-A1*exp(-time/alpha)-(1-A1)*exp(-time/beta), in which A is the normalized fluorescence intensity (dependent variable), time is the actual time (independent variable), and A1, alpha and beta are the three parameters to be determined. Table I list all the fitting parameters that were used for FRAP under the different conditions.
Table I.
Fitting parameters for FRAP under different conditions.
| A = 1–A1*exp(–time/alpha) – (1–A1)*exp(–time/beta) | |||
|---|---|---|---|
| A1 | alpha | beta | |
| Control | 0.41±0.01 | 3.0±0.2 | 1682±72 |
| Taxol | 0.34±0.01 | 2.7±0.2 | 4530±331 |
| Vinblastine | 0.76±0.01 | 3.2±0.2 | 1331±174 |
If our interpretation of the FRAP time course is correct, treatment with drugs that either stabilize or disassemble microtubules would be predicted to alter the slow phase of tubulin-GFP FRAP. In addition, treatments with drugs that disassemble microtubules should also increase the fast phase while those that promote microtubule assembly should decrease it. To test this hypothesis, we compared FRAP of tubulin-GFP in NMJ synaptic boutons that had been treated with either taxol or vinblastine to the control condition (Fig. 3). To determine whether the fast recovering phase is due to diffusion of tubulin subunits and slow recovering phase is rate limited by the turnover of microtubules, synaptic boutons were pretreated with either with 50μM taxol (30 mins) or 1μM vinblatine (60 mins) and the FRAP recovery kinetics were analyzed as above. Figure 3 and table II shows the comparison of tubulin-GFP FRAP under these three different conditions. Figure 3A shows representative images during a 10 minute interval of observation following local fluorescence bleaching in individual synaptic boutons in situ. Figure 3B shows the fluorescence recovery curves generated from 15-20 individual synaptic boutons recorded in each of three independent sets of experiments. Table II list the normalized fluorescence data at different times for each condition.
Table II.
Comparison of fluorescence recovery after photobleaching over time under different conditions.
| Right before photobleaching | Immediately after photobleaching | 10 seconds after photobleaching | 9.5 minutes after photobleaching | |
|---|---|---|---|---|
| Control | 100% | 23% ± 2% | 38% ± 2%. | 56% ± 2% |
| Taxol | 100% | 20% ± 2% | 32% ± 2% | 40% ± 2% |
| Vinblastine | 100% | 35% ± 2% | 70% ± 2% | 80% ± 2% |
Without drug treatment, the tubulin signal recovered gradually after being bleached (Fig. 3A, top row). Immediately after photobleaching, the tubulin-GFP fluorescence intensity in the photobleached region dropped to 23% ± 2% of the intensity immediately before photobleaching. During the first ten seconds after photobleaching, fluorescence recovered to 38% ± 2%. Finally, in the next ~10 minute observation period, fluorescence slowly recovered to 56% ± 2% of the initial intensity (Fig. 3B, red graph; Table II). With the taxol treatment, there is significantly less fluorescence recovery throughout the 10 minute monitoring period after photobleaching and no significant fluorescence recovery during the second FRAP phase (Fig. 3A, middle row). In the taxol condition, the fluorescence recovered from 20% ± 2% to 32% ± 2% of original intensity during the first ten seconds immediately after photobleaching (Fig. 3B, blue graph; Table II). By the end of the observation period, fluorescence recovery was 40% ± 2% of the original value (Fig. 3B; Table II). Thus, taxol treatment causes loss of both recovery phases, particularly the second phase of tubulin subunit incorporation into the microtubule polymer. In sharp contrast, with the vinblastine treatment fluorescence recovery was very rapid, with the qualitatively detectable bleached domain no longer discernable after 10 seconds post-bleaching (Fig. 3A, bottom row). In the vinblastine condition, quantification shows rapid tubulin-GFP fluorescence recovery during the first 10-seconds after photobleaching, from 35% ± 2% (first measurement) to 70% ± 2% (10 second measurement) of the initial intensity (Fig. 3B, green graph; Table II). During the next ~10 minute observation period, there was only around 10% additional fluorescence recovery (Fig. 3B; Table II). Thus, vinblastine treatment results in an increase of the fast recovery phase and a loss of the slow recovering phase. These data clearly support the proposed hypothesis showing that FRAP can be used to directly assay microtubule dynamics within living synaptic boutons in situ.
