Abstract
Tissue kallikrein acts on the substrate, low molecular weight kininogen, to liberate bradykinin in a variety of tissues. Bradykinin stimulation of B2 kinin receptors has been shown to initiate signaling in trabecular meshwork cells and increase conventional outflow facility. The objective of the present study was to determine if the components for kinin generation and response are expressed in tissues of the human anterior segment. Expression of mRNA encoding tissue kallikrein (KK), low molecular weight kininogen, and B1 and B2 kinin receptors was examined in human ciliary smooth muscle (CM), trabecular meshwork (TM) and non-pigmented epithelial (NPE) cells using RT-PCR. Expression of component proteins was also investigated by immunohistochemical analyses performed on parasagittal sections of human anterior segment and TM cells, and by immunoblot. KK mRNA was detected in NPE cells and in cultured CM and TM cells from multiple donors. Each cell type also expressed mRNAs encoding both B1 and B2 kinin receptors. Immunohistochemical analysis of KK protein in sectioned anterior segment supported the RT-PCR results. Intense KK immunofluorescence was observed in the epithelial lining of the ciliary body and KK protein was also detected in the ciliary muscle. KK protein expression within the TM was demonstrated by analyses of TM tissue and cultured TM cells. The presence of KK along with B1 and B2 receptor proteins was confirmed by immunoblots of cell lysates prepared from CM, NPE or TM cells. Finally, both CM and TM cells were found to possess enzymes for bradykinin inactivation. These data demonstrate that key components for kinin generation and regulation are localized within the human anterior segment. Further, multiple cell types express both B1 and B2 kinin receptors and are targets for kinin action. The results support the possibility that kinins produced within the eye may contribute to the regulation of aqueous outflow.
Keywords: anterior segment, tissue kallikrein, kinin receptor, kininogen
1. Introduction
The nanopeptide, bradykinin, has been shown to activate multiple signaling pathways in cells of the ocular trabecular meshwork. Stimulation of the B2 subtype of kinin receptors by bradykinin promotes inositol triphosphate formation, mobilizes intracellular free calcium, and produces shape change in both bovine and human trabecular meshwork cells (Sharif and Xu,1996;Llobet et al., 1999; Webb et al., 2003). Bradykinin activation of B2 receptors also serves to initiate PGE2 synthesis in trabecular cells (Polansky et al., 1989), and to synergistically enhance PGE2 stimulation of cyclic 3′, 5′-adenosine monophosphate (cAMP) formation (Webb et al., 2003). In addition, we recently demonstrated that bradykinin stimulation of B2 receptors promotes matrix metalloproteinase (MMP) secretion from trabecular meshwork cells, and associated this secretory response with an increase in conventional outflow facility (Webb et al., 2006). Taken together, these studies provide evidence that kinins can modulate aqueous dynamics by acting on trabecular meshwork cells or, possibly, other components within the anterior segment.
Endogenous kinins are produced locally within tissues by tissue kallikrein, a serine protease (Bhoola et al., 1992; Blais et al., 2000). Tissue kallikrein acts on low molecular weight kininogen, a circulating plasma protein produced primarily in the liver, to yield lysylbradykinin (kallidin) or bradykinin. Lysylbradykinin is subsequently converted to bradykinin by aminopeptidase activity. Once kininogen is activated to kallidin and bradykinin, these peptides act in an autocrine or paracrine fashion to produce similar biological effects by stimulation of B1 or B2 kinin receptors in a variety of cell types. Most kinin actions are mediated by B2 receptors. B1 receptors are generally expressed in low concentrations, but may be induced in certain pathophysiological states and preferentially respond to the desArg9 metabolites of kallidin or bradykinin (Bhoola et al., 1992; Blais et al., 2000). In the present study, experiments were performed to determine if the endogenous kallikrein/kinin system is expressed in tissues of the human anterior segment. The results demonstrate that key components for kinin generation and response are expressed by multiple cell types of the ciliary body and trabecular meshwork, and support the concept that kinins produced within the anterior segment may participate in the regulation of anterior segment function.
2. Materials and methods
2.1. Reagents
Dulbecco’s modified Eagle’s medium (DMEM) and amphotericin B were purchased from GIBCO-BRL (Grand Island, NY, USA). Medium 199 was obtained from Mediatech, Inc. (Manassas, VA, USA) and fetal bovine serum from HyClone Labs (Logan, UT, USA). Penicillin, streptomycin, and trypsin were acquired from Sigma (St. Louis, MO, USA).
