Abstract
Inflammatory bowel diseases (IBD) are associated with platelet activation and an increased risk for thromboembolism. While the mechanisms that underlie the altered platelet function and hypercoagulable state in IBD remain poorly understood, emerging evidence indicates that inflammation and coagulation are inter-dependent processes that can initiate a vicious cycle wherein each process propagates and intensifies the other. This review addresses the mechanisms that may account for the mutual activation of coagulation and inflammation during inflammation and summarizes evidence that implicates a role for platelets and the coagulation system in the pathogenesis of human and experimental IBD. The proposed link between inflammation and coagulation raises the possibility of targeting the inflammation-coagulation interface to reduce the morbidity and mortality associated with IBD.
Introduction
A variety of clinical conditions, including atherosclerosis, inflammatory bowel disease, sepsis, and rheumatoid arthritis involve both inflammation and coagulation, which combine to influence disease progression and severity. An intimate link between inflammation and coagulation has emerged from efforts to better understand the processes that underlie the coagulation and hemostasis associated with these pathological conditions. This work has revealed that many inflammatory mediators, and the signaling pathways that they activate, induce a hypercoagulable state and initiate clotting by reducing the activity of natural anticoagulant pathways and impairing the fibrinolytic system. There is also emerging evidence that the coagulation-anticoagulation pathways exert an influence on the inflammatory response. Different components of the coagulation pathways, including thrombin and tissue factor, appear to promote inflammation, while anticoagulants such as activated protein C and heparin exert anti-inflammatory effects. Platelets, which are recruited to and activated at sites of thrombus formation, also produce and release a myriad of substances that promote inflammation. The interdependence of inflammation and coagulation creates the potential for a vicious cycle wherein each process propagates and intensifies the other. The interdependence of these two processes also offers the opportunity for development of novel therapeutic strategies for chronic inflammatory diseases and the associated risk for thrombosis with drugs that target the interface between inflammation and coagulation.1
The inflammatory bowel diseases (IBD) are associated with a hypercoagulable state and an increased risk for thromboembolism, which remains a significant cause of death in IBD patients.2–5 Thrombus formation during IBD occurs both within the inflamed bowel and in extra-intestinal tissues.6–8 The hypercoagulable state and enhanced thrombus formation that is observed in human IBD has been reproduced in animal models of colitis. Animal studies have also demonstrated that elevated levels of pro-coagulants (e.g., thrombin) and reduced levels of endogenous anticoagulants (e.g., activated protein C) contribute to propagation of gut inflammation in experimental IBD.9 There observations, coupled to a large amount of clinical evidence for an imbalance in coagulation-anticoagulation systems, provide support for the view that a vicious cycle of inflammation and coagulation is initiated in IBD. In this review, we summarize the evidence that implicates a role the coagulation system and platelets in the pathogenesis of human and experimental IBD. Potential mechanisms underlying the mutual activation of coagulation and inflammation in IBD are addressed as well as the consequences of these responses to the initiation and propagation of IBD. Understanding the link between inflammation and coagulation in IBD should improve ongoing and future efforts to assess the feasibility of targeting the inflammation-coagulation interface to reduce the morbidity and mortality associated with this disease.
Evidence implicating platelets and platelet activation in human IBD
Platelets are generally considered the first line of defense in sealing off injured blood vessels. The accumulation of activated platelets at sites of vessel injury is also associated with an increased expression of platelet receptors for coagulation proteases and cofactors, the release of a variety of bioactive substances from alpha- and dense granules that recruit additional platelets and activate other cell types (e.g., leukocytes, endothelial cells), and an amplification of the procoagulant response that is characterized by explosive thrombin generation.10–12 Although hemostasis is inherently geared towards localizing procoagulant events to sites of vessel injury, significant numbers of activated platelets and their products of activation often gain access to the systemic circulation where they can be used to monitor (as surrogate markers) the localized hemostatic response. In addition, the appearance of these cells and activation products in systemic blood may enable the localized hemostatic response to exert pro-coagulant and/or pro-inflammatory effects in distant tissues.