DISCUSSION
We know very little about microtubule cytoskeleton dynamics within neurons and especially locally within the highly specialized synaptic compartment. Previous studies have made strong inferences about altered microtubule dynamics in different genetic mutants and disease models based wholly on very indirect assays of the local microtubule state within synapses (Zhang et al., 2001; Huot et al., 2001; McDermott et al., 2003; Lu et al., 2004; Sherwood et al., 2004; Trotta et al., 2004; Zhang and Broadie, 2005; Orso et al., 2005). The goal of this study was to develop techniques to directly assay microtubule dynamics, locally at synapses, in living neurons in situ. Our focus has been on the heavily used Drosophila NMJ synapse, given its central role as a genetic model of synaptic mechanisms and neurological diseases. In this study, we demonstrate that FRAP of tubulin-GFP in the presynaptic bouton has fast and slow components that probably correspond to the diffusion of tubulin subunits and the dynamic exchange between microtubules and subunits, respectively. These two phases were confirmed by comparing FRAP analyses under known drug conditions that alter microtubule stability. Thus, FRAP analysis of tubulin-GFP can be used to study microtubule dynamics in the presynaptic bouton directly.
Mathematical models have been successful in the quantitative analysis of FRAP curves involving protein binding reactions (Carrero et al., 2004a; Carrero et al., 2004b; McDonald et al., 2006). These models share one important assumption: the fluorescent proteins have spatially homogeneous distributions. In contrast, the neuronal microtubule cytoskeleton is clearly non-homogeneous (Fig. 1). In Drosophila presynaptic boutons, tubulin is non-uniformly distributed and microtubules (and their associate proteins) can form a distinctive cytoskeletal architecture (cortex “loops”) in the axonal varicosity (Roos et al., 2000; Packard et al., 2002; Pennetta et al., 2002; Franco et al., 2004; Ruiz-Canada et al., 2004; Sherwood et al., 2004). Thus, tubulin pools are not equal across the bouton, which makes mathematical modeling very difficult. This limitation affects our ability to derive exact information about the rates of microtubule assembly and disassembly under different conditions. We are currently working to develop a mathematical model of the quantitative FRAP analysis for tubulin-GFP in synaptic boutons, and other subcellular regions of the neuron, to provide more detailed information about local microtubule dynamics in living neurons.
We have previously proposed altered synaptic microtubule dynamics as a causative defect in Drosophila genetic models of Fragile X Syndrome (FXS) and Hereditary Spastic Paraplegia (HSP) (Zhang et al., 2001; Trotta et al., 2004; Zhang and Broadie 2005): FXS is caused by loss of function of the FMR1 gene, which encodes a negative translational regulator of Futsch/microtubule associated protein 1B (MAP1B), and 40% of HSP cases are linked to mutations of the spastin gene, which encodes a microtubule severing protein. Indirect evidence suggests that synaptic microtubules are hyper-stabilized in both mutants, but several mechanisms could be responsible. The FRAP method developed here can be used to differentiate between these mechanisms. First, there may be no change in the relative distribution of tubulin subunit and polymer pools, but the turn-over rate may be slower. In FRAP analysis of these mutants, we would expect to observe no change in the first diffusion phase of the recovery curve but a decrease in the second turnover phase. Second, there may be relatively more polymeric tubulin in the mutant conditions, but no difference in the microtubule turn-over rate. In FRAP analysis of these mutants, we would expect a decreased diffusion phase and no change in the turn-over phase. Third, there may be relatively more polymeric tubulin and microtubules turn-over slower in the mutant condition. In FRAP analysis of these mutants, we would expect a loss in both phases or the recovery curve. Thus, the technical approaches established in the present study will be instrumental in elucidating the molecular dysfunction in the microtubule cytoskeleton implicated in these disease states. Our current efforts are focused on expanding FRAP analyses to these genetic disease models.
