Abstract
The role of H2O2 as a second messenger in signal transduction pathways is well established. We show here that the NADPH oxidase-dependent production of and H2O2 or respiratory burst in alveolar macrophages (AM) (NR8383 cells) is required for ADP-stimulated c-Jun phosphorylation and the activation of JNK1/2, MKK4 (but not MKK7) and apoptosis signal-regulating kinase-1 (ASK1). ASK1 binds only to the reduced form of thioredoxin (Trx). ADP induced the dissociation of ASK1/Trx complex and thus resulted in ASK1 activation, as assessed by phosphorylation at Thr845, which was enhanced after treatment with aurothioglucose (ATG), an inhibitor of Trx reductase. While dissociation of the complex implies Trx oxidation, protein electrophoretic mobility shift assay detected oxidation of Trx only after bolus H2O2 but not after ADP stimulation. These results demonstrate that the ADP-stimulated respiratory burst activated the ASK1-MKK4-JNK1/c-Jun signaling pathway in AM and suggest that transient and localized oxidation of Trx by the NADPH oxidase-mediated generation of H2O2 may play a critical role in ASK1 activation and the inflammatory response.
Keywords: NADPH, alveolar macrophages, adenine nucleotides, MAPK
Introduction
Alveolar macrophages (AM) are the resident phagocytes of the lungs and the primary line of defense against inhaled particles and microorganisms. AM play a key role in orchestrating the inflammatory and immune responses through secretion of a variety of cytokines. In addition, AM express a multicomponent NADPH oxidase (NOX2), which could be activated by phagocytosis of pathogens and particles, or by soluble agents such as cytokines and adenine nucleotides [1]. NOX2 activation results in the production of and H2O2 (from spontaneous dismutation), a process referred to as the respiratory burst. In recent years, NOX2 has been shown to be the prototype of a large family of NOX homologues that are expressed in many non-phagocytic cells and be responsible for ROS production in these cells [2]. It is now well documented that H2O2 can act as a second messenger in many cells, modulating the activity of several signaling molecules and pathways such as the mitogen-activated protein kinase (MAPK) pathways, and the transcription factors AP-1 and NF-κB [3–16]. We previously showed that H2O2 generated by stimulation of the AM respiratory burst with various agents was required for the activation of ERK1/2 [8], NF-κB [6] and AP-1 DNA binding [7], indicating that activation of the NADPH oxidase is upstream of these pathways in rat AM and that H2O2 may represent a point of convergence by which several stimuli can modulate downstream signaling pathways.
AP-1 proteins are homodimers or heterodimers composed of proteins that belong to the Jun, Fos, Jun dimerization partners (JDP 1 and 2), and ATF subfamilies [17]. Upon activation by various stresses, AP-1 dimers bind to DNA recognition elements known as TPA response elements (TRE) to regulate the transcription of many genes. AP-1 activity is regulated through changes in gene transcription and protein stability, interactions with other proteins and post-translational modifications, in particular the phosphorylation of c-Jun by c-Jun-NH2 terminal kinase (JNK). JNK isoforms are activated through sequential phosphorylation of kinase modules where one of several serine-threonine MAP3Ks activates the dual-specificity kinases MAP2K, MKK4 and/or MKK7, which in turn phosphorylate JNK in their activation motif (TPY) [18,19].
The MAP3K, apoptosis signal-regulating kinase-1 (ASK1), directly phosphorylates MKK4/7 and MKK3/6, thereby activating the JNK and p38 MAPK pathways, respectively [20,21]. ASK1 is activated by proinfiammatory and stress signals such as TNF-α, Fas, ER stress and H2O2, and plays a pivotal role in apoptosis, as demonstrated in ASK1 null mice [22], but it is also implicated in cell survival and differentiation [20,23]. ASK1 activity is tightly controlled by several mechanisms including oligomerization, phosphorylation and protein-protein interactions. It has been reported that ASK1 could associate with TRAF2 [24,25], 14-3-3 [26], GSTMu [27,28], HSP72 [29], Daxx [24,30], the serine–threonine phosphatase PP5 [22], thioredoxin (Trx) [24] and glutaredoxin (Grx) [31]. Both Trx and Grx contain redox-active cysteine residues in the CXXC Trx-fold of their catalytic center [32]. In resting cells, the association between the N-terminus of ASK 1 and reduced Trx inhibits ASK1 activity [24,33]. The association of Grx with the C-terminus of ASK1 also inhibits ASK1 activity [31]. It has been suggested that Trx may be directly oxidized by H2O2 (or via catalysis by a peroxiredoxin (Prx)) while Grx, which is critical for reversible protein S-glutathionylation, may first interact with GSSG to form a mixed disulfide intermediate, and subsequently an intramolecular disulfide bridge [34]. The oxidized form of Trx or Grx dissociates from ASK1, which then undergoes changes in conformation and homo-oligomerization. Prior release of Trx appears critical for the binding of ASK1 to other proteins such as TRAF2 and Daxx that promotes ASK1 oligomerization and increases its kinase activity [24,30]. Phosphorylation is also a critical regulatory mechanism for ASK1 activation. Oligomerized ASK1 autophosphorylates at Thr845 in the kinase domain activation loop and results in increased kinase activity [35]. ASK1 can also be phosphorylated at other serine residuals such as Ser967, Ser83 or Ser1034, which may differentially affect ASK1 protein interactions and kinase activity [36–39].
In this study, we investigated the signaling pathways leading to AP-1 activation upon stimulation of the rat AM respiratory burst with ADP, an inflammatory mediator. We demonstrate that H2O2 generated by the ADP-stimulated respiratory burst causes Trx oxidation and rapid dissociation from ASK1, and the phosphorylation of ASK1 at Thr845. Our data show that respiratory burst increased AP-1 activity through the ASK1-Trx/MKK4/JNK pathway under physiological stimulation where cells generate low level of H2O2.