2.2. Cell Culture
Human tissue was handled in accordance with the Declaration of Helsinki. Experiments were performed with SV40- transformed, human non-pigmented epithelial cells and primary cultures of ciliary muscle or trabecular meshwork cells isolated from normal human eyes. Human eyes were obtained from the National Disease Research Interchange (Philadelphia, PA, USA) and Life-Point Ocular Tissue Division (Storm Eye Institute; MUSC, Charleston, SC, USA), and human non-pigmented epithelial cells were kindly provided by Dr. M. Coca-Prados (New Haven, CT, USA). Primary cultures of trabecular meshwork and ciliary muscle cells were isolated as performed previously (Webb et al., 2003; Husain, et al., 2005). Once established in culture, cells were maintained on polypropylene cell culture plates in Dulbecco’s modified Eagle’s medium (DMEM) (trabecular meshwork and non-pigmented epithelia cells) or Medium 199 (ciliary muscle cells) containing 10% fetal calf serum, 100 U/mL penicillin and 0.1 mg/mL streptomycin in a humidified atmosphere of 5% CO2 in air at 37°C. The medium was routinely changed every 48 hr and cells were subcultured weekly after detachment with 0.05% trypsin in phosphate-buffered saline (PBS).
2.3. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
To determine the presence of mRNA encoding tissue kallikrein, low molecular weight kininogen and B1 and B2 receptors, qualitative RT-PCR was performed on total RNA isolated from ocular cells. Total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The poly(A) fraction was then purified using Oligotex mRNA Maxi Kit (Qiagen, Valencia, CA, USA), and cDNA synthesized using the Superscript system (Invitrogen, Carlsbad, CA, USA). Specific primers were designed for kallikrein/kinin components and PCR performed using Taq DNA Polymerase (Takara Biotechnology, Madison, WI, USA). Component expression was examined in primary cultures of human ciliary muscle and trabecular meshwork cells, and in transformed non-pigmented epithelial cells (NPE). Primers specific for actin were used to confirm the efficiency of RT-PCR for each experiment. All PCR products were verified by sequence analysis.
The primers used were: tissue kallikrein, 5′-GCCAAGCAGACGAGG ACTAC-3′ (upstream), 5′-TTTGAG GTCCACACACTGGA-3′ (downstream); low molecular weight kininogen, 5′-TGC AAACGAATTGTTCCAAA-3′ (upstream), 5′-CGCAAATCTTGGTAG GTGGT-3′(downstream); B1 kinin receptor, 5′-CAGAGTGCTGCCGACATTTA-3′(upstream), 5′-GCCCAAGAC AAACACCAGAT-3′(downstream); B2 kinin receptor, 5′ –TGGTTGTGC TGC TGCTATTC- 3′ (upstream), 5′-GCACACTCC CTGGTACACCT- 3′ (downstream); actin 5′-AAAAGCCACCCCACTTCTCT-3′ (upstream), 5′-CTCAAGTTGGGGGACAAAAA-3′ (downstream). PCR amplifications were performed in a final volume of 25 μL with 1 μl cDNA, 1.25 units of Taq polymerase, 1 mM of each primer, and 200 mM dNTP in 1 X PCR buffer. The cycling conditions were denaturation at 94 ° C for 1 min, followed by 35 amplification cycles of 94 ° C for 0.5 min, 55 ° C for 1 min, 72 ° C for 1 min and a final extension of 10 min at 72 ° C. PCR products were separated on a 1.5 % agarose gel and visualized with ethinium bromide staining.
2.4. Immunohistochemistry
Tissue expression of kallikrein protein was determined in frozen parasagittal sections of human anterior segment and in cultured trabecular meshwork cells. To prepare anterior segment sections, donor eyes were fixed for 24 hours in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) at 4°C, bisected at the ora serrata and fixation continued for an additional 24 hours. Tissue was then washed three times in PBS and cryopreserved by placing it in a 30% sucrose solution overnight. Tissue was then mounted in optimal cutting temperature medium and frozen. Parasagittal sections (10 μm) were cut and mounted on gelatin-coated slides for immunohistochemical analysis. Primary cultures of human trabecular meshwork cells were first washed and then fixed by incubation for 0.5 hours in 4 % paraformaldehyde. Prior to incubation with antibodies, cells were permeabilized by incubation in PBS containing 0.3 % Triton X-100.
Tissue kallikrein expression in sectioned anterior segment or fixed trabecular meshwork cells was visualized by sequential incubation with anti-kallikrein antibody (Calbiochem, La Jolla, CA, USA) and anti-rabbit IgG conjugated to Alexa Fluor 488. Tissue sections and cells were viewed and imaged using an Axioplan fluorescence microscope (Carl Zeiss, Thornwood, NY, USA).