Acknowledgments
Special thanks to Dr. Shane Hutson and Xiaoyan Ma for advice on fitting FRAP analysis data. This work was supported by a grant from the Spastic Paraplegia Foundation and grant HD40654 from the National Institute for Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner M. Drosophila: A laboratory manual. Cold Spring Harbor Press. 1989 [Google Scholar]
- Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005;25:5943–55. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Brown A. Slow axonal transport: the polymer transport model. Trends in Cell Bio. 1997;7:380–84. doi: 10.1016/S0962-8924(97)01148-3. [DOI] [PubMed] [Google Scholar]
- Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–90. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- Black MM, Keyser P, Sobel E. Interval between the synthesis and assembly of cytoskeletal proteins in cultured neurons. J Neurosci. 1986;6:1004–12. doi: 10.1523/JNEUROSCI.06-04-01004.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brown A, Slaughter T, Black MM. Newly assembled microtubules are concentrated in the proximal and distal regions of growing axons. J Cell Biol. 1992;119:867–82. doi: 10.1083/jcb.119.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol. 2003;160:817–21. doi: 10.1083/jcb.200212017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrero G, McDonald D, Crawford E, de Vries G, Hendzel MJ. Using FRAP and mathematical modeling to determine the in vivo kinetics of nuclear proteins. Methods. 2003;29:14–28. doi: 10.1016/s1046-2023(02)00288-8. [DOI] [PubMed] [Google Scholar]
- Carrero G, Crawford E, Hendzel MJ, de Vries G. Characterizing fluorescence recovery curves for nuclear proteins undergoing binding events. Bull Math Biol. 2004a;66:1515–45. doi: 10.1016/j.bulm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Carrero G, Crawford E, Th’ng J, de Vries G, Hendzel MJ. Quantification of protein-protein and protein-DNA interactions in vivo, using fluorescence recovery after photobleaching. Methods Enzymol. 2004b;375:415–42. doi: 10.1016/s0076-6879(03)75026-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lagerholm BC, Yang B, Jacobson K. Methods to measure the lateral diffusion of membrane lipids and proteins. Methods. 2006;39:147–53. doi: 10.1016/j.ymeth.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Danuser G, Waterman-Storer CM. Quantitative fluorescent speckle microscopy of cytoskeleton dynamics. Annu Rev Biophys Biomol Struct. 2006;35:361–87. doi: 10.1146/annurev.biophys.35.040405.102114. [DOI] [PubMed] [Google Scholar]
- Dundr M, Hoffmann-Rohrer U, Hu Q, Grummt I, Rothblum LI, Phair RD, Misteli T. A kinetic framework for a mammalian RNA polymerase in vivo. Science. 2002;298:1623–6. doi: 10.1126/science.1076164. [DOI] [PubMed] [Google Scholar]
- Eng H, Lund K, Campenot RB. Synthesis of beta-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures. J Neurosci. 1999;19:1–9. doi: 10.1523/JNEUROSCI.19-01-00001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco B, Bogdanik L, Bobinnec Y, Debec A, Bockaert J, Parmentier ML, Grau Y. Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila. J Neurosci. 2004;24:6573–7. doi: 10.1523/JNEUROSCI.1580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigant B, Wang C, Ravelli RB, Roussi F, Steinmetz MO, Curmi PA, Sobel A, Knossow M. Structural basis for the regulation of tubulin by vinblastine. Nature. 2005;435:519–22. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]
- Grieder NC, de Cuevas M, Spradling AC. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development. 2000;127:4253–64. doi: 10.1242/dev.127.19.4253. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Terada S, Funakoshi T, Takeda S. Slow axonal transport: the subunit transport model. Trends in Cell Bio. 1997;7:384–8. doi: 10.1016/S0962-8924(97)01133-1. [DOI] [PubMed] [Google Scholar]
- Hummel T, Krukkert K, Roos J, Davis G, Klambt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26:357–70. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- Huot ME, Mazroui R, Leclerc P, Khandjian EW. Developmental expression of the fragile X-related 1 proteins in mouse testis: association with microtubule elements. Hum Mol Genet. 2001;10:2803–11. doi: 10.1093/hmg/10.24.2803. [DOI] [PubMed] [Google Scholar]
- Kneussel M. Postsynaptic scaffold proteins at non-synaptic sites. The role of postsynaptic scaffold proteins in motor-protein-receptor complexes. EMBO Rep. 2005;6:22–7. doi: 10.1038/sj.embor.7400319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Sugaya K, Cook PR. The transcription cycle of RNA polymerase II in living cells. J Cell Biol. 2002;159:777–82. doi: 10.1083/jcb.200206019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–42. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Black MM. Microtubule assembly and turnover in growing axons. J Neurosci. 1996;16:531–44. doi: 10.1523/JNEUROSCI.16-02-00531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O’donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–6. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques G. Morphogens and synaptogenesis in Drosophila. J Neurobiol. 2005;64:417–34. doi: 10.1002/neu.20165. [DOI] [PubMed] [Google Scholar]