Results
Stimulation of the NADPH oxidase by ADP activates the ASK1/MKK4/JNK1/2 signaling pathway in a redox-dependent manner
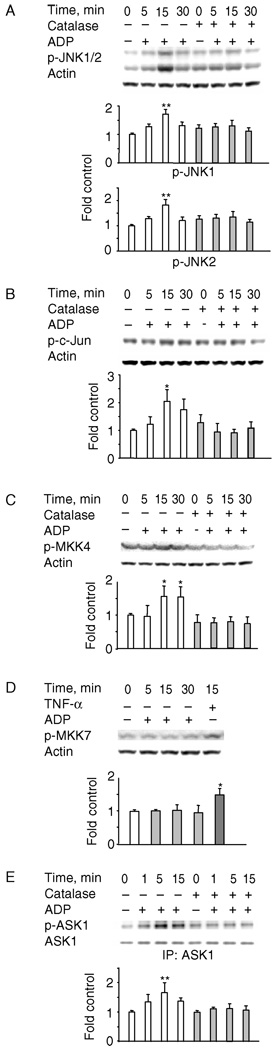
ADP is an important inflammatory mediator and a known activator of the NADPH oxidase in AM [40], and the respiratory burst of rat AM (NR8383 cells) is maximally stimulated by 400 µM ADP [41]. We previously showed that the increase in AP-1 DNA binding stimulated by ADP was inhibited by catalase, suggesting its dependence on the H2O2 produced by the respiratory burst [7]. Here, we extend these observations by delineating the ADP-stimulated signaling pathway upstream of AP-1. As seen in Figure 1, ADP (400 µM) increased the phosphorylation of JNK1/2 (Figure 1A), c-Jun (Figure 1B) and MKK4 (Figure 1C). In contrast, ADP did not activate MKK7, another MAP2K for JNK, while TNF-α, used as a control, induced a 1.5-fold increase in MKK7 phosphorylation (Figure 1D).
Figure 1.
ADP activates ASK1/MKK4/JNK1/2 in an H2O2-dependent manner. NR8383 cells were pre-incubated ± catalase (100 U/ml) for 15 min before stimulation with 400 µM ADP for the indicated times. Whole cell lysates (A–D) or ASK1 immunoprecipitates (E) were analyzed by Western blotting. ADP induced the increase in phosphorylation of JNK1/2 (A), c-Jun (B), MKK4 (C) and ASK1 (E) that was abrogated by exogenous catalase. ADP did not stimulate the phosphorylation of MKK7 (D) but TNF-α did, as previously reported. Shown are representative blots and bar graphs (B–E) represent the mean and standard deviation of photon counts obtained as described in Methods; n = 3, *p < 0.05, **p < 0.01.
Several MAP3K upstream of MKK4/7 have been identified, among which ASK1 was the most frequently studied. To test whether ADP activates ASK1, lysates from ADP-stimulated cells were immunoprecipitated with ASK1 antibody and the immunoprecipitates were analyzed by immunoblotting with antibodies to ASK1 phosphorylated at Thr845, which correlates with activation [35]. ASK1 phosphorylation at Thr845 increased as early as 1 min after ADP treatment and remained elevated by 15 min (Figure 1E). Pre-incubation of cells with catalase (100 U/ml), which inhibited the ADP-stimulated increase in AP-1 DNA binding activity [7], reduced the ADP-induced phosphorylation of JNK1/2, MKK4 and ASK1 to baseline levels (Figure 1). These data demonstrate that H2O2 generated by the ADP-stimulated respiratory burst is upstream of the activation of the ASK1/MKK4/JNK1/2 signaling pathway.
Thioredoxin dissociation from ASK1 is involved in ASK1 activation by ADP-stimulated respiratory burst
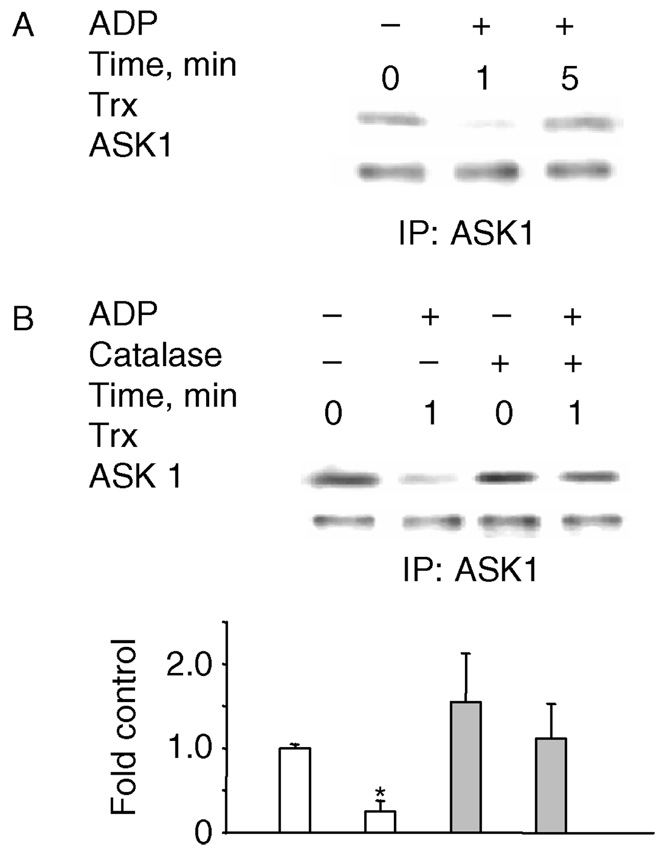
Previous studies have suggested that disruption of the complex formed by ASK1 with reduced Trx in resting cells precedes ASK1 activation and phosphorylation [42]. Whether ASK1 is activated through this mechanism by respiratory burst, however, remains unclear. Thus, we tested the Trx/ASK1 association with/without ADP stimulation. ASK1 was immuno-precipitated and the association of ASK1 with Trx was assessed by immunoblotting with a Trx antibody. At unstimulated condition, Trx is associated with ASK1 (Figure 2A); and stimulation with ADP reduced the amount of Trx associated with ASK1 (Figure 2A). The ADP-mediated dissociation of Trx from ASK1 was observed after 1 min and was followed by re-association. Pre-incubation with catalase prevented ADP-mediated Trx dissociation from ASK1 (Figure 2B), suggesting that Trx oxidation may be involved in the Trx-ASK1 dissociation process. Although the redox-regulated association of ASK1 with Grx has been reported in cells transfected with Grx and ASK1 constructs [31], we could not detect any Grx in the ASK1 immunoprecipitates prior to stimulation in AM, despite that Grx is detected in the whole cell lysates (data not shown). These data suggest that the H2O2-mediated oxidation of Trx and dissociation of the Trx/ASK1 complex are involved in ASK1 activation by ADP.
Figure 2.