There are several lines of evidence that are consistent with altered platelet function in IBD. A notable response to IBD is thrombocytosis.13 The larger number and smaller size of platelets in IBD patients is well characterized and it has been proposed that platelet count and size are useful indicators of disease activity.14,15 The underlying cause of the thrombocytosis remains unclear. Although plasma thrombopoietin levels are elevated in IBD patients, the absence of a correlation between platelet counts and thrombopoietin levels suggest that circulating factors other than thrombopoietin mediate the thrombocytosis.16 The platelet dysfunction in IBD is also manifested as an increased expression of activation-dependent surface antigens on circulating platelets, including P-selectin, GP53, and CD40-ligand.17,18 Soluble CD40L levels are significantly higher in IBD patients (both CD and UC) compared to normal controls, which largely reflects the shedding of this pro-inflammatory signaling molecule from the surface of activated platelets.18 Plasma CD40L levels exhibit a positive correlation with the extent of anatomical involvement in IBD.18 Alpha-granule derived platelet factors such as beta-thromboglobulin and platelet factor-4 are also increased in the plasma of IBD patients, although their appearance in plasma is not correlated with disease activity.19
Changes in platelet function during IBD are also manifested as a tendency for platelets to spontaneously aggregate in vitro20 and to exhibit an increased sensitivity to endogenous pro-aggregation molecules such as collagen and adenosine diphosphate.19 Intravascular platelet aggregates have been detected in mucosal biopsies of patients with UC and there is an increased number of circulating platelet aggregates in the mesenteric venous circulation draining the inflamed bowel in UC.21 The increased appearance of platelet-leukocyte aggregates (PLA) in systemic blood is another manifestation of the platelet activation in IBD.22 The increased expression of P-selectin on activated platelets enables these cells to bind to leukocytes, which constitutively express P-selectin glycoprotein ligand-1 (PSGL-1), the major ligand for platelet P-selectin. Although PLA formation is not correlated with disease activity, this heterotypic cell-cell interaction appears to yield platelets that are more intensely activated than their counterparts that participate in homotypic (platelet-platelet) interactions.23
Procoagulant state and thrombogenesis in human IBD
Soluble circulating biomarkers of the procoagulant state
In addition to platelet abnormalities, human IBD is associated with changes in plasma levels of different hemostatic biomarkers that are consistent with subclinical activation of the coagulation system.5,24 These biomarker responses to IBD reveal an imbalance between procoagulant and anti-coagulant pathways and changes in the fibrinolytic system that favors thrombosis. While some inconsistencies in biomarker responses are evident in the large number of clinical reports that address this issue, the findings are generally consistent with activation of tissue factor, impairment of the protein C pathway, and enhanced generation of thrombin. A reduction in fibrinolytic capacity is also evident and it appears to reflect both a decreased activation (reduced tPA) and increased inhibition (elevated PAI) of the fibrinolytic system. Autoantibodies against different components of the coagulation system (e.g., phospholipids, protein S) also exhibit higher titers in IBD patients, however, the contribution of these antibodies to the hypercoagulable state in IBD remains unclear.
Intestinal and extra-intestinal thrombus formation
The hypercoagulability of blood in IBD patients is associated with the appearance of thrombi in the vasculature of the intestine and extra-intestinal tissues. Within the chronically inflamed gut, microinfarctions are often detected by histology. Platelet aggregates which stain positively for glycoprotein IIb/IIIa appear to occur at sites of granulomatous destruction of mesenteric blood vessels and there is evidence for intravascular fibrin deposition and complete thrombotic occlusion.6 The fibrin clots are seen within arteries supplying the inflamed gut and in capillaries and venules. The vessel obstruction that results from the formation of these clots likely contributes to the impaired tissue perfusion, cell necrosis, and organ dysfunction described in the colon of patients with IBD.25
Systemic thromboembolic events (TE) represent a major cause of morbidity and mortality associated with IBD. The mortality rate from the thromboembolic complications of IBD ranges between 8–25% during the acute phase of the thrombotic events, with a two-year mortality of approximately 25%.3,4 The results of recent clinical studies indicate that the incidence of TE events in IBD patients is ~6.2%, with a 3.6-fold increase in risk for TE complications compared to the general population.26 Postmortem studies reveal a much higher incidence of systemic TE (41% of IBD patients) compared to clinical studies, suggesting that many cases are not detected.23 The absence of an increased risk for TE in other chronic inflammatory diseases such as rheumatoid arthritis and in other chronic bowel diseases (e.g., celiac disease) suggests that this hemostatic risk is unique to IBD.
Both the arterial and venous circulations appear to be involved in IBD-associated TE. While venous complications occur more frequently, the mortality associated with the less frequent arterial thrombus is comparable to that noted when veins are involved.27 TE is usually manifested as deep vein thrombosis (DVT) or pulmonary embolism (PE), although thromboses have been detected in other regional circulations, including brain, retina, and liver. Studies on ulcerative colitis and Crohn’s patients who experienced TE events have revealed that the risk of TE may correlate with disease severity and the extent of colonic involvement, with stronger correlations noted for CD than UC.3,26,28 However, large clincal studies have noted that up to one-third of TE complications occured during disease quiescence, suggesting that the procoagulant tendency is independent of disease activity.4,7 No single biomarker of coagulation appears to reliably correlate with the occurrence of TE in IBD.