2.5. Western Blot
Freshly-isolated trabecular meshwork tissue and cultured ciliary muscle, trabecular meshwork or non-pigmented epithelial cells were washed in ice-cold PBS and suspended in 250 μl of lysis buffer containing 20 mM β-glycerophosphate, 1% Triton X-100, 20 mM EGTA, 20 mM NaF, 15 mM MgCl2, 1 mM NaVO4, 0.3 % mercaptoethanol, pH 7.5, and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Total lysate was prepared by centrifugation of disrupted tissue or cells for 10 min at 6000 × g. Supernatant protein (25 – 50 μg) was then loaded onto 12% SDS-polyacrylamide gels, and proteins were separated according to molecular weight using standard SDS-polyacrylamide gel electrophoresis protocols and then transferred to a nitrocellulose membrane. Membranes were probed for component proteins by immunoblot using antibodies to tissue kallikrein (Novus Biologicals, Littleton, CO, USA), B1 kinin receptor (Santa Cruz Biotechnology, Santa Cruz, CA, USA)), or B2 kinin receptor (BD Biosciences/Transduction Laboratories, San Jose, CA, USA). Immunoreactive bands were visualized by the addition of horseradish peroxidase-conjugated secondary antibodies (New England Biolabs, Inc., Beverly, MA, USA) and enhanced chemiluminescent reagents (Amersham, Buckinghamshire, UK).
2.6. Bradykinin determination
Studies were performed to determine bradykinin degradation by cultured human trabecular meshwork or ciliary muscle cells. For these experiments, cells were cultured in serum-free DMEM containing 500 pg/ml bradykinin. At 30 minute intervals, media was removed from the cells and assayed for bradykinin content. Bradykinin was measured by radioimmunoassay using a commercial kit (Peninsula Laboratories, San Carlos, CA, USA) and performed according to the manufacturer instructions.
3. Results
3.1. Expression of kallikrein/kinin components in cells of the ciliary body and trabecular meshwork
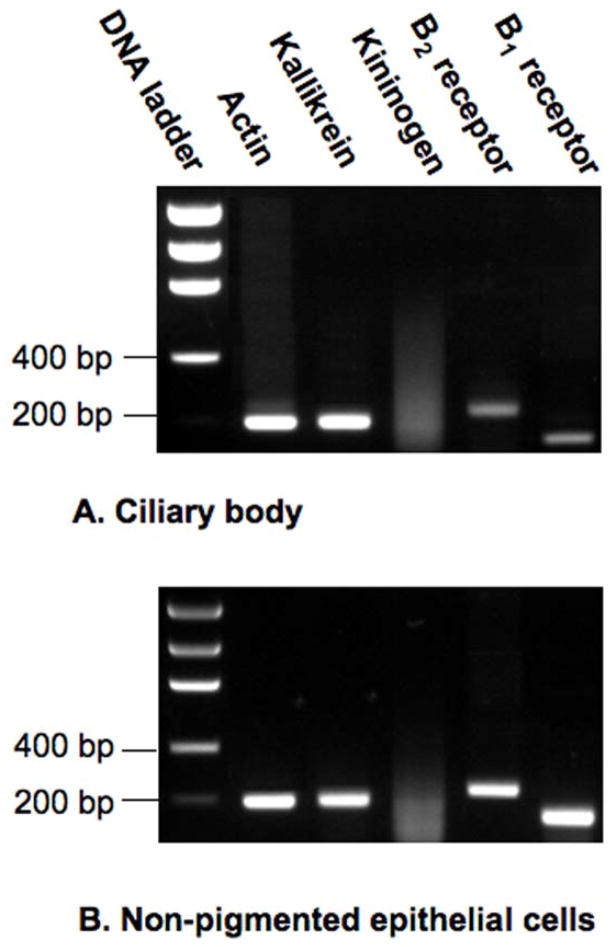
Expression of mRNA encoding the principal components of the kallikrein/kinin system was initially examined in RNA isolated from total human ciliary body as determined by qualitative RT-PCR and subsequent DNA sequence confirmation (Fig. 1A). Actin expression was monitored as a measure of method efficiency. Based on the specific primers employed for each component, anticipated product sizes of the PCR amplifications were 200 bp for tissue kallikrein, 188 bp for low molecular weight kininogen, 235 bp for the B2 kinin receptor and 157 bp for the B1 kinin receptor. RT-PCR analysis of tissue extract provided evidence in ciliary body of mRNAs encoding tissue kallikrein, and both the B2 and B1 subtypes of kinin receptor. Message encoding low molecular weight kininogen was not detected, however. As a positive control, effectiveness of the kininogen primers was verified by detection of kininogen expression in human hepatoma cells (data not shown).
Fig. 1.
Expression of kallikrein/kinin components in (A) human ciliary body and (B) cultured human non-pigmented epithelial cells. Transcripts were detected by qualitative RT-PCR performed on total RNA isolated from the ciliary body or cultured cells. Primers specific for actin were used to confirm RT-PCR efficiency in each analysis.
