Abstract
Normal functions of mitochondria are required for physiological dynamics of cells, while their dysfunction contributes to development of various disorders including those of immune system. Here we demonstrate that exposure of mast cells to ragweed pollen extract increases production of H2O2 via mitochondrial respiratory complex III. These mitochondrial ROS (mtROS) enhance secretion of histamine and serotonin from mast cells, but not enzymes such as β-hexosaminidase, independently from FcεRI-generated stimuli. The release of biogenic amines is associated with inhibition of secretory granules' H+-ATPase activity, activation of PKC-δ and microtubule-dependent motility, and it is independent from intracellular free Ca2+ levels. To asses differences from IgE-mediated mast cell degranulation we show that mtROS decrease antigen-triggered β-hexosaminidase release, while they are synergistic with antigen-induced IL-4 production in sensitized cells. Taken together, these data indicate that mitochondrial dysfunction can act independently from adaptive immunity, as well as augments Th2-type responses. Pharmacological maintenance of physiological mitochondrial function could have clinical benefits in prevention and treatment of allergic diseases.
Keywords: Mast cells, Mitochondria, Degranulation, Reactive oxygen species
1. Introduction
Mast cells are one of the most important effectors involved in elicitation of allergic inflammation and immune responses to many pathogens including parasites (Metcalfe et al., 1997). Antigenic activation of mast cells via the high-affinity receptor for IgE (FcεRI) mediates exocytosis of cytoplasmic granules containing preformed mediators, secretion of lipid-derived factors, and de novo synthesis of cytokines, chemokines and growth factors (Burgoyne and Morgan, 2003; Logan et al., 2003; Metcalfe et al., 1997; Rivera and Gilfillan, 2006). In addition to FcεRI-mediated signals, exposure to a variety of stimuli can lead to the release of mast cell mediators (Frossi et al., 2004). Pathogen-associated molecules may activate mast cells and basophils via receptors selectively expressed on their surfaces (Kojima et al., 2007). Eosinophil-derived major basic protein, compound 48/80 or substance P also induces degranulation of mast cells (Munitz et al., 2003). Several lines of evidence indicate that oxidative stress is also a stimulus for mast cell activation (Frossi et al., 2003; Ohmori et al., 1979; Swindle et al., 2002). During allergic and other inflammatory reactions mast cells are exposed to an oxidative microenvironment because ROS are produced by various cell types in the peripheral tissues as a consequence of their effector function (Nagata, 2005). We have previously reported that pollen grains, sub-pollen particles, and pollen extracts contain intrinsic NAD(P)H oxidases, which generate ROS [superoxide anions (O2.-)] (Bacsi et al., 2006a; Boldogh et al., 2005). These radicals induce oxidative stress in cultured cells, as well as in airway and conjunctival epithelium within minutes of exposure (Bacsi et al., 2005; Boldogh et al., 2005).
There is a close correlation between the exclusively maternal inheritance of mitochondria and the fact that maternal history of atopy and asthma is one of the substantial risk factors for the development of asthma in children (Litonjua et al., 1998). A mitochondrial haplogroup has been shown to be associated with elevated total serum IgE levels in asthmatic patients (Raby et al., 2007). Oxidative stress and mitochondrial metabolism are involved in antigen-induced release of mast cell mediators, including IL-4, which is essential for naive T cell polarization toward Th2 phenotype (Frossi et al., 2003; Inoue et al., 2008). Studies with metabolic inhibitors have demonstrated a close link between mitochondrial energy production and mast cell degranulation (Johansen, 1987). Furthermore, release of Ca2+ from mitochondria is involved in antigen-induced mast cell degranulation (Suzuki et al., 2006).
Here we report for the first time that treatment with short ragweed (Ambrosia artemisiifolia) pollen extract (RWE) induces elevated mitochondrial ROS production in non-sensitized RBL-2H3 cells, a model of mucosal mast cells (Park and Beaven, 2009; Seldin et al., 1985). We show that increased production of ROS from mitochondrial respiratory complex III, but not intrinsic pollen NAD(P)H oxidase-generated ROS directly, enhances secretion of histamine and serotonin from non-sensitized mast cells. Mitochondrial ROS trigger the release of biogenic amines, but not enzymes such as β-hexosaminidase, via inducing PKC δ- and microtubule-dependent motility of secretory granules and inhibiting activity of vacuolar H+-ATPase independently from intracellular Ca2+ levels. We demonstrate that mtROS are also able to enhance FcεRI-mediated IL-4 production of mast cells. These findings may shed light on a new role for mitochondrial dysfunction in the regulation of mast cell activation.
2. Materials and methods
2.1. Reagents
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
2.2. Cell cultures
The RBL-2H3 cells were obtained from the American Type Cell Collection and cultured at 37°C in a humidified atmosphere with 5% CO2 in Minimum Essential Medium containing Earle's salts and L-glutamine (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum, penicillin (100 U/ml) and streptomycin (100 μg/ml).
2.3. Analysis of inflammatory mediator release
Cells were treated with RWE (Greer Laboratories, Lenoir, NC) at the indicated concentrations for 1 h at 37°C. To induce mitochondrial ROS generation, cells were incubated with antimycin A, an inhibitor of complex III. Mitochondrial ROS-induced inflammatory mediator release was determined in the presence or absence of Ca2+ or antigenic stimuli. For antigenic stimulation, cells were first sensitized with mouse IgE anti-DNP (0.1 μg/ml; Sigma, clone SPE-7) for 1 h at 37°C and then treated with antigen (DNP-BSA; 50 ng/ml). Collected supernatants were centrifuged at 400 × g in 4°C to remove any remaining cells. Histamine secretion was determined according to Shore's method (Alfonso et al., 2000). The fluorescence was measured in an Flx800 microplate fluorescence reader at 360/460 nm.
The release of radioactively-labeled serotonin ([3H]-serotonin) was measured as previously described (Isersky et al., 1978). Briefly, 1.5 × 104 cells per well (96-well plates) in 100 μl of culture medium were incubated with 1 μCi/ml of [3H]-serotonin for 18 h at 37°C and 5% CO2. Labeled cells were washed with pre-warmed (37°C) assay buffer, HBSS containing 0.1% BSA. Cells were then further incubated for 30 min at 37°C. Radioactivity in the supernatant fluids was determined by scintillation spectroscopy (Beckman Coulter, Fullerton, CA). Results are expressed as a fraction of analyzed mediator concentration in the supernatant with respect to its total content in a corresponding number of non-treated cells.
Release of β-hexosaminidase was assayed fluorimetrically with 4-methylumbelliferyl N-acetyl-β-D-glucosaminide (MUNAG) (Demo et al., 1999). Briefly, equal volumes (25 μl) of supernatant and substrate solution (2 mM MUNAG in 0.2 M citric buffer, pH 4.5) were added to wells of a 96-well plate, and the enzymatic reaction was developed for 30 min at 37°C and terminated with 100 μl of 1 M Na2CO3 solution (pH 10). The fluorescence of released 4-methylumbelliferone was measured in an Flx800 microplate fluorescence reader at 360/460 nm. Results were further confirmed by measuring the β-hexosaminidase enzymatic activity spectrophotometrically using 4-nitrophenyl-N-acetyl-β-D-glucosamine as substrate (Suzuki et al., 2006).
2.4. Isolation of mitochondria
Mitochondria were isolated and purified as we had previously described (Bacsi et al., 2006b). Briefly, cell pellets were incubated in a hypotonic buffer A (220 mM mannitol, 70 mM sucrose, 2 mM MOPS and 1 mM EGTA, pH 7.4) containing protease inhibitor cocktail (catalog No. P8340, Sigma). Cell suspensions kept in ice bath were sonicated with a Branson sonifier by 4 pulses of 20 % power and cell homogenates were centrifuged for 10 min at 4700 × g. Mitochondria were sedimented from supernatants by centrifugation at 7168 × g. Pellets were resuspended in buffer B (220 mM mannitol, 70 mM sucrose, 2 mM MOPS, pH 7.4) and centrifuged at 9072 × g. Crude mitochondrial solutions were layered on discontinuous sucrose gradients (1.5, 1.0 and 0.5 M sucrose in 10 mM MOPS and 1 mM EDTA, pH 7.4) and ultracentrifuged for 1.5 h at 82705 × g (SW28 rotor, Beckman Coulter, CA). All centrifugation procedures were carried out at 4°C. The band containing mitochondria was removed and washed in 10 times volume of buffer B. Mitochondrial pellets were resuspended in buffer B containing protease inhibitor cocktail (Sigma).