Platelets and experimental IBD
It is well established that platelets adhere and aggregate at sites of vascular injury in response to endothelial denudation and exposure of subendothelial collagen. The accumulation of platelets at the injury site serves to temporarily plug the damaged vessel and localize subsequent procoagulant events.29 In vitro and in vivo studies have revealed that endothelial denudation is not an absolute requirement for platelet attachment to the walls of blood vessels.30 Normal healthy endothelial cells prevent platelet adhesion by hiding components of the subendothelial matrix (collagen, fibronectin) from platelets, augmenting fibrinolysis via tPA, and producing platelet inactivators (nitric oxide, PGI2). However, during inflammation, endothelial cells assume a phenotype that is associated with an increased surface expression of adhesion glycoproteins that enhance their capacity to bind platelets as well as leukocytes, to which platelets can also bind.31
Dextran sodium sulfate (DSS) induced colonic inflammation is associated with an accumulation of platelets in colonic venules that is temporally correlated with the appearance of adherent leukocytes and with disease activity.32 Approximately 20% of the platelets that accumulate in the inflamed colonic vasculature are bound directly to endothelial cells, while the remaining 80% are attached to the surface of adherent leukocytes.33 Immunoblockade or genetic deletion of either P-selectin or its ligand PSGL-1 significantly reduces the colitis-associated recruitment of both platelets and leukocytes, suggesting a major role for this receptor-ligand pair. An increased expression of both P-selectin and PSGL-1 is noted in the inflamed colonic vasculature, with both adherent platelets and endothelial cells contributing to the expression of P-selectin while both adherent leukocytes and endothelial cells account for the PSGL-1 expression.32,33 Therefore, platelet recruitment in DSS-induced colitis appears to be mediated by adhesive interactions between platelet-associated P-selectin and PSGL-1 expressed on both endothelial cells and adherent leukocytes. While vessel injury and exposure of the underlying extracellular matrix may contribute to platelet recruitment, this appears unlikely in view of the inability of a GPIIb/IIIa antibody to blunt DSS-induced platelet adhesion.32
DSS colitic mice rendered thrombocytopenic with anti-platelet serum exhibit a marked reduction in the numbers of rolling and adherent leukocytes in colonic venules,33 indicating that platelets exert a significant influence on the recruitment of leukocytes in the inflamed bowel. The modulating influence of platelets on leukocyte adhesion may reflect the ability of platelets to alter the activation state of leukocytes and/or endothelial cells. For example, the binding of platelets to neutrophils is known to enhance the activation state of the leukocytes, resulting in an increased production of superoxide. Platelet-induced leukocyte activation has been demonstrated in studies that incubate isolated unstimulated human neutrophils with platelets derived from patients with UC.34 Activated P-selectin-positive platelets from IBD patients have also been shown to activate monolayers of human intestinal microvascular endothelial cells (HIMEC), with a resultant increase in the expression of endothelial cell adhesion molecules35 that could also account for the platelet-dependency of leukocyte adhesion in experimental colitis.
Activated (P-selectin positive) platelets may also contribute to the endothelial barrier dysfunction and tissue injury that are detected in experimental colitis. Endothelial barrier dysfunction, manifested as increased vascular permeability to albumin, is evident as early as day-2 on DSS and it remains greatly elevated (9-fold increase) through day-6.32 Hence, this response is temporally disassociated from the recruitment of platelets and leukocytes as well as disease severity. Nonetheless, the increased vascular permeability detected on day-6 is largely abolished in mice that are genetically deficient in P-selectin32 and in bone marrow chimeras created by transplantation of bone marrow from P-selectin deficient mice into wild type recipients (unpublished observations by authors). Because P-selectin deficient platelets do no accumulate in colonic venules during DSS colitis, these observations suggest that the recruitment of P-selectin positive platelets is largely responsible for the endothelial barrier dysfunction observed in this model of experimental colitis. Since immunoblockade of either P-selectin36 or PSGL-137 significantly attenuates inflammation and histologic tissue injury in murine DSS colitis, it is possible that the recruitment of adherent platelets and endothelial barrier dysfunction are important early events in the induction of DSS-induced inflammation that ultimately results in tissue injury and the clinical signs of colitis that are observed in this experimental model.