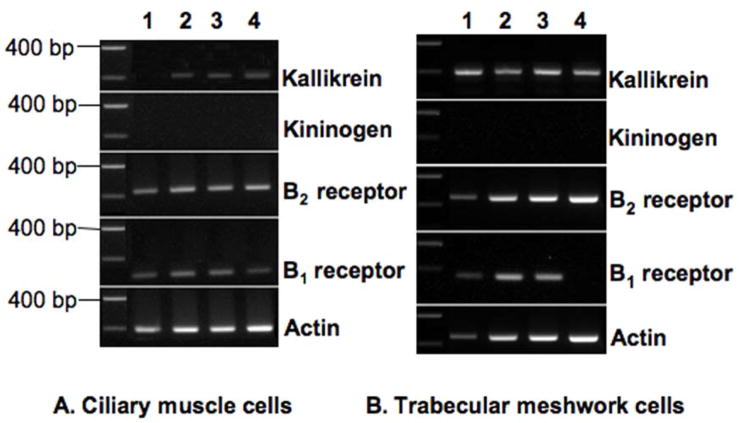
Tissue kallikrein mRNA and mRNA for each kinin receptor subtype was also observed in transformed non-pigmented epithelial cells ( Fig. 1B), while message encoding kininogen was again absent. Primary cultures of ciliary smooth muscle cells were prepared from 4 different human donors. Evaluation of these cell populations revealed mRNAs for B2 and B1 receptors in all 4 preparations, and expression of tissue kallikrein in 3 of the 4. Message encoding kininogen was not observed in cells from any of the 4 donors (Fig. 2A). Primary cultures of trabecular meshwork cells were prepared from 4 additional human donors. Examination of message for expression of kallikrein/kinin component in these cells gave results similar to those obtained with the ciliary body and its associated cell types. Expression of tissue kallikrein and the B2 receptor was observed in each cell population (Fig. 2B). Message encoding the B1 receptor was found in 3 of the 4, while kininogen expression was not detected in trabecular cells from any of the 4 different donors.
Fig. 2.
Expression of kallikrein/kinin components in cultured (A) human ciliary smooth muscle cells and (B) human trabecular meshwork cells. Transcripts were detected by qualitative RT-PCR performed on total RNA isolated from ciliary muscle and trabecular meshwork cells cultured from 2 sets of 4 different donors (lanes 1–4). Primers specific for actin were used to confirm RT-PCR efficiency in each analysis.
3.2. Expression of kallikrein protein in human anterior segment and cultured cells
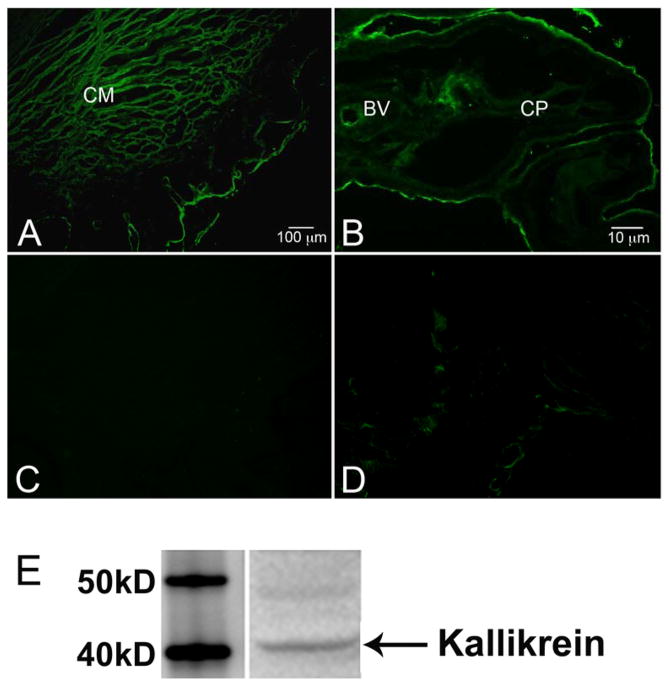
The results of the RT-PCR experiments indicate that message encoding tissue kallikrein is expressed in cells of the ciliary body and trabecular meshwork but do not establish the presence of kallikrein protein. To address this issue, fixed sections of anterior segment were probed with anti-kallikrein antibody and examined by immunohistochemistry. The distribution of kallikrein protein observed with this approach was entirely consistent with the RT-PCR data. Expression of tissue kallikrein was prominent in the longitudinal smooth muscle cells that project from ciliary body to the anterior portion of the eye and insert within close proximity of the trabecular meshwork (Fig. 3A). Kallikrein immunofluorescence was also evident in the non-pigmented epithelial layer lining the ciliary body and its processes (Fig. 3B), and in the endothelial lining of blood vessels within the ciliary body (Fig. 3B). Control sections where primary antibody was omitted showed no significant staining in any of these regions (Figs. 3C, 3D).
Fig. 3.
Immunofluorescence and immunoblot of tissue kallikrein in human anterior segment. A and B. Fixed 10 μm sections of anterior segment were probed with anti-kallikrein antibody followed by secondary antibody conjugated to Alexa Fluor 488. C and D. Adjacent control segments for A and B, respectively, that were probed with secondary antibody alone. Calibration lines are (A) 100 μm and (B) 10 μm. BV: Blood vessel; CM: ciliary muscle; CP: ciliary process. E. Western blot analysis of tissue kallikrein in lysate prepared from freshly-isolated trabecular meshwork tissue.