ADP stimulation of the respiratory burst induces the dissociation of Trx from ASK1. NR8383 cells were stimulated with 400 µM ADP and ASK1 was immunoprecipitated. Catalase (100 U/ml) was added 15 min before stimulation. (A) ADP induces Trx dissociation from ASK1. (B). Catalase eliminated ADP-induced dissociation of Trx from ASK1. Experiments were repeated at least three times and the blots shown are representative of the results obtained. The bar graph (B) represents the mean and standard deviation of photon counts as described in Methods; n = 4, *p < 0.05.
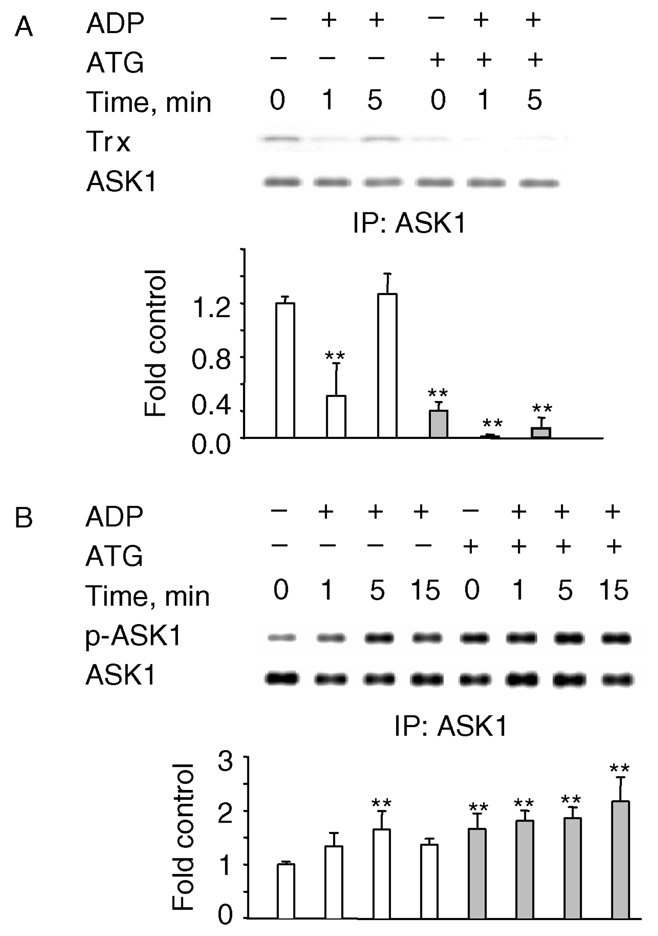
A link between the activity of thioredoxin reductase (TrxR), the selenocysteine enzyme that restores reduced Trx, and the redox state of Trx has been established. In yeast, TrxR deletion results in greatly decreased levels of reduced Trx [43]. Treatment with gold compounds such as aurothioglucose (ATG), which inhibit TrxR [44] would be expected to increase the levels of oxidized Trx, reduce ASK1 association with Trx, and increase ASK1 phosphorylation. Indeed, hardly any Trx was found associated with ASK1 in ATG-treated cells (Figure 3A). Consistent with this, ATG-treated cells exhibited an increase in the phosphorylation of ASK1 in control cells (time 0) and at all time points after ADP treatment (Figure 3B). These data further support the hypothesis that oxidation of Trx is involved in the activation of ASK1/MKK4/JNK1/2 pathway by the ADP-stimulated respiratory burst.
Figure 3.
Trx dissociation from ASK1 is enhanced by ATG. NR8383 were pretreated with 20 µM ATG for 15 min before stimulation with ADP and ASK1 was immunoprecipitated. ASK1 phosphorylation and Trx association were determined by Western blotting of the immunoprecipitates. ATG enhances ADP-mediated Trx dissociation (A) and ASK1 phosphorylation (B). The experiments were repeated three times and the blots shown are representative of the results obtained. Bar graphs represent the mean and standard deviation of photon counts obtained as described in Methods; n = 3, *p < 0.05, **p < 0.01.
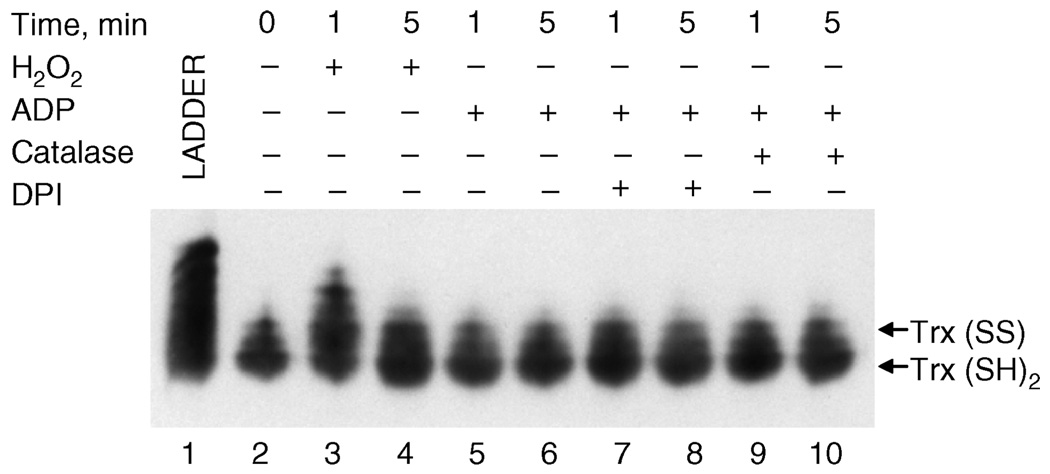
The oxidation state of proteins can be analyzed in vivo by determining the thiol adducts with protein mobility shift (PEMSA) assay [43]. Using this method, we determined the redox state of total cellular Trx in AM exposed to ADP. H2O2 exposure and fully alkylated rat Trx was used as controls (Figure 4, lane 1). Bolus addition of H2O2 resulted in the rapid and transient oxidation of Trx, as indicated by slower migrating bands (Figure 4; compare lane 2 with lanes 3 and 4). In contrast, after ADP stimulation, Trx appeared to remain primarily in the reduced state at all time points (lanes 5 and 6) and conditions (lanes 7–10), despite the fact that ADP induced the dissociation of Trx from ASK1 in a catalase-inhibitable manner. Because only a small portion of total cellular Trx associates with ASK1, this may reflect an inability to detect localized Trx oxidation by PEMSA.