2.5. Assessment of ROS
To measure the release of H2O2 from mitochondria, we used Amplex® Red (Molecular Probes) assay as we previously described (Bacsi et al., 2006b). Briefly, mitochondria (100 μg/ml) were suspended in 50 μl (per well) reaction buffer and incubated with 0.25 U/ml Amplex® Red and 1 U/ml of HRP at 25°C for 30 min. The changes in fluorescence intensity were measured using a microplate reader (SpectraMass M2, Molecular Devices, Sunnyvale, CA) at 530/590 nm.
Changes in intracellular ROS levels were determined by 2',7'-dichlorodihydro-fluorescein diacetate (H2DCF-DA; Molecular Probes, Invitrogen, Carslbad, CA) as we previously described (Bacsi et al., 2006b; Boldogh et al., 2005). Changes in DCF fluorescence intensity were determined using an Flx800 microplate fluorescence reader (BioTek Instruments, Winooski, VT) at 485 nm excitation and 528 nm emission.
2.6. Assessment of V-ATPase activity
Secretory granule fraction from RBL-2H3 cells was collected as it has previously been described (Lindstedt and Kovanen, 2006). ATP hydrolysis of vacuolar membranes was assayed by using an ATP determination kit (Molecular Probes) (Ha and Snyder, 1999). Secretory granules in ATPase buffer (Lu et al., 2002) were preincubated for 15 min at room temperature in the presence and absence of bafilomycin A. The reaction was initiated by addition of ATP at a final concentration of 2 mM and luminescence was measured using an Flx800 reader by adding 90 μl of luciferase reagent to 10 μl of sample. ATP values were calculated from the standard curve based on a series of ATP concentrations.
2.7. Annexin V binding assay
Fusion of secretory granules with the cytoplasmic membrane was determined as previously described (Demo et al., 1999). Briefly, stimulated cells (7.5 × 105/dish) were washed with HBSS and stained with 25 μl of Annexin V-Phycoerythrin (PE) in the manufacturer's 1 × binding buffer (Annexin V-PE Apoptosis Detection Kit I, Becton Dickinson, San Jose, CA) for 15 min. Fluorescence was analyzed directly by a FACSCanto™ Flow Cytometer (BD Becton Dickinson). A minimum of 15,000 cells per sample was acquired.
2.8. Assessment of pH in acidic cellular compartments
Cells adhered to cloverslips were loaded with 5 μg/ml acridine orange for 10 min at 37°C and treated with AA (10 μM) or bafilomycin A (100 nM), a specific inhibitor of vacuolar ATPases. Acridine orange fluorescence was visualized by a NIKON Eclipse TE 200 UV microscope (Lewisville, TX). Images were taken with a Photometrix CoolSNAP Fx digital camera using Metamorph software (version 6.09; Universal Imaging, Downingtown, PA).
In parallel experiments, changes in acridine orange-mediated fluorescence intensity at 10 min upon treatment were assessed in an FLx800 micro plate reader (Bio-Tek Instruments, Winooski, VT) at 485 nm excitation and 528 nm emission.
2.9. Measurement of extracellular IL-4 levels
Levels of IL-4 were measured in supernatant fluids of plated cells 5 h after stimulation using ELISA CytoSet™ from BioSource International (Camarillo, CA) specific for rat IL-4 according to manufacturer's instructions.
2.10. Statistical analysis
Data were analyzed by analysis of variance with post hoc tests: Bonferroni's (samples with equal variances) and Dunnett's T3 (samples with unequal variances) with SPSS 14.0 software. Differences were considered to be statistically significant at P < 0.05.
3. Results
3.1. RWE induces the release of biogenic amines in an IgE-independent manner
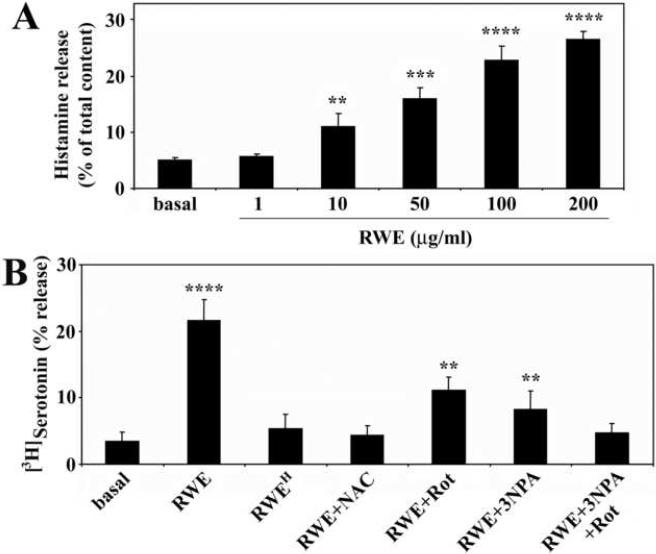
Previous studies have demonstrated that exposure to a variety of non-antigenic stimuli leads to the activation of mast cells (Frossi et al., 2004; Stassen et al., 2002; Yoshimaru et al., 2006). Here we investigated whether RWE induces degranulation of RBL-2H3 cells in the absence of sensitization with IgE antibodies. Treatment of the cells with RWE induced the release of histamine in a dose-dependent manner (Fig 1A). Administration of RWE (100 μg/ml) to the cells also increased the secretion of serotonin (Fig. 1B); however, it did not change the level of the β-hexosaminidase (data not shown). Elimination of NAD(P)H oxidase activity from RWE by heat-treatment (10 min at 72°C) (Boldogh et al., 2005), or pre-treatment of the cells with the antioxidant N-acetyl-L-cysteine (10 mM), abrogated the effect of RWE on the biogenic amine release (Fig. 1B). Unexpectedly, inhibitors of mitochondrial complex I (10 μM rotenone, an inhibitor of NADH-decylubiquinone reductase activity) or complex II (3 mM 3-nitropropionic acid, a succinate dehydrogenase inhibitor) significantly (p<0.05) decreased the release of serotonin from RWE-treated cells (Fig. 1B). Combined use of rotenone and 3-nitropropionic acid lowered the serotonin secretion to the basal level (Fig. 1B). These inhibitors block ROS generation only from mitochondria, suggesting that mtROS [but not pollen NAD(P)H oxidase-generated ROS directly] are implicated in the biogenic amine release.
Figure 1.
Ragweed pollen extract (RWE) induces release of biogenic amines from RBL-2H3 cells in the absence of IgE antibodies. Exposure to RWE triggers release of histamine in a dose-dependent manner (A). Heat-treatment of RWE (RWEH), administration of antioxidant (N-acetyl-L-cysteine; NAC) as well as mitochondrial complex I and complex II inhibitors together (rotenone; Rot, 3-nitropropionic acid; 3-NPA) abrogates the effect of RWE on serotonin release (B). Data are presented as means ±SEM of 5-6 measurements. **P< 0.01, ***P< 0.001, ****P< 0.0001.
3.2. Mitochondrial ROS induce histamine and serotonin release
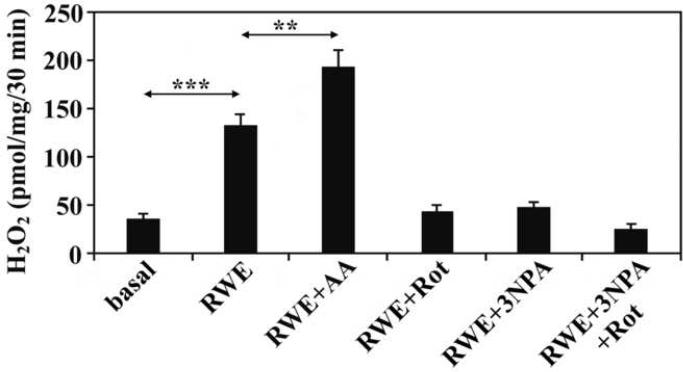
Oxidative stress has been shown to induce mitochondrial dysfunction leading to elevated mitochondrial O2.- production (Xia et al., 2007), which is rapidly converted to H2O2 by enzymatic or non-enzymatic pathways (Loschen et al., 1974). To test whether exposure of the cells to RWE leads to mitochondrial dysfunction, mitochondria were isolated from RWE-treated and mock-treated cells 60 min after exposure and changes in their H2O2 production were determined. As shown in Fig. 2, mitochondria from RWE-treated cells released significantly (p<0.05) higher amounts of H2O2 than those from mock-treated cells, suggesting that exposure to RWE enhances mtROS generation in the RBL-2H3 cells and mtROS may be involved in the release of biogenic amines.