While the adhesion molecules that mediate the accumulation of activated platelets in inflamed colonic venules have been extensively studied, relatively little attention has been devoted to defining the factors that initiate or modulate this recruitment response. Neutrophils appear to modulate the binding of platelets directly to venular endothelium in the inflamed colon33 inasmuch as DSS-treated mice rendered neutropenic exhibit a significant increase in platelet-endothelial cell adhesion (compared to untreated DSS mice), while platelet-leukocyte adhesion is profoundly attenuated. The enhanced direct binding of platelets to endothelial cells appears to be mediated by an interactions between platelet P-selectin and endothelial cell PSGL-1. These observations indicate that neutrophils: 1) represent the dominant leukocyte population that binds platelets in experimental colitis, and 2) interfere with the binding of platelets directly to venular endothelium. The latter role of neutrophils suggests these cells liberate substances that induce an anti-thrombogenic phenotype in endothelial cells and/or occupy or cover receptors (e.g., PSGL-1) on endothelial cells that would normally sustain the adhesion of platelets in the absence of neutrophil adhesion.
The CD40/CD40L signaling pathway has also been implicated in the recruitment of platelets in inflamed colonic venules.38 Mice that are genetically deficient in either CD40 or CD40L exhibit a marked reduction in total platelet adhesion in colonic venules of DSS colitic mice. However, CD40 deficiency reduces platelet-leukocyte adhesion without affecting platelet-endothelial interactions. CD40L deficiency more profoundly reduces DSS-induced platelet-leukocyte adhesion while completely inhibiting the platelet-endothelial cell interactions. Since DSS colitis is also associated with an increased endothelial cell expression of CD40 in the colonic microcirculation, and leukocyte-endothelial cell adhesion is comparably affected by CD40 or CD40L deficiency,38 it is likely that platelet-associated CD40L (or its circulating soluble form) is an important regulator of platelet interactions with both adherent leukocytes and venular endothelium.
The P-selectin-dependent platelet-leukocyte complexes that are observed in colonic microvessels during experimental colitis may be a precursor of the free-flowing platelet-leukocyte aggregates (PLA) that are detected at increased levels in the blood of IBD patients.22 It has been proposed that PLA are initially formed on the endothelial surface of inflamed mesenteric microvessels,22 where they are subsequently dislodged by shear forces generated from the movement of blood. Some of the PLA released into mesenteric venous blood may not appear in systemic blood due to entrapment of the aggregates in capillaries of the liver and/or lung. Since leukocyte-free P-selectin positive platelets also appear in systemic blood, then it is also possible that PLA are formed in flowing blood due to engagement of platelet P-selectin with PSGL-1 that is constitutively expressed on leukocytes. Whether leukocyte activation contributes to the formation of PLA remains unclear. However, platelet activators such as thromboxane and platelet activating factor (PAF) generated by the inflamed gut may predispose platelets to PLA formation. It has been proposed that the PLA may represent an important circulating source of inflammatory mediators that can sustain or amplify an inflammatory response.39 Leukocytes with attached platelets appear to be primed for adhesion and can achieve a more activated state than their platelet-free counterparts.40 Nonetheless, it remains unclear whether circulating PLA contribute to extra-intestinal responses to IBD, including thromboembolism.
Mechanisms and consequences of platelet activation
While there is clear evidence for platelet activation in human and experimental IBD, the mechanisms that underlie this activation process remain poorly understood. Platelets coursing through the microvasculature of the inflamed bowel are likely exposed to a variety of substances that either prime the cells for activation or directly activate them. In regions of the bowel with tissue damage, platelets may be exposed to collagen, which is a potent stimulant for activation. The direct contact of platelets with cytokine-activated endothelial cells can also lead to platelet activation.18 Similarly, a variety of soluble substances released from injured resident cells and/or recruited inflammatory cells may also participate in the activation of platelets within the intestinal vasculature. Adenosine diphosphate accumulation due to diminished capillary perfusion, as well as arachidonic acid and PAF produced in response to phospholipase A2 activation, and cytokines released from activated leukocytes and macrophages are also potential mediators of platelet activation in the inflamed bowel. An attenuated production of endogenous inhibitors of platelet activation, including prostacyclin (PGI2) and nitric oxide, may also contribute to the activation response. Once platelet activation is initiated, then a myriad of substances that are either produced (e.g., thromboxane A2, ADP, serotonin) by platelets, released from granules or shed (CD40L) from the cell surface serve to greatly amplify the activation and accumulation of platelets. The relative importance of these different factors in the activation of platelets as they course through the inflamed gut remains unknown.