Figure 4.
PEMSA determination of Trx redox state. NR8383 cells were exposed to 100 µM H2O2 (3–4) or 400 µM ADP (5–10) for 1 or 5 min and harvested for Trx redox determination as described in Methods. Where indicated, cells were pre-incubated for 15 min with 100 U/ml catalase (7 – 8) or 2 µM DPI (9 – 10). Lane 1 represents the rat Trx alkylation ladder control. Arrows indicate the fraction of Trx in fully reduced (Trx(SH)2) or dithiol (Trx(SS)) state. PEMSA was repeated three times and the blots shown are representative of the results obtained.
Discussion
ROS, particularly H2O2, are now considered bona fide participants in the signaling pathways of many cells [3–16] and a consensus has emerged that signaling proteins with critical thiols are targets for H2O2, especially if the cysteine is in the thiolate form [10,45]. Previous studies of respiratory burst in phagocytes have mainly concentrated on the participation of ROS in bacterial killing and tissue injury; nevertheless, ROS were shown to be involved in signaling some 10 years ago [6,46,47]. We previously demonstrated that production of H2O2 by the respiratory burst triggered by ADP and other inflammatory mediators was necessary for the stimulated increase in AP-1 DNA binding activity in rat AM [7], and in this paper we try to identify the upstream signaling events of AP-1 activation during respiratory burst in AM.
The redox sensitivity of AP-1 activation is mediated by conserved cysteine residues in the DNA binding domain of the Fos and Jun proteins that must be reduced for DNA binding activity. Oxidation of these cysteines is reversed by the multifunctional protein redox factor-1 [[1]], which contains a redox active cysteine residue [48–50]. AP-1 redox sensitivity can also be the result of JNK activation by oxidants. Multiple mechanisms have been invoked for JNK activation by H2O2. It was proposed that JNK remains inactive in quiescent cells through its complex with GSTpi, which can be oxidized by H2O2, resulting in its oligomerization and dissociation from JNK that is then activated [51]. Lack of GSTpi in null mice resulted in increased JNK constitutive activity, confirming its role [52]. Another study proposed that H2O2 activated JNK via recruitment to tumor necrosis factor receptor 1, although the mechanism of JNK activation and the target for H2O2 oxidation remain unclear [53]. Recently, a novel mechanism was demonstrated in yeast where the H2O2-mediated formation of a disulfide complex between Tpxl, a 2-Cys Prx and Styl, the JNK/p38MAPK homolog, through an evolutionary conserved cysteine in Styl resulted in JNK activation [54]. Nevertheless, the best characterized mechanism for JNK activation by H2O2 involves ASK1 and Trx [24,34]. The importance of ASK1 in this pathway was demonstrated by the significant decrease in H2O2-mediated JNK activation observed in cells derived from ASK1 −/− mice [55]. Here, we show that H2O2 generated by the ADP-stimulated respiratory burst induces JNK activation via MKK4 and ASK1 – Trx (Figure 1), a pathway that is most likely responsible for the increase in AP-1 DNA binding activity previously observed [7].
Both MKK4 and MKK7 can activate JNK at the TPY activation motif, although MKK4 preferentially phosphorylates the Tyr residue while MKK7 targets the Thr [56,57]. Both kinases contribute to JNK activation by UV irradiation and anisomycin while only MKK7 is essential for JNK activation by TNF-α, which does not activate MKK4 [58]. Few studies reporting JNK activation by H2O2 investigated the activation of the dual-specificity kinases, except for one study in U937 cells showing that bolus addition of 1 mM H2O2 activated both MKK4 and MKK7 [59]. The fact that only MKK4 was activated by the respiratory burst in our studies (Figure 1C) may be due to differences between immature human monocytic cells and rat AM or to differential activation pathways by lower levels of H2O2.
ASK1 activation is known to be regulated by protein–protein interactions and by phosphorylation. It is well accepted that the binding of ASK1 to reduced Trx inhibits its kinase activity in resting cells. This was confirmed in experiments where down regulation of Trx-1 or Trx-2 with antisense oligonucleotides resulted in increased activation of endogenous ASK1 [24], although Trx-2 interaction with ASK1 is not required for JNK activation [60]. Others have suggested that Trx binding to ASK1 may inhibit its kinase activity in a redox-independent manner by increasing ASK1 ubiquitination and degradation [61]. Nevertheless, Trx oxidation has been shown to be an essential step, pre-requisite to ASK1 release from the complex and activation by H2O2 [35]. Association of reduced Grx with ASK1 has also been shown to inhibit constitutive kinase activity of ASK1. As for Trx, oxidation of Grx precedes ASK1 activation, although these data have only been obtained so far with both Grx and ASK1 overexpression [31]. We did not detect any associated Grx in the ASK1 immuno-precipitates from quiescent AM, despite the significant expression of Grx in the cells (data not shown). One can speculate that this association may not occur with endogenous proteins or that the Grx/ASK1 complexes are too few to be easily detected. Alternatively, this association may be cell type-specific. This issue will await further confirmatory studies.
Most studies investigating the mechanism of ASK1 activation by oxidants have used ASK1-overexpression system and bolus addition of high doses of H2O2. Only one study reported the activation of endogenous ASK1 by H2O2 using pulmonary artery endothelial cells and 250 µM H2O2 [62]. In rat AM, ADP induced the rapid dissociation of Trx from ASK1, which was inhibited by catalase, as was the phosphorylation of MKK4 and c-Jun. In addition, ADP treatment induced the rapid phosphorylation of ASK1 on Thr845, which has been associated with activation [35], and catalase inhibited the increase in ASK1 phosphorylation. Another residue, S967, is highly phosphorylated in quiescent cells, and bound to 14-3-3, which inhibits ASK1 kinase activity [36–38]. A recent study demonstrated that H2O2 activated ASK1 through dephosphorylation of S967 by an okadaic acid-sensitive phosphatase, inducing dissociation of 14-3-3 [63]. While the authors did not determine whether Trx was released from ASK1 under their experimental conditions using transfected cells, they argued that their results were consistent with a model in which Trx oxidation by H2O2 and its release from ASK1 preceded the dephosphorylation of S967.