Figure 2.
Mitochondria isolated from RWE-treated cells produce higher amount of H2O2 than those from mock-treated cells. Co-administration of complex I or complex II inhibitors (Rot as well as 3-NPA) with RWE decreases the mtROS generation; however, co-administration of a complex III inhibitor (antimycin A; AA) enhances RWE-induced mtROS. Data are presented as means ±SEM of 3 measurements. **P< 0.01, ***P< 0.001.
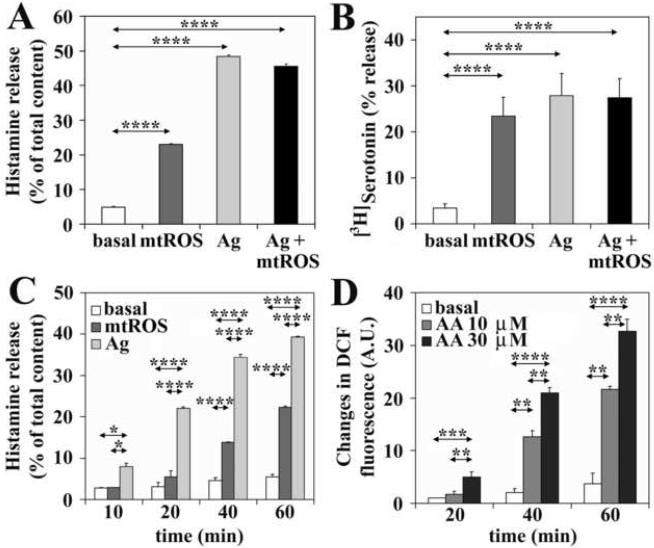
Pollen grains and their extracts contain several compounds, which can alter the function of human cells (Bagarozzi et al., 1998; Gunawan et al., 2008; Traidl-Hoffmann et al., 2002). To evaluate if increases in mtROS levels are the cause of the release of biogenic amines (histamine and serotonin), cells were treated with 10 μM of antimycin A (AA, an inhibitor of cytochrome b reoxidation), which generates mtROS by blocking electron transport at complex III resulting in mitochondrial dysfunction (Bacsi et al., 2006b; Panduri et al., 2004). Elevated mtROS levels induced a significant (p<0.05) increase in the extracellular histamine (from 4.9%±0.2 to 23.0%±0.2) and serotonin (from 3.4%±0.9 to 23.4%±4.3) levels (Fig. 3A and B). Mitochondrial ROS did not further enhance antigen-induced histamine or serotonin release from sensitized cells (Fig. 3A and B). Since cell loading with radio-labeled serotonin was shown to change the properties of secretory granules (Pihel et al., 1998), we investigated the effect of mtROS on histamine release in detail. Time course experiments showed that a statistically significant (p<0.05) increase in histamine secretion correlated well with the kinetics of increases in intracellular ROS levels (Fig. 3C and D).
Figure 3.
Mitochondrial dysfunction induces biogenic amine release. Treatment of RBL-2H3 cells with antimycin A (AA) induces histamine (A) and serotonin (B) release. Mitochondrial ROS-induced histamine release is delayed compared to that triggered by the antigen (Ag) in sensitized cells (C). Kinetics of increase in intracellular ROS levels after induction of mitochondrial dysfunction by AA treatment (D). Data are presented as means ± SEM of 7-10 measurements. *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001.
3.3. Inhibition of mtROS generation abolishes the release of histamine
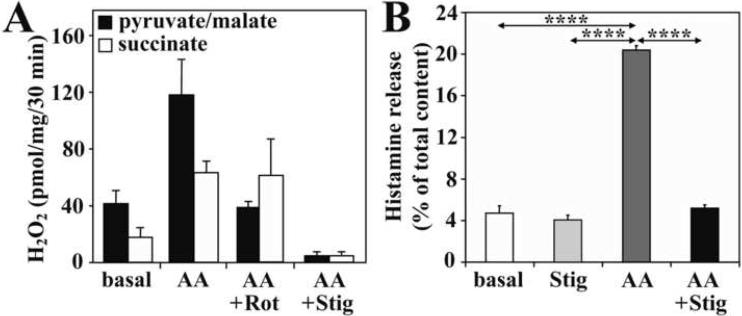
Addition of AA increased the release of H2O2 from isolated mitochondria respiring from pyruvate+malate or succinate (Fig. 4A). Administration of rotenone prevented AA-induced H2O2 production in the presence of complex I substrates (pyruvate/malate); however, it did not affect AA-induced ROS generation in mitochondria respiring on succinate, a complex II substrate (Fig. 4A). Stigmatellin, which inhibits complex I at 10 μM and the Qo site of complex III at 0.06 μM concentration (Degli Esposti et al., 1993), abolished mitochondrial H2O2 generation mediated by AA (Fig. 4A). As expected, stigmatellin (10 μM) inhibited AA-induced ROS production and subsequent histamine release (Fig. 4B).
Figure 4.
Inhibition of mtROS generation abolishes release of histamine. Rotenone (Rot) or stigmatellin (Stig) blocks electron flow to the ubiquinone pool, consequently preventing antimycin A (AA)-induced mitochondrial H2O2 production (A). Results are representative of 3 independent experiments. Stigmatellin inhibits the AA-induced histamine release (B). Data are presented as means ±SEM of 6 measurements. ****P< 0.0001.
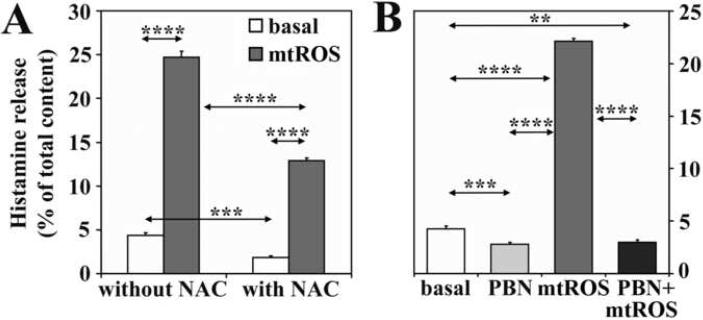
To provide additional evidence that the histamine release from RBL-2H3 cells is due to mtROS, antioxidants with distinct mechanisms of action were used. Treatment of cells with N-acetyl-L-cysteine, a glutathione precursor, significantly decreased spontaneous and mtROS-induced release of histamine (Fig. 5A). Phenyl N-tert- butylnitrone (1 mM), a direct scavenger of superoxide anion and hydroxyl radicals (Kotake, 1999), completely abolished mtROS-triggered release of histamine and decreased its basal secretion (Fig. 5B). In parallel experiments, we confirmed that these inhibitors decreased intracellular ROS levels to or below basal levels (data not shown). These data together suggest that inhibition of mtROS generation or overall decrease in intracellular ROS levels was sufficient to block histamine release.
Figure 5.
Antioxidants decrease the mtROS-induced histamine release. N-acetyl-L-cysteine (NAC)(A) or phenyl N-tert-butylnitrone (PBN)(B) decrease both mtROS-triggered and basal release of histamine. Mitochondrial dysfunction was induced by AA treatment (10 μM). Data are presented as means ±SEM of 6-9 measurements. **P< 0.01, ***P< 0.001, ****P< 0.0001.