Activated platelets produce and release a variety of substances that have the potential to influence the quality and intensity of an inflammatory response. These platelet-derived factors act on both leukocytes and endothelial cells to induce an inflammatory phenotype. Some products of platelet activation contribute to transcellular metabolic reactions in leukocytes, such as neutrophils using arachidonic acid released by platelets to produce increased quantities of inflammatory leukotrienes.41 The attachment of activated platelets to neutrophils also enables the latter produce larger quantities of superoxide34 and PAF42 than either cell is capable of producing alone.
Endothelial cells are also an important target for platelets and their activation products. When CD40L-positive platelets are co-incubated with cultured HIMEC, the endothelial cells become activated, as evidenced by an increased surface expression of ICAM-1 and VCAM-1, an enhanced production of IL-8 (a neutrophil chemoattractant), and increased leukocyte-endothelial cell adhesion. These platelets also release the chemokine RANTES, which binds to glycosaminoglycans on the endothelial cell surface to further promote leukocyte.35 Platelet activation products such as histamine, PAF, and cationic proteins may also contribute to the endothelial barrier dysfunction and increased vascular permeability that are characteristic of colonic inflammation.32 Since RANTES has been recently implicated as a mediator of impaired endothelium-dependent vasodilation associated with hypertension43, it is possible that this platelet-derived chemokine may contribute to the reduced capacity of colonic arterioles from IBD patients and DSS colitic mice to dilate in response to acetylcholine and other endothelium-dependent vasodilators.25,32
Evidence for activation of coagulation and enhanced thrombosis in experimental IBD
Relatively little attention has been devoted to determining whether the procoagulant state that is detected in human IBD is recapitulated in animal models of this disease. However, emerging evidence from rodent models of chemically-induced colitis (e.g., DSS, TNBS) suggest that the balance between anti-coagulant and procoagulant factors is tipped in favor of coagulation and thrombosis. For example, thrombocytosis and increased plasma fibrinogen levels, along with a corresponding reduction in antithrombin III concentration has been reported in rats with TNBS-induced colitis.44 Similarly, thrombin-antithrombin (TAT) complexes are three-times higher in mice with DSS colitis compared to their control (non-colitic) counterparts, implicating an accelerated production of thrombin in the diseased mice.45 DSS colitic mice also exhibit a significantly reduced capacity for protein C activation, which would also favor enhanced thrombin production.9
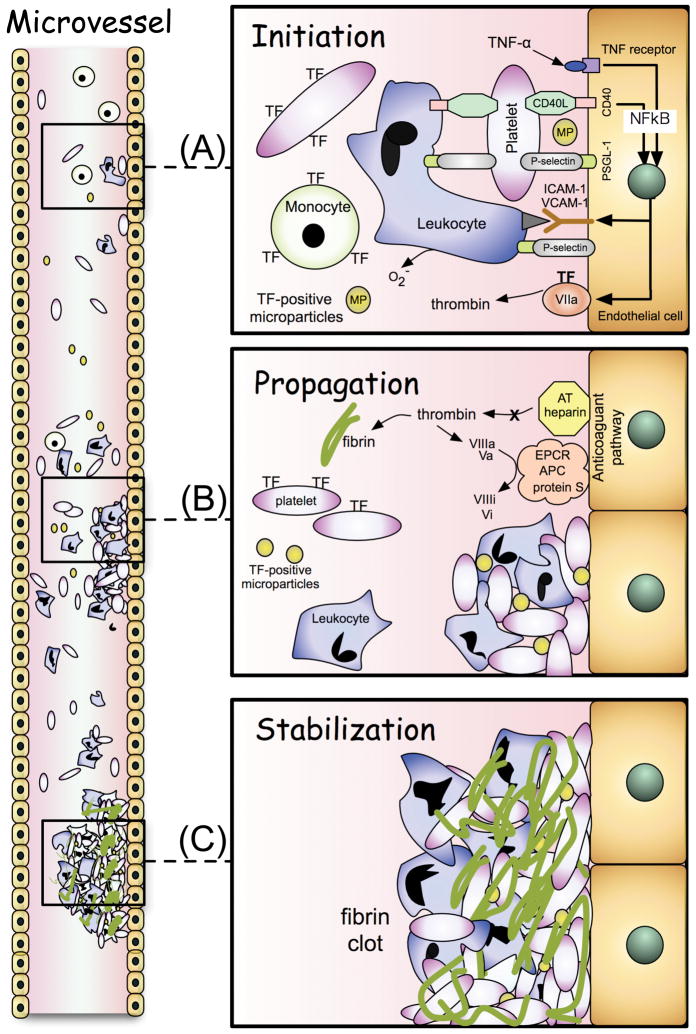
Enhanced microvascular thrombosis has also been demonstrated in extra-intestinal tissues (cremaster muscle) of mice with DSS colitis.45–47 Following photochemical injury to endothelial cells in arterioles and venules of mouse cremaster muscle, thrombus formation is initiated. Thrombogenesis is believed to occur in three stages: initiation, propagation, and stabilization (Figure 1).48 The initiation phase involves the rapid binding of platelets to the injured vessel wall and activation of the coagulation cascade by tissue factor. The time of onset of platelet deposition/aggregation within the microvessel (onset time) is used experimentally as a measure of the initiation phase. The propagation phase involves the recruitment of more platelets and amplification of the coagulation cascade via the intrinsic pathway, while stabilization of the thrombus occurs as a result of fibrin deposition. Experimentally, the time to flow cessation within an injured microvessel is considered to reflect the time for thrombus stabilization and is likely to include both the propagation and stabilization phases of thrombogenesis.46 Mice with DSS-induced colonic inflammation exhibit a significant acceleration of both the initiation (onset time) and propagation/stabilization (time to flow cessation) phases of thrombus formation in cremaster muscle microvessels following photochemical (light/dye) injury,45–47 which is consistent with the view that IBD enhances thrombus formation at extra-intestinal sites.