In this study we observed that phosphorylation at Thr845 of ASK1 upon ADP treatment was transient (Figure 1E); i.e. maximum ASK1 phosphorylation occurred at 5 min of ADP treatment and was diminished after 15 min. The transient nature of ASK1 activation by ADP may be related to several factors. Besides the rapid re-association of ASK-1 with reduced Trx (Figure 2A), protein phosphatase 5 (PP5) may also play a role in this process. PP5 is a Ser/Thr protein phosphatase that can bind to ASK1 and downregulate its activity by dephosphorylating p-ASK1 [67–70]. Zhou et al. [69] reported that hypoxia, induces the expression of PP5 and the activation of the ASK1/MKK-4/JNK signaling cascade, which was turned-off by binding of PP5 to ASK1. This supports the previous report by Morita et al. [67] where PP5 was found to act as a physiological inhibitor of the ASK1/JNK/p38 pathway, inhibiting the sustained activation of ASK1 by H2O2. Based on these published observations, PP5 may play a role in the transient activation of ASK1 by ADP in addition to the dissociation and re-association of Trx, although its constitutive expression has not been documented in NR8383 cells.
Treatment with ATG (Figure 3), a TrxR inhibitor that would increase the levels of oxidized Trx [64,65], reduced the association of Trx with ASK1 and enhanced ASK1 phosphorylation, consistent with the contention that dissociation from ASK1 involves Trx oxidation and precedes phosphorylation. Nevertheless, when the redox status of Trx was examined by PEMSA (Figure 4), transient Trx oxidation was only detected with bolus addition of H2O2 (lanes 3 and 4), at a dose that is approximately 1000 times greater (100 µM H2O2 ~ 100 nmoles in the 1 ml incubation volume) than the amount generated by the ADP-stimulated respiratory burst (data not shown). As signaling involves local gradients of second messengers ranging from very high at the point of production to zero at the point of destruction, bolus addition may need to be high to achieve sufficient concentration locally. Other studies using addition of cAMP to permeabilized cells also require much higher concentrations to achieve signaling than are produced in the total cell volume through stimulated production [66]. Thus, the local concentration of H2O2 between the NADPH oxidase and the ASK1 – Trx complex may be as high as 100 µM, although globally the increase would be a small fraction of that. In fact, catalase inhibited the ADP-stimulated Trx release from ASK1, which only binds reduced Trx, indicating that the low levels of H2O2 produced by the respiratory burst result in Trx oxidation. It is very unlikely that the inability to detect oxidized Trx in ADP-treated cells by PEMSA is due to a difference in the mechanism by which ADP or bolus H2O2 caused Trx dissociation. Instead, only the small portion of Trx that was associated with ASK1 and in close vicinity to the NADPH oxidase where sufficiently high H2O2 is being generated may have undergone oxidation that PEMSA could not detect.
As summarized in Figure 5, ADP binding to its G protein-coupled receptor induces the assembly of the NADPH oxidase and production of superoxide that is dismutated to H2O2. While the redox active cysteine of Trx is potentially susceptible to oxidation by H2O2, the non-enzymatic reaction might be too slow to play a significant role in signaling. Trx participates in protein disulfide/dithiol exchange and is the reducing cofactor in the elimination of H2O2 by enzymes first called thiol-specific antioxidant, then thioredoxin peroxidases, and now referred to as Prx [71]. It has been suggested that Prx effectively control the levels of H2O2 in cells and may affect redox signaling through its transient inhibition so that the H2O2 concentration can reach signaling levels [71,72]; however, it is possible that Prx in catalyzing the oxidation of Trx by H2O2 activates ASK1. Regardless, H2O2, either through a Prx-catalyzed reaction or directly, induces the oxidation of Trx and activation of the ASK1/MKK4/JNK pathway. This pathway is most likely responsible for the increased in AP-1 DNA binding activity stimulated by the low doses of H2O2 produced by the respiratory burst we previously demonstrated in rat AM [73]. AP-1 activation in AM has been shown to be necessary for the induction of lung inflammation in an in vivo model [74]. Thus, activation of this pathway by the respiratory burst may participate in the induction of gene expression for proinflammatory mediators, in conjunction with the NF-κB pathway.
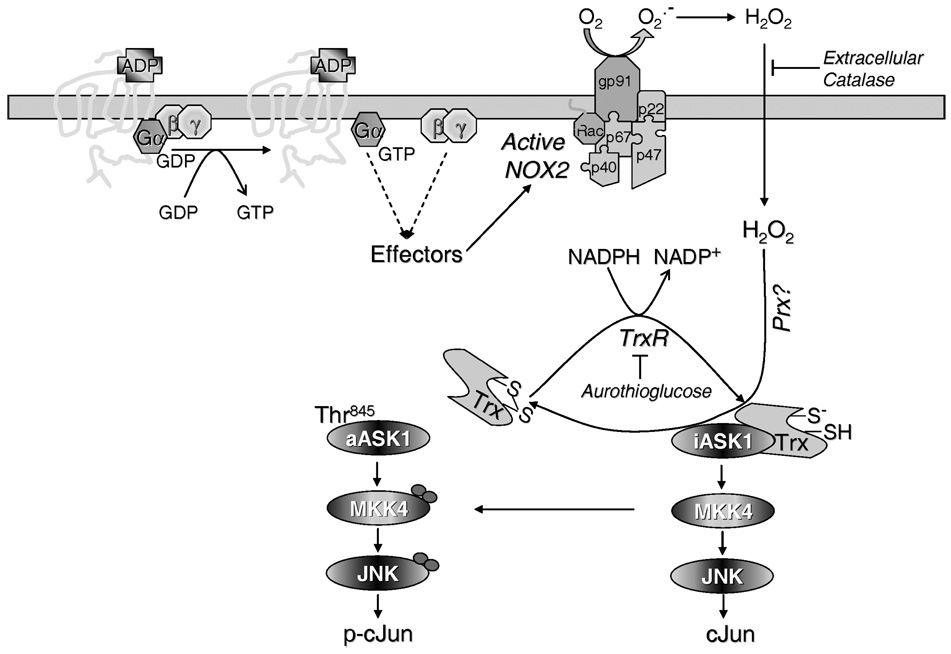
Figure 5.