3.4. Mitochondrial ROS-induced histamine release does not require complete exocytosis
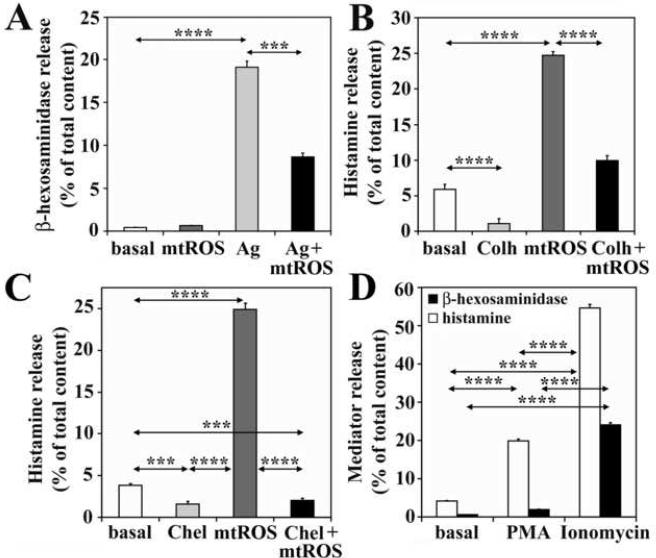
In antigen-exposed, sensitized cells histamine release is mediated via a series of events including migration, fusion, and release of granule/vesicle contents into the cell exterior (Nishida et al., 2005). Since β-hexosaminidase was not released from cells with mitochondrial dysfunction (Fig. 6A), it suggests that migration of secretory granules and exocytosis may not required for histamine liberation. When cells were treated with colchicine, an inhibitor of granule motility (Smith et al., 2003) (100 μM, optimal concentration was determined in preliminary studies), mtROS-induced release of histamine was decreased (from 24.7%±0.6 to 15.9%±0.7). These data suggest that granule migration to the proximity of cytoplasmic membrane is required for histamine release (Fig. 6B).
Figure 6.
Protein kinase C (PKC)-dependent migration of secretory granules is a requirement for mtROS-induced histamine release. Mitochondrial dysfunction does not increase β-hexosaminidase secretion from non-sensitized cells and it inhibits release of this enzyme from antigen (Ag)-treated sensitized cells (A). Colchicine (Colh), an inhibitor of the motility of secretory granules, decreases both basal and mtROS-induced release of histamine (B). Chelerythrine chloride (Chel), an inhibitor of PKC, decreases spontaneous and mtROS-induced histamine release (C). A PKC activator, 4 beta-phorbol 12-myristate 13-acetate (PMA), imitates the effects of mtROS (D). Mitochondrial dysfunction was induced by AA treatment (10 μM). ***P< 0.001, ****P< 0.0001.
PKC δ regulates movement of endosomes, lysosomes and secretory vesicles (Chen et al., 2004; Llado et al., 2004; Ma et al., 2008). Because mtROS-induced histamine release required vesicles migration (Fig. 6B), we investigated a potential involvement of PKC. Treatment of cells with chelerythrine chloride (5 μM), a specific, but not isoform-selective inhibitor of PKC, decreased mtROS-induced histamine release indicating the participation of PKC in this process (Fig. 6C). Furthermore, PMA (100 nM), a PKC activator (Wrede et al., 2003), significantly increased histamine release, but did not influence the β-hexosaminidase secretion (Fig. 6D). In control, ionomycin induced release of both histamine and β-hexosaminidase (Fig. 6D). Together these data indicate that PKC-dependent migration of secretory granules is a requirement for mtROS-induced histamine release and they are in line with oxidative stress-mediated activation of PKC δ via tyrosine phosphorylation (Konishi et al., 1997).
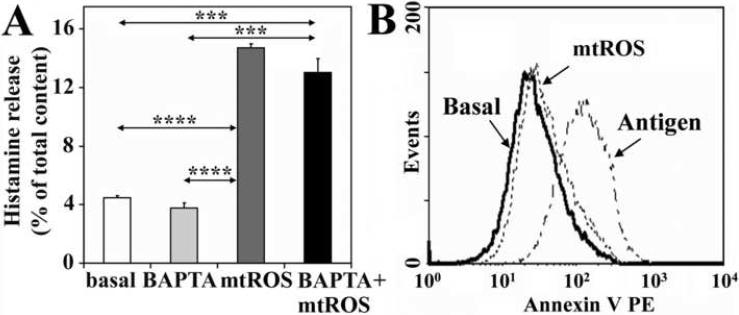
To sort out whether PKC-dependent histamine liberation requires free cytoplasmic Ca2+ ([Ca2+]i), we investigated effects of Ca2+ chelators. Administration of EGTA (1 mM, data not shown) or BAPTA [1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetrakis(acetoxymethyl ester); 10 μM for 30 min], chelators of extracellular Ca2+ and [Ca2+]i, respectively resulted in insignificant changes in histamine release (Fig. 7A). These results indicate that [Ca2+]i is not an absolute requirement for mtROS-mediated release of biogenic amines in RBL-2H3 cells, and also support the involvement of PKC δ, a Ca2+-independent isoform of PKC family (Pernas-Sueiras et al., 2006), in these processes.
Figure 7.
Mitochondrial dysfunction-induced histamine release does not require intracellular free Ca2+ and complete exocytosis. Administration of BAPTA, an intracellular Ca2+ chelator, causes only a slight decrease in the mtROS-mediated release of histamine (A). Low levels of Annexin V binding indicate that mtROS-induced release of histamine does not require the full fusion of secretory granules with the plasma membrane (B). Antigen-induced exocytosis was used as positive control. Mitochondrial dysfunction was induced by AA treatment (10 μM). Data are presented as means ±SEM of 8-10 measurements. ***P< 0.001, ****P< 0.0001.
Appearance of phosphatidylserine at the outer side of the cytoplasmic membrane is a hallmark of exocytosis (Demo et al., 1999). To test the requirement of exocytosis for histamine release by mtROS, we analyzed the appearance of phosphatidylserine by Annexin V assay. In cells with mitochondrial dysfunction, there was only insignificant change in Annexin V binding, suggesting the lack of complete exocytosis (Fig. 7B). These results are consistent with non-degranulative secretion of biogenic amines as reported previously (Uvnas, 1991). In control, intense binding of Annexin V to the cell surface after addition of antigen to IgE-sensitized cells indicated exocytosis of granules (Fig. 8A). These results imply that mtROS-generated signals induce granule migration, but are not sufficient for release of the granules' total content.
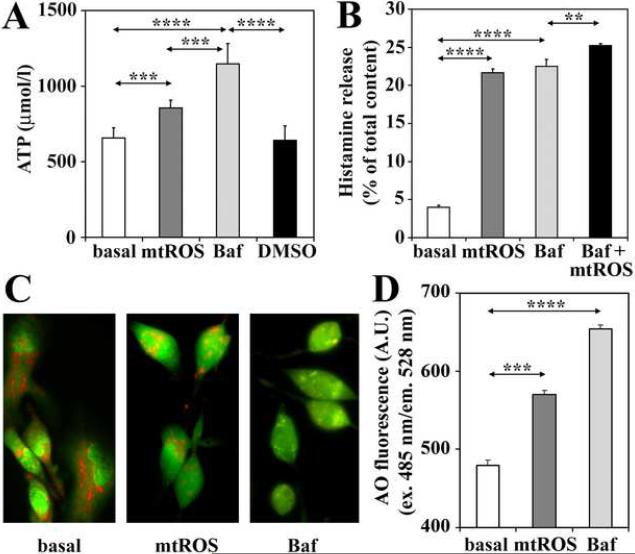
Figure 8.
Mitochondrial ROS induce histamine release via inhibition of vacuolar H+-ATPases. Secretory granules from cells with mitochondrial dysfunction utilize significantly lower amounts of externally added ATP, compared to those from untreated control cells (A). Bafilomycin A (Baf), a specific inhibitor of vacuolar-type H+-ATPases, induces histamine release from the RBL-2H3 cells (B). Cells exhibiting mitochondrial dysfunction show increases in the pH of cellular acidic compartments, which is visualized by fluorescence microscopy (C), and assessed by means of fluorimetry using acridine orange (AO) (D). In control experiments Baf was used. Mitochondrial dysfunction was induced by AA treatment (10 μM). Data are presented as means ±SEM of 6-8 measurements. **P< 0.01; ***P< 0.001, ****P< 0.0001.