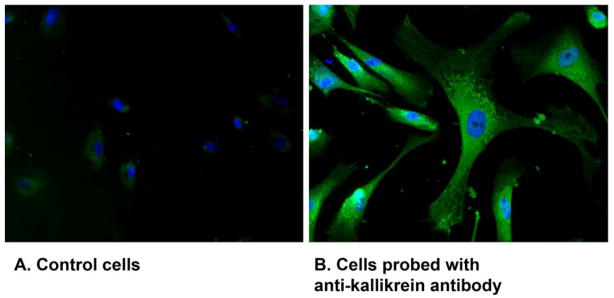
It was difficult to determine kallikrein expression within the trabecular meshwork by immunohistochemistry in sectioned anterior segment. However, presence of a 40 kD protein identified as tissue kallikrein was readily detected when lysate prepared from freshly-dissected trabecular meshwork was examined by immunoblot (Fig. 3E). To assess the source of kallikrein within the meshwork, immunohistochemical determinations were performed on primary cultures of human trabecular meshwork cells. Fixed cells probed with anti-kallikrein antibody exhibited intense immunofluorescence throughout the cell, while no fluorescence was observed in cells incubated with only secondary antibody (Fig. 4).
Fig. 4.
Immunofluorescence of tissue kallikrein in cultured human trabecular meshwork cells. A. Control cells were probed with secondary antibody alone. B. Cells were probed with anti-kallikrein antibody followed by secondary antibody conjugated to Alexa Fluor 488.
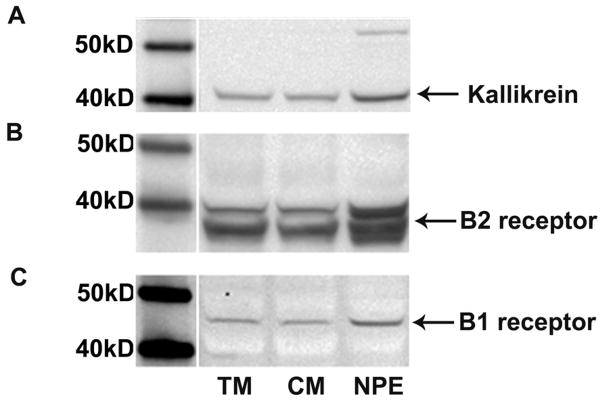
Finally, expression of tissue kallikrein as well as B1 and B2 kinin receptors in different cell types was verified by immunoblot. A 40 kD protein identified as tissue kallikrein was observed in cell lysates produced from trabecular meshwork, ciliary muscle and non-pigmented epithelial cells (Fig. 5A). In addition, a 40 kD protein and a 45 kD protein were also detected in each cell type using antibodies to kinin B2 and B1 receptors, respectively (Figs. 5B, 5C). The estimated sizes for the tissue kallikrein and receptor proteins observed in the cell lysates are in agreement with previous reports of tissue kallikrein (Finlay et al., 1999) and kinin receptors (Perosa et al., 2007) identified by western blot of preparations from human tissues.
Fig. 5.
Western blot analysis of (A) tissue kallikrein, (B) kinin B2 receptor and (C) kinin B1 receptor in cell lysates from human trabecular meshwork (TM) cells, ciliary muscle (CM) cells and non-pigmented epithelial (NPE) cells.
3.3. Bradykinin degradation by trabecular meshwork and ciliary muscle cells
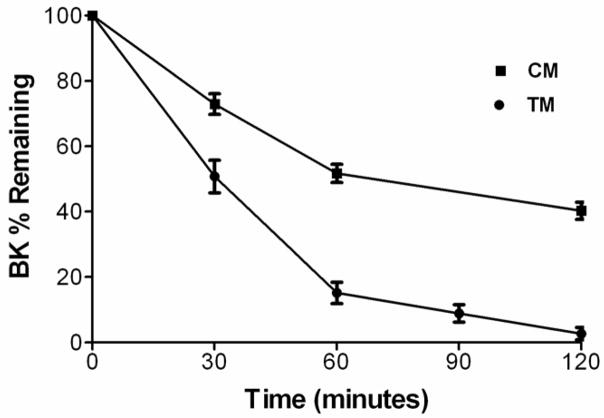
Kinins are degraded and rapidly inactivated by multiple peptidases including angiotensin converting enzyme (kininase II). The relative importance of specific enzymes in kinin inactivation varies with species and tissue site. Trabecular meshwork and ciliary muscle cells are potential targets for bradykinin actions to influence conventional outflow facility. To determine if these cells express peptidases for terminating kinin actions, exogenous bradykinin was added to cultured human trabecular meshwork or ciliary muscle cells and levels in the media were monitored as a function of time (Fig. 6). Trabecular meshwork cells rapidly degraded added peptide. After 30 minutes, 51 ± 5 % of bradykinin remained and by 90 minutes, only 9 ± 3 % of the peptide was detected. After 60 minutes of incubation in control media, bradykinin levels were reduced to 15 ± 3% of the initial value. In comparison, when cells were incubated for 60 minutes in media containing captopril (100 μM), 46 ± 2 % of the bradykinin remained intact, suggesting that angiotensin converting enzyme has a role in kinin inactivation by human trabecular meshwork cells. Bradykinin degradation was also observed in ciliary muscle cells. After 30 minutes, 73 ± 3% of peptide was present in the media and 52 ± 3 % was detected after 60 minutes of incubation. When captopril was included in the media, bradykinin degradation by ciliary muscle cells was decreased only slightly with 67 ± 7% of peptide remaining after 60 minutes. While equal numbers of trabecular meshwork and ciliary muscle cells were cultured for these studies, quantitative differences between the cells should be viewed with caution, particularly since each cell type was isolated from different sets of donors.