Figure 1.
Stages of thrombus formation caused by inflammation. A) Initiation phase: In the absence of collagen exposure, a thrombus is likely initiated by the recruitment of tissue factor (TF)-expressing blood cells (platelets, leukocytes) and TF-positive microparticles, as well as an increased expression of TF by endothelial cells. Cytokines (eg, TNF-alpha) and CD40/CD40L interactions can elicit the expression of the TF and adhesion molecules (ICAM-1, VCAM-1, P-selectin) via NFkB-mediated, transcription-dependent mechanisms. Platelet-endothelial cell and platelet-leukocyte adhesion are mediated by P-selectin (platelet)-PSGL-1 (endothelial cell or leukocyte) interactions. ICAM-1, VCAM-1 and P-selectin expression by activated endothelium mediates the adhesion of leukocytes in the vasculature. B) Propagation phase: Thrombin generation from the extrinsic (TF-VIIa) pathway, activation of the intrinsic pathway, and the recruitment of additional TF-bearing platelets, TF-positive microparticles and leukocytes greatly amplifies thrombus formation. Inhibition of the activated protein C pathway and antithrombin (AT)-heparin system further increases thrombin generation. C) Stabilization phase: The fibrin deposition resulting from the action of thrombin on fibrinogen serves to stabilize the thrombus.
The enhanced thrombus formation observed in DSS colitic mice is largely exhibited in arterioles but not venules. This contrasts with the response noted in cremaster muscle of mice after administration of bacterial endotoxin (LPS) wherein venules (not arterioles) exhibit the accelerated thrombosis. The different responses to experimental colitis and LPS suggests that gut-derived endotoxin may not be an important factor that enables colonic inflammation to exert an influence on thrombus formation at extra-intestinal sites.
Mechanisms underlying the enhanced extra-intestinal thrombosis in experimental IBD
Animal studies have been undertaken to define the pro-and anti-coagulant factors that contribute to the accelerated light/dye-induced thrombosis in cremaster microvessels during DSS colitis.45–47 These studies have revealed a role for tissue factor (TF), an impaired protein C pathway, and thrombin in the extra-intestinal thrombosis associated with experimental colitis. Immunoneutralization of tissue factor largely prevents the reduction in the time to onset of thrombus formation in arterioles of DSS colitic mice, without altering the time to flow cessation.45 The responses to TF immunoblockade are consistent with the proposal that tissue factor expression is a key event in the initiation phase (onset) of arteriolar thrombogenesis.48 TF is known to activate coagulation by binding to and activating factor VII. Monocytes, neutrophils, platelets, and endothelial cells can produce and express TF in vivo. Microparticles (0.05 to 1.0 um) shed from monocytes and platelets are also a rich source of TF that can initiate coagulation.48 Since exposure collagen due to endothelial cell lifting/injury is an unlikely initiating event in the enhanced extra-intestinal thrombosis elicited during colitis, the TF that initiates this process is likely to be either expressed on the surface of locally activated endothelial cells or delivered to site of thrombosis via circulating leukocytes, platelets, microparticles, and/or PLA (Figure 1).