Since mtROS did not induce β-hexosaminidase release from RBL-2H3 cells, we investigated whether mtROS can alter the β-hexosaminidase release from antigen-treated sensitized cells. Unexpectedly, mtROS inhibited β-hexosaminidase release from antigen-treated sensitized cells; thus, its level in the supernatant was decreased by 50% (Fig. 6A). Although this phenomenon was also observed in RWE-treated RBL-2H3 cells and warrants further investigations, these results suggest that mitochondrial dysfunction (mtROS) inhibits events required for fusion of vesicles with cytoplasmic membrane, which could be a possible explanation for the lack of β-hexosaminidase release.
3.5. Vacuolar H+-ATPase is inhibited by mtROS
Release of histamine and serotonin from secretory granules thought to be regulated by changes in intravesicular pH (Williams and Webb, 2000). Intraluminal pH of granules is maintained by vacuolar H+-ATPase (V-ATPase) activities (Schumacher, 2006). Therefore, we assessed V-ATPase activity by determining ATP consumption of isolated secretory granules. Secretory granules isolated from cells with mitochondrial dysfunction, utilized significantly lower amounts of externally added ATP, compared to those from untreated control cells, so we found higher levels of unutilized ATP in the reaction mixture (Fig. 8A). Treatment of cells with bafilomycin A (100 nM), a specific inhibitor of V-ATPases (Camacho et al., 2008), resulted in similar decrease in ATP utilization (Fig. 8A). Suppression of ATP utilization by mtROS is consistent with H2O2-mediated inhibition of V-ATPases (Wang and Floor, 1998). In control, treatment of cells with bafilomycin A increased histamine release from the RBL-2H3 cells, confirming that a decrease in V-ATPase activity results in secretion of biogenic amines (Fig. 8B). In support, V-ATPase-dependent acidification of secretory granules was shown by microscopic imaging (Fig. 8C) and fluorimetrically (Fig. 8D) using acridine orange, a dye that accumulates in secretory granules and other cellular acidic compartments (Williams and Webb, 2000). In acridine orange-loaded, untreated cells, secretory granules emitted red fluorescence (Fig. 8C), while green fluorescence was observed in cells with mitochondrial dysfunction or treated with bafilomycin A, indicating an increase in intraluminal pH (Fig. 8C and D). Together, these data indicate that inhibition of V-ATPase activity, consequently an increase in pH of secretory vesicles is a possible cause of mtROS-induced release of biogenic amines from mast cells.
3.6. Mitochondrial ROS do not induce de novo synthesis of enhance IgE-mediated IL-4 production
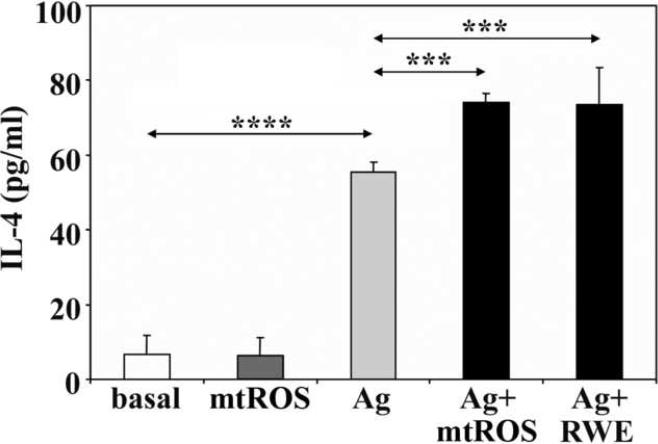
Antigenic stimuli result in release of preformed low and high molecular weight inflammatory mediators, as well as de novo synthesis of cytokines in mast cells (Rivera and Gilfillan, 2006). Next, we investigated whether mtROS affect the production of IL- 4, which is characterized as one of the key cytokines of allergic responses (Corry and Kheradmand, 2002). As shown in Figure 9, mtROS alone did not enhance synthesis of IL-4 in RBL-2H3 cells; however, they further increased IL-4 production of antigen-treated sensitized cells. When RWE was administered to the antigen-treated, sensitized cells, it also significantly enhanced the level of IL-4 in the culture supernatant (Fig. 9). These data suggest that mtROS are synergistic with FcεRI-induced cell activation signals for IL-4 production and release. These data are in line with previous findings showing that environmental oxidative stress- and FcεRI-mediated signals are additive, enhancing IL-4 production from mast cells (Frossi et al., 2007).
Figure 9.
Mitochondrial dysfunction enhances IgE-mediated IL-4 production. Mitochondrial dysfunction was induced by AA or RWE. Data are presented as means ± SEM of 5 measurements. ***P< 0.001, ****P< 0.0001.
4. Discussion
Mitochondria are involved in regulation of various cellular functions as they store/release/generate signaling mediators including Ca2+ and ROS. These mediators are required in differentiation and function of various immune cells participating in innate and adaptive responses (Del Prete et al., 2008; Hunt et al., 1991). In this study, we show that increased production of mtROS, induced by treatment with RWE, triggers secretion of biogenic amines, i.e. histamine and serotonin, but not β-hexosaminidase from RBL-2H3 cells, independently from FcεRI-mediated signals. This phenomenon occurs in the absence of complete exocytosis of secretory granules and without changes in intracellular Ca2+ levels, but it is exclusively associated with activation of protein kinase C-δ and inhibition of vacuolar H+-ATPase. We also show that mtROS significantly increase FcεRI-mediated IL-4 production. These findings provide evidence for a relationship between mitochondrial dysfunction and antigen dependent and independent activation of mast cells. Thus, pharmacological protection from mitochondrial dysfunction may have clinical significance in prevention and treatment of allergic diseases.
In our studies, we utilized extract from ragweed pollen, which known to cause severe allergic symptoms (Hunt et al., 2002). Ragweed pollen-induced clinical symptoms are highly dependent on ROS production by pollen grains' intrinsic NADPH oxidases, which are activated after hydration on mucosal membranes of airways or conjunctiva (Bacsi et al., 2005; Boldogh et al., 2005), raising the possibility that these ROS are implicated in mast cell degranulation. However, our results showing that mitochondrial respiratory chain inhibitors (rotenone and 3NPA), which have no effect on pollen grains' NADPH oxidases, prevented RWE-induced release of allergic mediators were unexpected. These results strongly suggest that ROS generated by pollen grains' NADPH oxidase have no direct implication in degranulation processes, but induce damage to mitochondrial macromolecules (Boldogh et al., unpublished data), thereby dysfunction and elevated release of ROS from mitochondria. Indeed, mitochondria isolated from RWE-treated cells released nearly 3-fold higher amount of H2O2 (the major product of O2.- dismutation (Turrens, 2003)) compared to control mitochondria. Using site-specific inhibitors we identified the complex III-Qi as site of ROS generation in the respiratory chain. Indeed, previous studies have been identified complex III as one of the main sites of O2.- production (Raha and Robinson, 2000; St-Pierre et al., 2002).
Antimycin A, by binding to cytochrome b, inhibits the electron flow from semiquinone to ubiquinone, consequently increasing the steady state concentration of semiquinone, and resulting in electron escape from complex III Qi site (Turrens, 2003). Stigmatellin does not allow entry of electrons into complex III at the Qo site and thus it abolishes the AA (and RWE)-induced ROS production. Because complex III-Qi is the site of ROS generation in RWE treated cells and exposure to RWE may trigger a myriad of cellular effects, we utilized AA, a complex III inhibitor, to study effects of mtROS on release of the content of secretory vesicles.
Our data demonstrated the release of biogenic amines by mtROS (AA or RWE exposure). Inhibitors of mitochondrial H2O2 release (and antioxidants NAC or PBN) significantly decreased histamine/serotonin release, confirming that both AA- and RWE-induced mast cell activations are specific to mtROS. These results are in line with previous findings showing that H2O2 induce histamine release from mast cells (Menon et al., 1989; Ohmori et al., 1979). Interestingly, there was no β-hexosaminidase release detectable by mtROS (after AA or RWE exposure) from RBL-2H3 cells. However, these results are not surprising because oxidative stress did not trigger release of β-hexosaminidase from either RBL-2H3 cells (Bachelet et al., 2002) or murine bone marrow-derived mast cells (Mortaz et al., 2008). Similarly, organophosphate compounds induce histamine but not β-hexosaminidase release from RBL-1 cells after 1 h of exposure (Xiong and Rodgers, 1997). Worm (e.g. Trichinella) antigens induce the release of histamine but not β-hexosaminidase from sensitized rat mast cells (Yepez-Mulia et al., 2009). Although our data suggest that mtROS inhibit complete exocytosis required for β-hexosaminidase release, an alternative explanation for the lack of its secretion could be the existence of various compartments storing different allergic mediators (Puri and Roche, 2008; Rajotte et al., 2003; Smith et al., 2003).