Proposed model for activation of the JNK pathway by ADP. ADP, acting via its G-protein coupled receptor, stimulates the assembly of the NADPH oxidase (NOX2) at the plasma membrane, resulting in extracellular generation of superoxide that dismutates to hydrogen peroxide. H2O2 freely diffuses into cells but can be eliminated by addition of extracellular catalase. Inactive ASK1 (iASK1) is bound to reduced thioredoxin (Trx-(SH)(S−)), which is released from ASK1 upon its oxidation to a disulfide (Trx(S)2) by H2O2, a reaction likely catalyzed by a Prx. Free ASK1 undergoes activation (aASK1) via several mechanisms including phosphorylation of Thr845, resulting in activation of the JNK module and phosphorylation of c-Jun. Reduction of oxidized Trx by NADPH and TrxR is followed by Trx re-association with ASK1, which can be delayed upon inhibition of TrxR by gold compounds such as ATG.
Material and methods
Reagents and materials
Unless otherwise noted, chemicals were from Sigma (St. Louis, MO, USA) and at least of analytical grade. The Trx antibody was previously described [75]. The antibodies to ASK1, p-JNK, p-c-Jun and actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), pThr845-ASK1 from Cell Signaling Technology (Beverly, MA, USA), p-MKK4 from Upstate Biotech. Inc. (Lake Placid, NY, USA), Grx antibody from Labfrontier (E-mail: bio-orders@labfrontier.com) and protein A-Sepharose from Amersham Biosciences (Piscataway, NJ, USA).
Cell culture
The NR8383 rat AM cell line [76] was a generous gift from Dr G. H. Zhang, University of Texas Health Science Center at San Antonio. Cells were cultured in F-12K medium (Life Technologies, Grand Island, NY, USA), supplemented with 15% heat-inactivated fetal bovine serum (Omega Sci., CA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin in 5% CO2 at 37°C.
ASK1 immunoprecipitation
After treatment for various times, cells were lyse d with buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1% Triton X100, 0.5% NP40, 50 mM beta-glycerophosphate, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM EGTA, 1 mM PMSF, 10% glycerol, 1 mM DTT, 100 mM sodium fluoride, 10 ng/ml leupeptin and aprotinin. Clarified lysates were immunoprecipitated with anti-ASK1 at 4°C overnight and then incubated for 1 h with protein A-Sepharose (Amersham Biosciences, Piscataway, NJ, USA). After washing with lysis buffer and re-suspension in 2X Laemmli sample buffer, the immunoprecipitates were analyzed by SDS-PAGE and Western blotting.
Western blotting
Whole cell lysates were prepared after stimulation by extracting proteins with M-PER buffer (Pierce, Rockford, IL, USA). Immunoprecipitates or 30 µg protein from cell lysates were separated by SDS-PAGE (4–20%; InVitrogen, Carlsbad, MA, USA) and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Membranes were blocked at room temperature in 5% nonfat dry milk (NFDM) for 1 h, incubated overnight at 4°C with the appropriate primary antibody in 5% NFDM in Tris buffer saline (TBS) and subsequently for 2 h at room temperature with a peroxidase-labeled anti-species antibody. Protein bands were detected using a chemiluminescent system (ECL Plus, Amersham Biosciences), imaged and quantified by photon counting using the charged-coupled device (CCD) camera of a Kodak Image Station 2000R (Kodak, Rochester, NY, USA) and Kodak 1D 3.6 Image Analysis Software. Photon counting was used for graphing and statistical analysis.
Thioredoxin redox state determination
The redox state of Trx was determined by PEMSA [43]. Briefly, cells were stimulated under several conditions and incubated for 15 min at 37°C in 400 µl alkylation buffer (8 M urea, 100 mM Tris (pH 8.2) and 1 mM EDTA) containing 30 mM iodoacetate (IAA). Samples were then frozen/thawed, precipitated, washed with cold acetone, and incubated for 30 min at 37°C in 400 µ1 alkylation buffer containing 3.5 mM DTT. Samples received iodoacetamide (IAM) to 10 mM and were incubated for 30 min at 37°C. An alkylation ladder, showing the seven charge isomers of rat Trx, was prepared by harvesting cells in alkylation buffer containing 3.5 mM DTT and treating the reduced lysate with IAA and IAM mixtures. Protein concentration was determined by the Bradford method [77] and equal amounts of protein (10 or 20 µg) were analyzed by urea-PAGE, and transferred to Hybond membrane (Amersham). After blocking overnight in 5% NFDM in TBS-T (15 mM Tris (pH 7.6), 140 mM NaCl and 0.1% Tween), membranes were incubated with rabbit anti-mouse Trx antisera, washed and incubated with HRP-conjugated goat-anti-rabbit IgG (Jackson Immunol, West Grove, PA, USA). Protein bands were visualized with ECL reagents. An alkylation ladder was prepared that showed the seven possible charge isomers of rat Trx, which has six cysteine residuals. Fully reduced Trx migrates more rapidly to the anode than fully oxidized Trx.
Statistical analysis
SigmaStat software was used for statistical analysis and statistical significance was accepted when p < 0.05. Comparison of variants between experimental groups was performed with one way ANOVA followed by Tukey’s test.
Acknowledgements
This work was supported by grant HL37556 to HJF. KEI is supported by a Parker B. Francis Fellowship from the Francis Families Foundation and funding from AHA-BGA.