- McDermott CJ, Grierson AJ, Wood JD, Bingley M, Wharton SB, Bushby KM, Shaw PJ. Hereditary spastic paraparesis: disrupted intracellular transport associated with spastin mutation. Ann Neurol. 2003;54:748–59. doi: 10.1002/ana.10757. [DOI] [PubMed] [Google Scholar]
- McDonald D, Carrero G, Andrin C, de Vries G, Hendzel MJ. Nucleoplasmic beta-actin exists in a dynamic equilibrium between low-mobility polymeric species and rapidly diffusing populations. J Cell Biol. 2006;172:541–52. doi: 10.1083/jcb.200507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JR, Lasek RJ. Monomer-polymer equilibria in the axon: direct measurement of tubulin and actin as polymer and monomer in axoplasm. J Cell Biol. 1984;98:2064–76. doi: 10.1083/jcb.98.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso G, Martinuzzi A, Rossetto MG, Sartori E, Feany M, Daga A. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J Clin Invest. 2005;115:3026–34. doi: 10.1172/JCI24694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–30. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D, Jordan MA, Chu KC, Wilson L. Differential effects of vinblastine on polymerization and dynamics at opposite microtubule ends. J Biol Chem. 1996;271:29807–12. doi: 10.1074/jbc.271.47.29807. [DOI] [PubMed] [Google Scholar]
- Pearson CG, Gardner MK, Paliulis LV, Salmon ED, Odde DJ, Bloom K. Measuring nanometer scale gradients in spindle microtubule dynamics using model convolution microscopy. Mol Biol Cell. 2006;17:4069–79. doi: 10.1091/mbc.E06-04-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennetta G, Hiesinger PR, Fabian-Fine R, Meinertzhagen IA, Bellen HJ. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron. 2002;35:291–306. doi: 10.1016/s0896-6273(02)00769-9. [DOI] [PubMed] [Google Scholar]
- Poskanzer KE, Fetter RD, Davis GW. Discrete residues in the c(2)b domain of synaptotagmin I independently specify endocytic rate and synaptic vesicle size. Neuron. 2006;50:49–62. doi: 10.1016/j.neuron.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Reits EA, Neefjes JJ. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat Cell Biol. 2001;3:E145–E147. doi: 10.1038/35078615. [DOI] [PubMed] [Google Scholar]
- Roos J, Hummel T, Ng N, Klambt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–82. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Ruiz-Canada C, Ashley J, Moeckel-Cole S, Drier E, Yin J, Budnik V. New synaptic bouton formation is disrupted by misregulation of microtubule stability in aPKC mutants. Neuron. 2004;42:567–80. doi: 10.1016/s0896-6273(04)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schulze E, Kirschner M. Microtubule dynamics in interphase cells. J Cell Biol. 1986;102:1020–31. doi: 10.1083/jcb.102.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2004;2:e429. doi: 10.1371/journal.pbio.0020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague BL, Pego RL, Stavreva DA, McNally JG. Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys J. 2004;86:3473–95. doi: 10.1529/biophysj.103.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardy Y, McGrath JL, Hartwig JH, Dewey CF. Interpreting photoactivated fluorescence microscopy measurements of steady-state actin dynamics. Biophys J. 1995;69:1674–82. doi: 10.1016/S0006-3495(95)80085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S, Hirokawa N. Moving on to the cargo problem of microtubule-dependent motors in neurons. Curr Opin Neurobiol. 2000;10:566–73. doi: 10.1016/s0959-4388(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Trotta N, Orso G, Rossetto MG, Daga A, Broadie K. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr Biol. 2004;14:1135–47. doi: 10.1016/j.cub.2004.06.058. [DOI] [PubMed] [Google Scholar]
- Wang L, Brown A. Rapid movement of microtubules in axons. Curr Biol. 2002;12:1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. How microtubules get fluorescent speckles. Biophys J. 1998;75:2059–69. doi: 10.1016/S0006-3495(98)77648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Fluorescent speckle microscopy of microtubules: how low can you go? FASEB J. 1999;(Suppl 2):S225–S230. doi: 10.1096/fasebj.13.9002.s225. [DOI] [PubMed] [Google Scholar]
- Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA. Insights into the mechanism of microtubule stabilization by Taxol. Proc Natl Acad Sci U S A. 2006;103:10166–73. doi: 10.1073/pnas.0603704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Baas PW. The growth of the axon is not dependent upon net microtubule asssembly at its distal tip. J Neurosci. 1995;15:6827–33. doi: 10.1523/JNEUROSCI.15-10-06827.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Broadie K. Fathoming fragile X in fruit flies. Trends Genet. 2005;21:37–45. doi: 10.1016/j.tig.2004.11.003. [DOI] [PubMed] [Google Scholar]