Upon activation of RBL-2H3 cells, secretory granules migrate to cytoplasmic membrane, the sites of exocytosis, and this event is microtubule-dependent and can be inhibited by colchicine (Smith et al., 2003). Release of histamine/serotonin by mtROS required colchicine-sensitive movement of secretory granules, suggesting microtubule-dependent release of these mediators. During typical degranulation process, migration of secretory granules is followed by exocytotic events resulting in release of vesicles content into extracellular milieu. However, in mtROS activated RBL-2H3 cells full exocytosis may not occur, as β-hexosaminidase was not released. It is possible that granules may fuse with the plasma membrane transiently (Hide et al., 1993; Obermuller et al., 2005; Williams and Webb, 2000) or the size of the fusion pores between vesicle and plasma membrane (Alvarez de Toledo et al., 1993; Fernandez-Chacon and Alvarez de Toledo, 1995) does not allow release of β-hexosaminidase.
As activation of PKC δ is required for oxidant-induced regulation of microtubule cytoskeleton (Banan et al., 2002), granule movement and exocytosis (Ma et al., 2008), we showed that chelerythrine chloride, a PKC inhibitor decreased mtROS-induced histamine release, while depletion of intracellular Ca2+ had no effect. Chelerythrine chloride is a specific but not a PKC isoform-selective inhibitor; however, only PKC δ and PKC θ from the novel subfamily have been identified as regulators of antigen-induced mast cell degranulation (Cho et al., 2004; Liu et al., 2001; Parravicini et al., 2002). Presently there are no data demonstrating any effects of oxidative stress on PKC θ activity; therefore, PKC δ could be the key kinase in regulation of microtubule-dependent granule motility. In support, PKC δ is a Ca2+-independent isoform and it can be activated by PMA (Pernas-Sueiras et al., 2006) as well as by ROS (Inoue et al., 2008), suggesting that mtROS regulate activity of PKC-δ in microtubule-dependent migration of secretory granules for release of biogenic amines from non-sensitized mast cells.
Biogenic amines are low molecular weight, positively charged mediators retained by low intravacuolar pH (Williams and Webb, 2000), and an increase in pH may be sufficient for their liberation as suggested by bafilomycin A-mediated release of histamine. Bafilomycin A is specific inhibitor of vacuolar H+-ATPase (V-ATPase) (Camacho et al., 2008). V-ATPases function exclusively as ATP-dependent proton pumps, and the proton-motive force generated by them (in organelles' membranes including secretory vesicles) maintains pH gradient toward the cytosol (Nelson et al., 2000) and it is utilized as a driving force for transport processes (Beyenbach and Wieczorek, 2006; Saroussi and Nelson, 2008). Changes in pH gradient due to altered activity of V-ATPases in the vesicles' membrane thought to be responsible for the accumulation and release of amines (Camacho et al., 2008). Correspondingly, a decrease in the V-ATPases' function causes granule alkalization leading to release of serotonin from cells (Williams and Webb, 2000). Alkalization of secretory vesicles shown by acridine orange fluorescence suggests inhibition of the V-ATPase in mtROS producing cells. The fact that secretory vesicles isolated from these cells utilized lower amount of ATP than those from control cells strongly suggests the inhibition of V-ATPase activity by mtROS. These observations are in line with previous data showing inhibition of the V-ATPase by H2O2 due to oxidation of reactive cysteine sulfhydryl group in the ATP binding site (Wang and Floor, 1998).
Mast cell activation leads to increased synthesis of various cytokines (Metcalfe et al., 1997) and oxidative stress exacerbates their production (Frossi et al., 2007). Thus, mtROS-mediated augmentation of IL-4 production from antigen-activated cells is not particularly surprising. H2O2 is a well-characterized signaling molecule, which influence mast cell behavior (Guerin-Marchand et al., 2001; Matsui et al., 2000). Narrow concentration range of externally added H2O2 (5-50 nM) alone was sufficient to trigger IL-4 release from RBL-2H3 cells (Frossi et al., 2003), while in our hand mtROS (H2O2) did not do so. This phenomenon could be explained simply by concentration or by the site of H2O2 action. Although, it is highly speculative, but it is possible that externally added H2O2 could trigger gene transcription via membrane receptor(s) and signals amplified by second messengers, while mtROS act directly on redox-sensitive kinases, phophatases, thereby signal intensity could be lower. Together these results raise the possibility that mitochondrial dysfunction is directly implicated in early events of allergic responses via inducing release of biogenic amines and it is indirectly involved in Th2 differentiation, B cell activation, and IgE production by its synergistic effect on IL-4 production.
In conclusion, we show that mitochondrial dysfunction triggers release of histamine/serotonin from non-sensitized mast cells by inducing PKC δ- and microtubule-dependent motility of secretory granules and inhibiting activity of vacuolar H+-ATPase independently from intracellular Ca2+ levels. The synergistic effect of mtROS on antigen-induced IL-4 production warrants further investigation and suggests that mitochondrial dysfunction may also have a role in the development of adaptive immune responses. These findings are particularly important because mitochondrial dysfunction was induced by extract of ragweed pollen, one of the most potent inducers of severe allergic symptoms. Thus, therapeutic strategies that decrease mitochondrial dysfunction will offer benefits in intervention and prevention of allergic symptoms.
Acknowledgements
This work was supported by NIAID, P01 AI062885-01 (I.B., S.S., T.H), NIH HL071163 (S.S., I.B), NIEHS Center Grant, EOS 006677 and the Hungarian Scientific Research Fund (73347).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfonso A, Cabado AG, Vieytes MR, Botana LM. Calcium-pH crosstalks in rat mast cells: cytosolic alkalinization, but not intracellular calcium release, is a sufficient signal for degranulation. Br. J. Pharmacol. 2000;130:1809–1816. doi: 10.1038/sj.bjp.0703490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Bachelet M, Marchand F, Souil E, Francois D, Mariethoz E, Weyer A, Polla BS. Expression and localization of heat shock proteins in rat basophilic leukemia cells: differential modulation by degranulation, thermal or oxidative stress. Allergy. 2002;57:791–797. doi: 10.1034/j.1398-9995.2002.23665.x. [DOI] [PubMed] [Google Scholar]
- Bacsi A, Choudhury BK, Dharajiya N, Sur S, Boldogh I. Subpollen particles: carriers of allergenic proteins and oxidases. J. Allergy Clin. Immunol. 2006a;118:844–850. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacsi A, Dharajiya N, Choudhury BK, Sur S, Boldogh I. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. J. Allergy Clin. Immunol. 2005;116:836–843. doi: 10.1016/j.jaci.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacsi A, Woodberry M, Widger W, Papaconstantinou J, Mitra S, Peterson JW, Boldogh I. Localization of superoxide anion production to mitochondrial electron transport chain in 3-NPA-treated cells. Mitochondrion. 2006b;6:235–244. doi: 10.1016/j.mito.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagarozzi DA, Jr., Potempa J, Travis J. Purification and characterization of an arginine-specific peptidase from ragweed (Ambrosia artemisiifolia) pollen. Am. J. Respir. Cell Mol. Biol. 1998;18:363–369. doi: 10.1165/ajrcmb.18.3.2825. [DOI] [PubMed] [Google Scholar]