Fig. 6.
Degradation of bradykinin by cultured human trabecular meshwork (TM) or ciliary muscle (CM) cells. Bradykinin (500 pg/ml) was added to cells in serum-free media and bradykinin concentration was measured at 30-min intervals. Values are means ± S.E.M. from 4 experiments with cells isolated from 2 different donors.
4. Discussion
This study investigated whether primary components of the kallikrein/kinin system are expressed within the human anterior segment with the potential for modulating outflow of aqueous humor. The principal enzyme for generation of tissue kinins is the serine protease, tissue kallikrein (Bhoola et al., 1992; Blais et al., 2000). Message encoding this enzyme was detected by qualitative RT-PCR in multiple cell types within the ciliary body and anterior segment. These sites included non-pigmented epithelial cells, ciliary smooth muscle, and cells of the trabecular meshwork. Messenger RNA for both the B2 and B1 subtypes of kinin receptors was also found in non-pigmented epithelial, ciliary muscle and trabecular meshwork cells. These results are in general agreement with the study of Ma et al. (1996) that also observed mRNA encoding components of the kallikrein/kinin system, including B1 and B2 receptors, in the stroma of human ciliary body as well as in multiple layers of the retina, and in the choroid.
To date, all of the effects described for bradykinin on cell signaling in trabecular meshwork cells or outflow facility in bovine or human eyes have been attributed to stimulation of B2 receptors (Sharif and Xu, 1996;Llobet et al., 1999; Polansky et al., 1989; Webb et al., 2003; Webb et al., 2006) as determined by antagonism of these actions by the B2 selective antagonist, Hoe-140. Consequently, the finding that mRNA encoding B1 receptor was present in various ocular cell types isolated from multiple different human donors was unanticipated and clearly merits further exploration. Enzymes for inactivating kinins were also demonstrated in both ciliary muscle and trabecular meshwork cells as evidenced by degradation of bradykinin added to the culture media, and the data obtained support a significant role for angiotensin converting enzyme in kinin inactivation by trabecular cells.
Immunohistochemical analysis of the distribution of kallikrein protein within sectioned anterior segment supported the results of the RT-PCR experiments. Tissue kallikrein expression was observed in the non-pigmented epithelial cells lining the ciliary body and its processes, and was also prominent in the longitudinal smooth muscle extending from the stroma of the ciliary body to the trabecular meshwork of the anterior chamber. Kallikrein immunofluorescence was also demonstrated in cells cultured from the trabecular meshwork. The presence of kallikrein protein, as well as B1 and B2 receptors, in trabecular meshwork, ciliary muscle and non-pigmented epithelial cells was confirmed by immunoblot of cell lysates from each cell type. The overall distribution of tissue kallikrein observed within the anterior segment supports the idea that the enzyme is positioned well for local formation of kinins that may then act on cells within the conventional and/or uveoscleral outflow pathways. This possibility is supported further by the finding that both B2 and B1 kinin receptors are expressed by cells within each pathway.