References
- 1.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004 doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 2.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 3.Forman HJ, Torres M. Signaling by the respiratory burst in macrophages. IUBMB Life. 2001;51:365–371. doi: 10.1080/152165401753366122. [DOI] [PubMed] [Google Scholar]
- 4.Simeonova PP, Luster MI. Iron and reactive oxygen species in the asbestos-induced tumor necrosis factor-alpha response from alveolar macrophages. Am J Respir Cell Mol Biol. 1995;12:676–683. doi: 10.1165/ajrcmb.12.6.7539275. [DOI] [PubMed] [Google Scholar]
- 5.Gossart S, Cambon C, Orfila C, Seguelas M, Lepert J, Rami J, Carre P, Pipy B. Reactive oxygen intermediates as regulators of TNF-a production in rat lung inflammation induced by silica. J Immunol. 1996;156:1540–1548. [PubMed] [Google Scholar]
- 6.Kaul N, Forman HJ. Activation of NF-κB by the respiratory burst of macrophages. Free Radic Biol Med. 1996;21:401–405. doi: 10.1016/0891-5849(96)00178-5. [DOI] [PubMed] [Google Scholar]
- 7.Iles KE, Dickinson DA, Watanabe N, Iwamoto T, Forman HJ. AP-1 activation through endogenous H(2)O(2) generation by alveolar macrophages. Free Radic Biol Med. 2002;32:1304–1313. doi: 10.1016/s0891-5849(02)00840-7. [DOI] [PubMed] [Google Scholar]
- 8.Torres M, Forman HJ. Activation of several MAP kinases upon stimulation of rat alveolar macrophages: Role of the NADPH oxidase. Arch Biochem Biophys. 1999;366:231–239. doi: 10.1006/abbi.1999.1225. [DOI] [PubMed] [Google Scholar]
- 9.Gozal E, Forman HJ, Torres M. ADP stimulates the respiratory burst without activation of ERK and AKT in rat alveolar macrophages. Free Radic Biol Med. 2001;31:679–687. doi: 10.1016/s0891-5849(01)00630-x. [DOI] [PubMed] [Google Scholar]
- 10.Forman HJ, Fukuto J, Torres M. Redox signaling—thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol. 2004;287:C246–C256. doi: 10.1152/ajpcell.00516.2003. [DOI] [PubMed] [Google Scholar]
- 11.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-κB activation: Distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima I, Takeda K, Kawamoto Y, Okuno Y, Kato M, Suzuki H. Redox control of catalytic activities of membrane-associated protein tyrosine kinases. Arch Biochem Biophys. 2005;434:3–10. doi: 10.1016/j.abb.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Chen F. Reactive oxygen species (ROS), trouble-makers between nuclear factor-κB (NF-κB) and c-Jun NH(2)-terminal kinase (JNK) Cancer Res. 2004;64:1902–1905. doi: 10.1158/0008-5472.can-03-3361. [DOI] [PubMed] [Google Scholar]
- 14.Cross JV, Templeton DJ. Thiol oxidation of cell signaling proteins: Controlling an apoptotic equilibrium. J Cell Biochem. 2004;93:104–111. doi: 10.1002/jcb.20202. [DOI] [PubMed] [Google Scholar]
- 15.Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol. 2003;14:S211–S215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- 16.Stone JR. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch Biochem Biophys. 2004;422:119–124. doi: 10.1016/j.abb.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 17.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 18.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 19.Torres M. Mitogen-activated protein kinase pathways in redox signaling. Front Biosci. 2003;8:D369–D391. doi: 10.2741/999. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Hatai T, Hamazaki TS, Nishitoh H, Saitoh M, Ichijo H. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J Biol Chem. 2000;275:9805–9813. doi: 10.1074/jbc.275.13.9805. [DOI] [PubMed] [Google Scholar]
- 21.Wang XS, Diener K, Jannuzzi D, Trollinger D, Tan TH, Lichenstein H, Zukowski M, Yao Z. Molecular cloning and characterization of a novel protein kinase with a catalytic domain homologous to mitogen-activated protein kinase kinase kinase. J Biol Chem. 1996;271:31607–31611. doi: 10.1074/jbc.271.49.31607. [DOI] [PubMed] [Google Scholar]
- 22.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzawa A, Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal. 2005;7:472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- 24.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masters SC, Subramanian RR, Truong A, Yang H, Fujii K, Zhang H, Fu H. Survival-promoting functions of 14-3-3 proteins. Biochem Soc Trans. 2002;30:360–365. doi: 10.1042/bst0300360. [DOI] [PubMed] [Google Scholar]
- 27.Cho SG, Lee YH, Park HS, Ryoo K, Kang KW, Park J, Eom SJ, Kim MJ, Chang TS, Choi SY, et al. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276:12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- 28.Dorion S, Lambert H, Landry J. Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-transferase Mu from Askl. J Biol Chem. 2002;277:30792–30797. doi: 10.1074/jbc.M203642200. [DOI] [PubMed] [Google Scholar]
- 29.Stonehouse MJ, Cota-Gomez A, Parker SK, Martin WE, Hankin JA, Murphy RC, Chen W, Lim KB, Hackett M, Vasil AI, et al. A novel class of microbial phosphocholine-specific phospholipases C. Mol Microbiol. 2002;46:661–676. doi: 10.1046/j.1365-2958.2002.03194.x. [DOI] [PubMed] [Google Scholar]
- 30.Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 31.Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277:46566–46575. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- 32.Holmgren A. Thiols of thioredoxin and glutaredoxin in redox signaling. In: Forman HJ, Fukuto J, Torres M, editors. Signal transduction by reactive oxygen and nitrogen species: Pathways and chemical principles. Dordrecht: Boston: Kluwer Academic Publishers; 2003. pp. 33–52. [Google Scholar]
- 33.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 34.Song JJ, Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem J. 2003;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Chen J, Fu H. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc Natl Acad Sci USA. 1999;96:8511–8515. doi: 10.1073/pnas.96.15.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miele L, Cordella-Miele E, Xing M, Frizzell R, Mukherjee AB. Cystic fibrosis gene mutation (DF508) is associated with an intrinsic abnormality in Ca2+-induced arachidonic acid release by epithelial cells. DNA Cell Biol. 1997;16:749–759. doi: 10.1089/dna.1997.16.749. [DOI] [PubMed] [Google Scholar]
- 38.Subramanian RR, Zhang H, Wang H, Ichijo H, Miyashita T, Fu H. Interaction of apoptosis signal-regulating kinase 1 with isoforms of 14-3-3 proteins. Exp Cell Res. 2004;294:581–591. doi: 10.1016/j.yexcr.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Fujii K, Goldman EH, Park HR, Zhang L, Chen J, Fu H. Negative control of apoptosis signal-regulating kinase 1 through phosphorylation of Ser-1034. Oncogene. 2004;23:5099–5104. doi: 10.1038/sj.onc.1207668. [DOI] [PubMed] [Google Scholar]