- Banan A, Fields JZ, Farhadi A, Talmage DA, Zhang L, Keshavarzian A. Activation of delta-isoform of protein kinase C is required for oxidant-induced disruption of both the microtubule cytoskeleton and permeability barrier of intestinal epithelia. J. Pharmacol. Exp. Ther. 2002;303:17–28. doi: 10.1124/jpet.102.037218. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 2006;209:577–589. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol. Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Camacho M, Machado JD, Alvarez J, Borges R. Intravesicular calcium release mediates the motion and exocytosis of secretory organelles: a study with adrenal chromaffin cells. J. Biol. Chem. 2008;283:22383–22389. doi: 10.1074/jbc.M800552200. [DOI] [PubMed] [Google Scholar]
- Chen YW, Lang ML, Wade WF. Protein kinase C-alpha and -delta are required for FcalphaR (CD89) trafficking to MHC class II compartments and FcalphaR-mediated antigen presentation. Traffic. 2004;5:577–594. doi: 10.1111/j.1600-0854.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Cho SH, Woo CH, Yoon SB, Kim JH. Protein kinase Cdelta functions downstream of Ca2+ mobilization in FcepsilonRI signaling to degranulation in mast cells. J. Allergy Clin. Immunol. 2004;114:1085–1092. doi: 10.1016/j.jaci.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Corry DB, Kheradmand F. Biology and therapeutic potential of the interleukin-4/interleukin-13 signaling pathway in asthma. Am. J. Respir. Med. 2002;1:185–193. doi: 10.1007/BF03256608. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M, Ghelli A, Crimi M, Estornell E, Fato R, Lenaz G. Complex I and complex III of mitochondria have common inhibitors acting as ubiquinone antagonists. Biochem. Biophys. Res. Commun. 1993;190:1090–1096. doi: 10.1006/bbrc.1993.1161. [DOI] [PubMed] [Google Scholar]
- Del Prete A, Zaccagnino P, Di Paola M, Saltarella M, Oliveros Celis C, Nico B, Santoro G, Lorusso M. Role of mitochondria and reactive oxygen species in dendritic cell differentiation and functions. Free Radic. Biol. Med. 2008;44:1443–1451. doi: 10.1016/j.freeradbiomed.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Demo SD, Masuda E, Rossi AB, Throndset BT, Gerard AL, Chan EH, Armstrong RJ, Fox BP, Lorens JB, Payan DG, Scheller RH, Fisher JM. Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-V binding assay. Cytometry. 1999;36:340–348. doi: 10.1002/(sici)1097-0320(19990801)36:4<340::aid-cyto9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Alvarez de Toledo G. Cytosolic calcium facilitates release of secretory products after exocytotic vesicle fusion. FEBS Lett. 1995;363:221–225. doi: 10.1016/0014-5793(95)00319-5. [DOI] [PubMed] [Google Scholar]
- Frossi B, De Carli M, Daniel KC, Rivera J, Pucillo C. Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur. J. Immunol. 2003;33:2168–2177. doi: 10.1002/eji.200323995. [DOI] [PubMed] [Google Scholar]
- Frossi B, De Carli M, Pucillo C. The mast cell: an antenna of the microenvironment that directs the immune response. J. Leukoc. Biol. 2004;75:579–585. doi: 10.1189/jlb.0603275. [DOI] [PubMed] [Google Scholar]
- Frossi B, Rivera J, Hirsch E, Pucillo C. Selective activation of Fyn/PI3K and p38 MAPK regulates IL-4 production in BMMC under nontoxic stress condition. J. Immunol. 2007;178:2549–2555. doi: 10.4049/jimmunol.178.4.2549. [DOI] [PubMed] [Google Scholar]
- Guerin-Marchand C, Senechal H, Pelletier C, Fohrer H, Olivier R, David B, Berthon B, Blank U. H2O2 impairs inflammatory mediator release from immunologically stimulated RBL-2H3 cells through a redox-sensitive, calcium-dependent mechanism. Inflamm. Res. 2001;50:341–349. doi: 10.1007/PL00000254. [DOI] [PubMed] [Google Scholar]
- Gunawan H, Takai T, Kamijo S, Wang XL, Ikeda S, Okumura K, Ogawa H. Characterization of proteases, proteins, and eicosanoid-like substances in soluble extracts from allergenic pollen grains. Int. Arch. Allergy Immunol. 2008;147:276–288. doi: 10.1159/000144035. [DOI] [PubMed] [Google Scholar]
- Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hide I, Bennett JP, Pizzey A, Boonen G, Bar-Sagi D, Gomperts BD, Tatham PE. Degranulation of individual mast cells in response to Ca2+ and guanine nucleotides: an all-or-none event. J. Cell. Biol. 1993;123:585–593. doi: 10.1083/jcb.123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LW, Gleich GJ, Kita H, Weiler DA, Schroeder DR, Vuk-Pavlovic Z, Sur S. Removal of bronchoalveolar cells augments the late eosinophilic response to segmental allergen challenge. Clin. Exp. Allergy. 2002;32:210–216. doi: 10.1046/j.1365-2222.2002.01228.x. [DOI] [PubMed] [Google Scholar]
- Hunt NH, Cook EP, Fragonas JC. Interference with oxidative processes inhibits proliferation of human peripheral blood lymphocytes and murine B-lymphocytes. Int. J. Immunopharmacol. 1991;13:1019–1026. doi: 10.1016/0192-0561(91)90056-d. [DOI] [PubMed] [Google Scholar]
- Inoue T, Suzuki Y, Yoshimaru T, Ra C. Reactive oxygen species produced up- or downstream of calcium influx regulate proinflammatory mediator release from mast cells: role of NADPH oxidase and mitochondria. Biochim. Biophys. Acta. 2008;1783:789–802. doi: 10.1016/j.bbamcr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Isersky C, Taurog JD, Poy G, Metzger H. Triggering of cultured neoplastic mast cells by antibodies to the receptor for IgE. J. Immunol. 1978;121:549–558. [PubMed] [Google Scholar]
- Johansen T. Energy metabolism in rat mast cells in relation to histamine secretion. Pharmacol. Toxicol. 1987;61(Suppl 2):1–20. doi: 10.1111/j.1600-0773.1987.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Kojima T, Obata K, Mukai K, Sato S, Takai T, Minegishi Y, Karasuyama H. Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J. Immunol. 2007;179:7093–100. doi: 10.4049/jimmunol.179.10.7093. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. U. S. A. 1997;94:11233–7. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake Y. Pharmacologic properties of phenyl N-tert-butylnitrone. Antioxid. Redox Signal. 1999;1:481–499. doi: 10.1089/ars.1999.1.4-481. [DOI] [PubMed] [Google Scholar]
- Lindstedt KA, Kovanen PT. Isolation of mast cell granules. Curr. Protoc. Cell Biol. 2006 doi: 10.1002/0471143030.cb0316s29. Chapter 3, Unit 3.16. [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am. J. Respir. Crit. Care Med. 1998;158:176–181. doi: 10.1164/ajrccm.158.1.9710014. [DOI] [PubMed] [Google Scholar]
- Liu Y, Graham C, Parravicini V, Brown MJ, Rivera J, Shaw S. Protein kinase C theta is expressed in mast cells and is functionally involved in Fcepsilon receptor I signaling. J. Leukoc. Biol. 2001;69:831–840. [PubMed] [Google Scholar]
- Llado A, Tebar F, Calvo M, Moreto J, Sorkin A, Enrich C. Protein kinaseCdelta-calmodulin crosstalk regulates epidermal growth factor receptor exit from early endosomes. Mol. Biol. Cell. 2004;15:4877–4891. doi: 10.1091/mbc.E04-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J. Allergy Clin. Immunol. 2003;111:923–932. quiz 933. [PubMed] [Google Scholar]
- Loschen G, Azzi A, Richter C, Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Lu M, Vergara S, Zhang L, Holliday LS, Aris J, Gluck SL. The amino-terminal domain of the E subunit of vacuolar H(+)-ATPase (V-ATPase) interacts with the H subunit and is required for V-ATPase function. J. Biol. Chem. 2002;277:38409–38415. doi: 10.1074/jbc.M203521200. [DOI] [PubMed] [Google Scholar]
- Ma JS, Haydar TF, Radoja S. Protein kinase C delta localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J. Immunol. 2008;181:4716–4722. doi: 10.4049/jimmunol.181.7.4716. [DOI] [PubMed] [Google Scholar]