The principal substrate for the action of tissue kallikrein to yield biologically active kinins is low molecular weight kininogen (Bhoola et al., 1992; Blais et al., 2000). In the present study there was no evidence of kininogen expression by any of the cell types investigated within the ciliary body or the trabecular meshwork. Consequently, some other source of substrate would appear necessary for kinin generation to occur within the anterior segment. While the existence of a blood aqueous barrier in the mammalian eye is well established (Raviola, 1977), it is now clear that plasma proteins may enter aqueous humor under normal physiological conditions. Studies in rabbits (Freddo et al., 1990), monkeys (Barsotti et al., 1992) and humans (Bert et al., 2006) have established a diffusional pathway for the movement of circulating proteins from plasma into the aqueous humor via the ciliary body and anterior portions of the iris. Low molecular weight kininogen is a 68 kDa circulating protein produced primarily in the liver (Nakaniski, 1987) and is a likely candidate for traversing this pathway, a possibility supported by recent proteomic analyses which have identified kininogen among the array of proteins present in human aqueous humor (Fautsch, M. P., personal communication). The current thinking is that plasma proteins move through the capillaries of the ciliary body stroma into the posterior root of the iris. Diffusion of solute from plasma into the posterior chamber is largely prevented by the tight junctions of the epithelial cells of the ciliary body and posterior iris, limiting the possibility for bulk flow of protein from the posterior to anterior chamber (Bert et al., 2006). Instead, it appears that the major route of protein diffusion is through the relatively porous stroma of the anterior iris and then directly into the anterior chamber. Since this model of protein movement suggests that plasma-derived protein is shunted initially to the root of the iris prior to diffusion into the anterior chamber, it is probable that a significant fraction of this protein would be immediately exposed to components of the trabecular meshwork within the anterior chamber angle (Bert et al., 2006). Such a pathway for the delivery of plasma kininogen would be optimal for exposure of substrate to the high concentrations of tissue kallikrein found in the trabecular meshwork, yielding kinins in close proximity to trabecular cells and/or the ciliary smooth muscle.
Kinins generated locally within tissues are believed to act in an autocrine or paracrine fashion and are short-lived because of rapid inactivation by kininases (Bhoola et al., 1992; Blais et al., 2000). Kininase activities have been demonstrated in aqueous humor (Igic, 1985) and also in association with the endothelial lining of collecting tubules and Schlemm’s canal (Laliberte, 1988). Further, results from the present study provide direct evidence of kininase activity in ciliary muscle and trabecular meshwork cells. Together, these data indicate that multiple sites exist for kinin inactivation within the conventional outflow pathway. Initiation of signaling by bradykinin in trabecular cells is generally a rapid process occurring in minutes (Sharif and Xu, 1996;Llobet et al., 1999; Polansky et al., 1989; Webb et al., 2003) but functional effects of the peptide appear to evolve more slowly. For example, in bradykinin’s action to increase outflow facility in perfused bovine anterior segments, peak response was not achieved until 4 hours after the initial exposure (Webb et al., 2006). In the same study, bradykinin was observed to promote secretion of matrix metalloproteinase from trabecular meshwork cells into the extracellular environment. Increased extracellular MMP was detectable within 1 to 2 hours and was linked to the enhancement of outflow facility produced by bradykinin perfusion. These data provide evidence that bradykinin stimulation of B2 kinin receptors initiates signaling to promote MMP release which, over a period of hours, alters the extracellular matrix to decrease outflow resistance within the trabecular meshwork. Such a sequence is consistent with established effects of MMPs on trabecular matrix and outflow facility (Bradley et al., 1998; Bradley et al., 2001) and a similar mechanism has been proposed for adenosine actions to increase the outflow of aqueous humor (Crosson et al., 2005; Shearer and Crosson, 2002).
In summary, a combination of RT-PCR, immunohistochemical experiments and immunoblots were used to demonstrate tissue kallikrein expression by multiple cell types within the anterior segment of the human eye. Kallikrein expression was prominent in the non-pigmented epithelial lining of the ciliary body and ciliary processes, as well as ciliary smooth muscle cells and cells of the trabecular meshwork. Further, expression of both B2 and B1 kinin receptors was detected in ciliary smooth muscle and trabecular meshwork cells from multiple donors. Viewed collectively, the data indicate that key components for kinin production and actions are localized within human ocular tissues and support the possibility that kinins produced within the eye may contribute to the regulation of aqueous outflow and the control of intraocular pressure.