Impairment of the protein C pathway, an anticoagulant system that limits the generation of thrombin, also appears to contribute to the enhanced extra-intestinal thrombosis associated with DSS colitis in mice. This contention is based on a recent report that demonstrates a blunted acceleration of the arteriolar thrombogenesis elicited by photochemical injury in colitic wild type mice receiving exogenous murine activated protein C (mAPC) and in mice that genetically overexpress the endothelial protein C receptor (EPCR).46 EPCR-overexpressing mice generate more APC in response to thrombin and are resistant to factor Xa-induced thrombosis.49 When DSS colitic mice are treated with an APC neutralizing antibody, the thrombosis response is further accelerated, suggesting that a small amount of APC is still available to offer some protection against thrombus formation in arterioles. Unlike TF, which specifically affects the initiation phase of thrombogenesis, APC-directed interventions (exogenous mAPC, EPCR-overexpression, anti-APC antibody) alter the time to flow cessation (propagation/stabilization phases of thrombogenesis), without affecting the onset of thrombosis (initiation).46 The significant role of the protein C pathway in experimental colitis enhanced thrombosis is consistent with reports describing a reduced expression of EPCR by intestinal microvascular endothelium derived from IBD patients and a reduced capacity for protein C activation in plasma of DSS colitic mice.9
An expected consequence of the tissue factor activation and an impaired protein C pathway that are associated with experimental colitis is the generation of thrombin, which promotes thrombus formation by converting fibrinogen to fibrin, and through feedback activation of factors V, VIII and XI. The role of thrombin in DSS colitis-enhanced thrombus formation in cremaster arterioles after light/dye-induced endothelial injury has been recently examined using three direct thrombin inhibitors (hirudin, heparin, antithrombin III) with distinct mechanisms of action.47 All three drugs were shown to be very effective in delaying colitis-enhanced arteriolar thrombogenesis. The significant extension of time to flow cessation, but not the time of onset of thrombosis, observed with each of the thrombin inhibitors is consistent with a role for thrombin in the propagation/stabilization phases (but not the initiation phase) of thrombogenesis.47
While progress has been made in understanding the contribution of different pro- and anti-coagulant factors towards the enhanced production in extra-intestinal vessels during experimental colitis, the identity of the chemical and/or cellular signal produced by the inflamed gut that initiates this distant organ thrombogenic response remains unknown. One possibility is that blood cells such as monocytes or platelets that flow through the microvasculature of the inflamed colon may be activated to express tissue factor, which is then delivered via blood to extraintestinal vascular beds to initiate thrombus formation. Gut-derived bacterial endotoxin may also play a role in this response, since exogenous LPS also tends to accelerate photochemical injury-induced thrombosis.50 However, unlike DSS colitis, E. coli endotoxin promotes a toll-like receptor-4 (TLR-4) dependent thrombosis response that is manifested in venules but not arterioles.51 Nonetheless, it is conceivable that the quality and/or quantity of LPS that gains access to the systemic circulation in experimental colitis yields a thrombogenic phenotype that more closely resembles that observed in colitic animals. Cytokines released into the blood stream by the inflamed bowel are also potential signaling molecule(s) that mediate the distant organ thrombogenic response in experimental IBD. Tumor necrosis factor-α and interleukin-1 β, which have been implicated in pathogenesis of IBD, are known to down-regulate the expression of endothelial protein C receptor on microvascular endothelial cells and reduce the capacity of these cells to activate protein C.9 Furthermore, it has been reported that TNF-α, IL-6 and IL-1 enhance tissue factor transcription via an NFkB-dependent mechanism.52–54 Preliminary results from our laboratory55 indicate that TNF- α and IL-1 β administered to control mice accelerates photochemical injury-induced thrombus formation in a manner similar to that noted in DSS-colitic mice. In addition, immunoblockade of the two cytokines in DSS colitic mice significantly attenuates the thrombogenic response that normally accompanies experimental IBD.55
Another candidate signaling pathway that may contribute to the distant thrombogenic response to colonic inflammation is the CD40/CD40L dyad. CD40L, a membrane glycoprotein in the TNF family that is expressed on circulating T-lymphocytes and platelets, has been implicated in the pathophysiology of human and experimental IBD.18,38,56 Platelet CD40L expression is increased in IBD patients and the plasma concentration of circulating soluble CD40L (sCD40L), a bioactive form of the membrane-bound protein that is shed from the surface of activated T-cells and platelets, is similarly increased in these patients. The increased plasma sCD40L detected in IBD patients is largely derived from activated platelets.18 Direct adhesion of activated platelets to endothelial cells has been shown to elicit the expression of TF on endothelial cells via a CD40/CD40L-dependent mechanism.57 Since sCD40L is known to promote hemostasis by binding to glycoprotein GPIIb/IIIa58 and CD40L deficient mice exhibit an attenuated thrombogenic response in arterial vessels that can be restored to normal after administration of sCD40L,59 it is tenable to propose that the elevated levels of sCD40L in IBD may also contribute to the extra-intestinal thrombosis associated with this disease. A role for the CD40/CD40L dyad is supported by a preliminary report describing a protective effect of CD40L deficiency against DSS-enhanced, photochemical injury-induced thrombus formation in mice.60 Whether the protection afforded by CD40L deficiency is related to a direct effect of platelet-associated or circulating soluble CD40L on extra-intestinal thrombus formation or an indirect effect due to attenuation of the colonic inflammation remains unclear.