- Matsui T, Suzuki Y, Yamashita K, Yoshimaru T, Suzuki-Karasaki M, Hayakawa S, Yamaki M, Shimizu K. Diphenyleneiodonium prevents reactive oxygen species generation, tyrosine phosphorylation, and histamine release in RBL-2H3 mast cells. Biochem. Biophys. Res. Commun. 2000;276:742–748. doi: 10.1006/bbrc.2000.3545. [DOI] [PubMed] [Google Scholar]
- Menon IA, Shirwadkar S, Ranadive NS. Nature of the oxygen species generated by xanthine oxidase involved in secretory histamine release from mast cells. Biochem. Cell Biol. 1989;67:397–403. doi: 10.1139/o89-064. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Mortaz E, Redegeld FA, Sarir H, Karimi K, Raats D, Nijkamp FP, Folkerts G. Cigarette smoke stimulates the production of chemokines in mast cells. J. Leukoc. Biol. 2008;83:575–580. doi: 10.1189/jlb.0907625. [DOI] [PubMed] [Google Scholar]
- Munitz A, Piliponsky AM, Levi-Schaffer F. IgE-Independent Activation of Human Mast Cells Indicates their Role in the Late Phase Reaction of Allergic Inflammation. Cell Tissue Bank. 2003;4:25–28. doi: 10.1023/A:1026307812980. [DOI] [PubMed] [Google Scholar]
- Nagata M. Inflammatory cells and oxygen radicals. Curr. Drug Targets Inflamm. Allergy. 2005;4:503–504. doi: 10.2174/1568010054526322. [DOI] [PubMed] [Google Scholar]
- Nelson N, Perzov N, Cohen A, Hagai K, Padler V, Nelson H. The cellular biology of proton-motive force generation by V-ATPases. J. Exp. Biol. 2000;203:89–95. doi: 10.1242/jeb.203.1.89. [DOI] [PubMed] [Google Scholar]
- Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, Nishizumi H, Kitamura D, Goitsuka R, Geha RS, Yamamoto T, Yagi T, Hirano T. Fc{epsilon}RI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J. Cell Biol. 2005;170:115–126. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermuller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J. Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- Ohmori H, Komoriya K, Azuma A, Kurozumi S, Hashimoto Y. Xanthine oxidase-induced histamine release from isolated rat peritoneal mast cells: involvement of hydrogen peroxide. Biochem. Pharmacol. 1979;28:333–334. doi: 10.1016/0006-2952(79)90524-0. [DOI] [PubMed] [Google Scholar]
- Panduri V, Weitzman SA, Chandel NS, Kamp DW. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L1220–1227. doi: 10.1152/ajplung.00371.2003. [DOI] [PubMed] [Google Scholar]
- Park SK, Beaven MA. Mechanism of upregulation of the inhibitory regulator, src-like adaptor protein (SLAP), by glucocorticoids in mast cells. Mol. Immunol. 2009;46:492–497. doi: 10.1016/j.molimm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O'Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- Pernas-Sueiras O, Alfonso A, Vieytes MR, Botana LM. PKC and cAMP positively modulate alkaline-induced exocytosis in the human mast cell line HMC-1. J. Cell. Biochem. 2006;99:1651–1663. doi: 10.1002/jcb.21009. [DOI] [PubMed] [Google Scholar]
- Pihel K, Hsieh S, Jorgenson JW, Wightman RM. Quantal corelease of histamine and 5-hydroxytryptamine from mast cells and the effects of prior incubation. Biochemistry. 1998;37:1046–1052. doi: 10.1021/bi9714868. [DOI] [PubMed] [Google Scholar]
- Puri N, Roche PA. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2580–2585. doi: 10.1073/pnas.0707854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raby BA, Klanderman B, Murphy A, Mazza S, Camargo CA, Jr., Silverman EK, Weiss ST. A common mitochondrial haplogroup is associated with elevated total serum IgE levels. J. Allergy Clin. Immunol. 2007;120:351–358. doi: 10.1016/j.jaci.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- Rajotte D, Stearns CD, Kabcenell AK. Isolation of mast cell secretory lysosomes using flow cytometry. Cytometry A. 2003;55:94–101. doi: 10.1002/cyto.a.10065. [DOI] [PubMed] [Google Scholar]
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- Saroussi S, Nelson N. Vacuolar H(+)-ATPase-an enzyme for all seasons. Pflugers Arch. 2009;457:581–587. doi: 10.1007/s00424-008-0458-9. [DOI] [PubMed] [Google Scholar]
- Schumacher K. Endomembrane proton pumps: connecting membrane and vesicle transport. Curr. Opin. Plant Biol. 2006;9:595–600. doi: 10.1016/j.pbi.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Seldin DC, Adelman S, Austen KF, Stevens RL, Hein A, Caulfield JP, Woodbury RG. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc. Natl. Acad. Sci. U. S. A. 1985;82:3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Pfeiffer JR, Zhang J, Martinez AM, Griffiths GM, Wilson BS. Microtubule-dependent transport of secretory vesicles in RBL-2H3 cells. Traffic. 2003;4:302–312. doi: 10.1034/j.1600-0854.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- Stassen M, Hultner L, Schmitt E. Classical and alternative pathways of mast cell activation. Crit. Rev. Immunol. 2002;22:115–140. [PubMed] [Google Scholar]
- Suzuki Y, Yoshimaru T, Inoue T, Ra C. Mitochondrial Ca2+ flux is a critical determinant of the Ca2+ dependence of mast cell degranulation. J. Leukoc. Biol. 2006;79:508–518. doi: 10.1189/jlb.0705412. [DOI] [PubMed] [Google Scholar]
- Swindle EJ, Hunt JA, Coleman JW. A comparison of reactive oxygen species generation by rat peritoneal macrophages and mast cells using the highly sensitive real-time chemiluminescent probe pholasin: inhibition of antigen-induced mast cell degranulation by macrophage-derived hydrogen peroxide. J. Immunol. 2002;169:5866–5873. doi: 10.4049/jimmunol.169.10.5866. [DOI] [PubMed] [Google Scholar]
- Traidl-Hoffmann C, Kasche A, Jakob T, Huger M, Plotz S, Feussner I, Ring J, Behrendt H. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J. Allergy Clin. Immunol. 2002;109:831–838. doi: 10.1067/mai.2002.124655. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas B. The molecular mechanism of nondegranulative release of biogenic amines. J. Physiol. Pharmacol. 1991;42:211–219. [PubMed] [Google Scholar]
- Wang Y, Floor E. Hydrogen peroxide inhibits the vacuolar H+-ATPase in brain synaptic vesicles at micromolar concentrations. J. Neurochem. 1998;70:646–652. doi: 10.1046/j.1471-4159.1998.70020646.x. [DOI] [PubMed] [Google Scholar]
- Williams RM, Webb WW. Single granule pH cycling in antigen-induced mast cell secretion. J. Cell Sci. 2000;113(Pt 21):3839–3850. doi: 10.1242/jcs.113.21.3839. [DOI] [PubMed] [Google Scholar]
- Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Fatty acid and phorbol ester-mediated interference of mitogenic signaling via novel protein kinase C isoforms in pancreatic beta-cells (INS-1) J. Mol. Endocrinol. 2003;30:271–286. doi: 10.1677/jme.0.0300271. [DOI] [PubMed] [Google Scholar]
- Xia T, Kovochich M, Nel AE. Impairment of mitochondrial function by particulate matter (PM) and their toxic components: implications for PM-induced cardiovascular and lung disease. Front. Biosci. 2007;12:1238–1246. doi: 10.2741/2142. [DOI] [PubMed] [Google Scholar]
- Xiong S, Rodgers K. Effects of malathion metabolites on degranulation of and mediator release by human and rat basophilic cells. J. Toxicol. Environ. Health. 1997;51:159–175. doi: 10.1080/00984109708984019. [DOI] [PubMed] [Google Scholar]
- Yepez-Mulia L, Montano-Escalona C, Fonseca-Linan R, Munoz-Cruz S, Arizmendi-Puga N, Boireau P, Ortega-Pierres G. Differential activation of mast cells by antigens from Trichinella spiralis muscle larvae, adults, and newborn larvae. Vet. Parasitol. 2009;159:253–257. doi: 10.1016/j.vetpar.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Yoshimaru T, Suzuki Y, Inoue T, Niide O, Ra C. Silver activates mast cells through reactive oxygen species production and a thiol-sensitive store-independent Ca2+ influx. Free Radic. Biol. Med. 2006;40:1949–1959. doi: 10.1016/j.freeradbiomed.2006.01.023. [DOI] [PubMed] [Google Scholar]