Acknowledgments
This study was supported by National Eye Institute grants EY014653 ( J.G.W.), EY09741( C.E.C.), EY01479 (C.E.C.) and Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barsotti MF, Bartels SP, Freddo TF, Kamm RD. The source of protein in the aqueous humor of the normal monkey eye. Invest Ophthalmol Vis Sci. 1992;33:581–595. [PubMed] [Google Scholar]
- Bert RJ, Caruthers SD, Jara H, Krejza J, Melhem ER, Kolodny NH, Patz S, Freddo TF. Demonstration of an anterior diffusional pathway for solutes in the normal human eye with high spatial resolution contrast-enhanced dynamic MR imaging. Invest Ophthalmol Vis Sci. 2006;47:5153–5162. doi: 10.1167/iovs.05-0372. [DOI] [PubMed] [Google Scholar]
- Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens and kininases. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- Blais C, Jr, Marceau F, Rouleau JL, Adam A. The kallikrein-kininogen-kinin system: lessons from the quantification of endogenous kinins. Peptides. 2000;21:1903–1940. doi: 10.1016/s0196-9781(00)00348-x. [DOI] [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- Bradley JM, Vranka J, Colvis CM, Conger DM, Alexander JP, Fisk AS, Samples JR, Acott TS. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- Crosson CE, Sloan CF, Yates PW. Modulation of conventional outflow facility by the adenosine A1 agonist, N6-cyclohexyladenosine. Invest Ophthalmol Vis Sci. 2005;46:3795–3799. doi: 10.1167/iovs.05-0421. [DOI] [PubMed] [Google Scholar]
- Erickson-Lamy K, Rohen JW, Grant WM. Outflow facility studies in the perfused bovine aqueous outflow pathways. Curr Eye Res. 1988;7:799–807. doi: 10.3109/02713688809033211. [DOI] [PubMed] [Google Scholar]
- Finlay JA, Day JR, Rittenhouse HG. Polyclonal and monoclonal antibodies to prostate-specific antigen can cross-react with human kallikrein 2 and human kallikrein 1. Urology. 1999;53:746–751. doi: 10.1016/s0090-4295(98)00573-1. [DOI] [PubMed] [Google Scholar]
- Freddo TF, Bartels SP, Barsotti MF, Kamm RD. The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci. 1990;31:125–137. [PubMed] [Google Scholar]
- Husain S, Jafri F, Crosson C. Acute effects of PGF2α on MMP-2 secretion from human ciliary muscle cells: A PKC- and ERK-dependent process. Invest Ophthalmol Vis Sci. 2005;46:1706–1713. doi: 10.1167/iovs.04-0993. [DOI] [PubMed] [Google Scholar]
- Igic R. Kallikrein and kininases in ocular tissues. Exp Eye Res. 1985;41:117–120. doi: 10.1016/0014-4835(85)90100-9. [DOI] [PubMed] [Google Scholar]
- Laliberte MF, Laliberte F, Alhenc-Gelas F, Chevillard C. Immunohistochemistry of angiotensin I-converting enzyme in rat eye structures involved in aqueous humor regulation. Lab Invest. 1988;59:263–270. [PubMed] [Google Scholar]
- Llobet A, Gual A, Pales J, Barraquer R, Tobias E, Nicolas JM. Bradykinin decreases outflow facility in perfused anterior segments and induces shape changes in passaged BTM cells in vitro. Invest Ophthalmol Vis Sci. 1999;40:113–125. [PubMed] [Google Scholar]
- Ma JX, Song Q, Hatcher HC, Crouch RK, Chao L, Chao J. Expression and cellular localization of the kallikrein-kinin system in human ocular tissues. Exp Eye Res. 1996;63:19–26. doi: 10.1006/exer.1996.0087. [DOI] [PubMed] [Google Scholar]
- Nakaniski S. Substance P precursor and kininogen: their structures, gene organizations and regulation. Physiol Rev. 1987;67:1117–1142. doi: 10.1152/physrev.1987.67.4.1117. [DOI] [PubMed] [Google Scholar]
- Perosa SR, Arganaraz GA, Goto EM, Costa LGP, Konno AC, Varella PPV, Santiago JFC, Pesquero JB, Canzian M, Amado D, Yacubian EM, Carrete H, Jr, Centeno RS, Cavalheiro EA, Silva JA, Jr, da Graca Naffah Mazzacoratti M. Kinin B1 and B2 receptors are overexpressed in the hippocampus of humans with temporal lobe epilepsy. Hippocampus. 2007;17:26–33. doi: 10.1002/hipo.20239. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Kurtz RM, Alvarado JA, Weinreb RN, Mitchell MD. Eicosanoid production and glucocorticoid regulatory mechanisms in cultured human trabecular meshwork cells. Prog in Clin Biol Res. 1989;312:113–138. [PubMed] [Google Scholar]
- Raviola G. The structural basis of the blood-ocular barriers. Exp Eye Res. 1977;25 (Suppl):27–64. doi: 10.1016/s0014-4835(77)80009-2. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Xu SX. Pharmacological characterization of bradykinin receptors coupled to phosphoinositide turnover in SV40-immortalized human trabecular meshwork cells. Exp Eye Res. 1996;63:631–637. doi: 10.1006/exer.1996.0157. [DOI] [PubMed] [Google Scholar]
- Shearer TW, Crosson CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002;43:3016–3020. [PubMed] [Google Scholar]
- Webb JG, Husain S, Yates PW, Crosson CE. Kinin modulation of conventional outflow facility in the bovine eye. J Ocul Pharmacol Ther. 2006;22:310–316. doi: 10.1089/jop.2006.22.310. [DOI] [PubMed] [Google Scholar]
- Webb JG, Shearer TW, Yates PW, Mukhin YV, Crosson CE. Bradykinin enhancement of PGE2 signaling in bovine trabecular meshwork cells. Exp Eye Res. 2003;76:283–289. doi: 10.1016/s0014-4835(02)00313-5. [DOI] [PubMed] [Google Scholar]