- 40.Murphy JK, Livingston FR, Gozal E, Torres M, Forman HJ. Stimulation of the rat alveolar macrophage respiratory burst by extracellular adenine nucleotides. Am J Respir Cell Mol Biol. 1993;9:505–510. doi: 10.1165/ajrcmb/9.5.505. [DOI] [PubMed] [Google Scholar]
- 41.Giron-Calle J, Forman HJ. Phospholipase D and priming of the respiratory burst by H2O2 in NR8383 alveolar macro-phages. Am J Respir Cell Mol Biol. 2000;23:748–754. doi: 10.1165/ajrcmb.23.6.4227. [DOI] [PubMed] [Google Scholar]
- 42.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. Embo J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bersani NA, Merwin JR, Lopez NI, Pearson GD, Merrill GF. Protein electrophoretic mobility shift assay to monitor redox state of thioredoxin in cells. Methods Enzymol. 2002;347:317–326. doi: 10.1016/s0076-6879(02)47031-0. [DOI] [PubMed] [Google Scholar]
- 44.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 45.Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- 46.Derevianko A, Graeber T, D’Amico R, Simms HH. The role of neutrophil-derived oxidants as second messengers in interleukin 1beta-stimulated cells. Shock. 1998;10:54–61. doi: 10.1097/00024382-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Kaul N, Choi J, Forman HJ. Transmembrane redox signaling activates NF-kappaB in macrophages. Free Radic Biol Med. 1998;24:202–207. doi: 10.1016/s0891-5849(97)00209-8. [DOI] [PubMed] [Google Scholar]
- 48.Xanthoudakis S, Miao G, Vang F, Pan Y-C, Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11:3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992;ll:653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 51.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, et al. Regulation of JNK signaling by GSTp. Embo J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elsby R, Kitteringham NR, Goldring CE, Lovatt CA, Chamberlain M, Henderson CJ, Wolf CR, Park BK. Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J Biol Chem. 2003;278:22243–22249. doi: 10.1074/jbc.M301211200. [DOI] [PubMed] [Google Scholar]
- 53.Pantano C, Shrivastava P, McElhinney B, Janssen-Heininger Y. Hydrogen peroxide signaling through tumor necrosis factor receptor 1 leads to selective activation of c-Jun N-terminal kinase. J Biol Chem. 2003;278:44091–44096. doi: 10.1074/jbc.M308487200. [DOI] [PubMed] [Google Scholar]
- 54.Veal EA, Findlay VJ, Day AM, Bozonet SM, Evans JM, Quinn J, Morgan BA. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell. 2004;15:129–139. doi: 10.1016/j.molcel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 55.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. 2001;2:222. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fleming Y, Armstrong CG, Morrice N, Paterson A, Goedert M, Cohen P. Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem J. 2000;352(Pt 1):145–154. [PMC free article] [PubMed] [Google Scholar]
- 57.Tournier C, Whitmarsh AJ, Cavanagh J, Barrett T, Davis RJ. The MKK7 gene encodes a group of c-Jun NH2-terminal kinase kinases. Mol Cell Biol. 1999;19:1569–1581. doi: 10.1128/mcb.19.2.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim DK, Cho ES, Seong JK, Um HD. Adaptive concentrations of hydrogen peroxide suppress cell death by blocking the activation of SAPK/JNK pathway. J Cell Sci. 2001;114:4329–4334. doi: 10.1242/jcs.114.23.4329. [DOI] [PubMed] [Google Scholar]
- 60.Zhang R, Al-Lamki R, Bai L, Streb JW, Miano JM, Bradley J, Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASKl-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 62.Machino T, Hashimoto S, Maruoka S, Gon Y, Hayashi S, Mizumura K, Nishitoh H, Ichijo H, Horie T. Apoptosis signal-regulating kinase 1-mediated signaling pathway regulates hydrogen peroxide-induced apoptosis in human pulmonary vascular endothelial cells. Crit Care Med. 2003;31:2776–2781. doi: 10.1097/01.CCM.0000098027.49562.29. [DOI] [PubMed] [Google Scholar]
- 63.Goldman EH, Chen L, Fu H. Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J Biol Chem. 2004;279:10442–10449. doi: 10.1074/jbc.M311129200. [DOI] [PubMed] [Google Scholar]
- 64.Hill KE, McCollum GW, Boeglin ME, Burk RF. Thioredoxin reductase activity is decreased by selenium deficiency. Biochem Biophys Res Commun. 1997;234:293–295. doi: 10.1006/bbrc.1997.6618. [DOI] [PubMed] [Google Scholar]
- 65.Gromer S, Arscott LD, Williams CH, Jr, Schirmer RH, Becker K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J Biol Chem. 1998;273:20096–20101. doi: 10.1074/jbc.273.32.20096. [DOI] [PubMed] [Google Scholar]
- 66.Almholt K, Tullin S, Skyggebjerg O, Scudder K, Thastrup O, Terry R. Changes in intracellular cAMP reported by a Redistribution assay using a cAMP-dependent protein kinase-green fluorescent protein chimera. Cell Signal. 2004;16:907–920. doi: 10.1016/j.cellsig.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. Embo J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H, Houghton PJ. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J Biol Chem. 2004;279:36490–36496. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- 69.Zhou G, Golden T, Aragon IV, Honk, anen RE. Ser/Thr protein phosphatase 5 inactivates hypoxia-induced activation of an apoptosis signal-regulating kinase 1/MKK-4/JNK signaling cascade. J Biol Chem. 2004;279:46595–46605. doi: 10.1074/jbc.M408320200. [DOI] [PubMed] [Google Scholar]
- 70.Kutuzov MA, Andreeva AV, Voyno-Yasenetskaya TA. Regulation of apoptosis signal-regulating kinase 1 (ASK1) by polyamine levels via protein phosphatase 5. J Biol Chem. 2005;280:25388–25395. doi: 10.1074/jbc.M413202200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 72.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 73.Iles KE, Dickinson DA, Watanabe N, Iwamoto T, Forman HJ. AP-1 activation through endogenous H2O2 generation by alveolar macrophages. Free Radic Biol Med. 2002;32:1304–1313. doi: 10.1016/s0891-5849(02)00840-7. [DOI] [PubMed] [Google Scholar]
- 74.Guo RF, Lentsch AB, Sarma JV, Sun L, Riedemann NC, McClintock SD, McGuire SR, Van Rooijen N, Ward PA. Activator protein-1 activation in acute lung injury. Am J Pathol. 2002;161:275–282. doi: 10.1016/S0002-9440(10)64179-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujii S, Nanbu Y, Konishi I, Mori T, Masutani H, Yodoi J. Immunohistochemical localization of adult T-cell leukaemia-derived factor, a human thioredoxin homologue, in human fetal tissues. Virchows Arch A Pathol Anat Histopathol. 1991;419:317–326. doi: 10.1007/BF01606523. [DOI] [PubMed] [Google Scholar]
- 76.Helmke RJ, Boyd RL, German VF, Mangos JA. From growth factor dependence to growth factor responsiveness: The genesis of an alveolar macrophage cell line. In Vitro Cell Dev Biol. 1987;23:567–574. doi: 10.1007/BF02620974. [DOI] [PubMed] [Google Scholar]
- 77.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]