Dependence of inflammatory response on coagulation pathways in experimental colitis
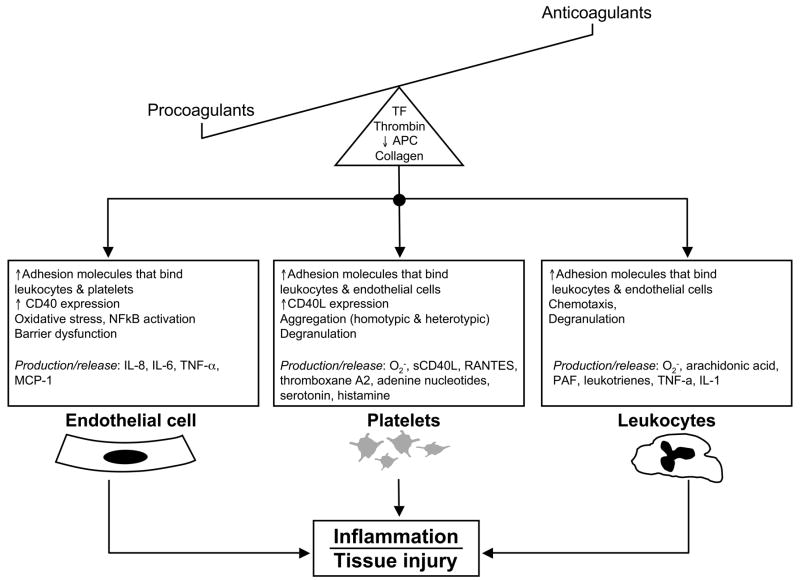
The influence of hemostasis on inflammation is supported by numerous reports that describe how different components of the coagulation-anticoagulation pathways can regulate inflammation by exerting an influence on endothelial cells, platelets, and/or leukocytes (Figure 2). Thrombin, for example, has been shown to increase the expression (via transcription-independent and -dependent mechanisms) of adhesion molecules on endothelial cells and to promote leukocyte-endothelial cell adhesion.61 The engagement of TF with its ligand (factor VIIa) activates the protease-activated receptors PAR 1 – 4, which elicits the production of pro-inflammatory cytokines (TNF-α, IL-6) and promotes leukocyte rolling in venules.62 Mice that lack the cytoplasmic domain of TF exhibit an attenuated recruitment of rolling, adherent and transmigrating leukocytes in postcapillary venules after LPS challenge.62 Furthermore, a small molecule inhibitor of the TF-VIIa complex (BCX-3607) has been shown to attenuate the LPS-induced production of IL-6 and IL-8 in vitro (by endothelial cells) and IL-6 in vivo.63 Similarly, APC has been shown to inhibit the production of adhesion molecules (VCAM-1, ICAM-1) and cytokines in endothelial cells, as well as agonist-induced leukocyte activation and LPS-induced production of TNF-α and other cytokines by cultured monocytes/macrophages.64 Finally, mice with single-allele targeted disruption of the protein C gene (heterozygous protein C deficient (PC+/−) mice) have higher levels of circulating cytokines, including a 4-fold increase in TNF-α, after endotoxin challenge,65 which is consistent with an anti-inflammatory action of APC.
Figure 2.
An imbalance between procoagulant and anticoagulant factors can promote inflammation. The excess production of tissue factor and thrombin, exposure of subendothelial collagen, and reduced levels of activated protein C that are associated with thrombus formation can lead to the activation of endothelial cells, platelets, and leukocytes. The activated cells respond to the coagulation factors by increasing the expression of adhesion molecules that facilitate their binding and interactions with other cells. Increased superoxide production (oxidative stress) and the generation/release of a variety of pro-inflammatory molecules also occur in all three cells. Some of these inflammatory products of cell activation (eg, cytokines, sCD40L, ADP, thromboxane A2, PAF) can also exert a positive feedback effect on the coagulation process, thereby creating a vicious cycle wherein inflammation intensifies coagulation and vice versa.
The view that coagulation and inflammation are interdependent processes that propagate and amplify each other is also supported by studies that examined the influence of anticoagulant strategies on the severity of gut inflammation in animal models of IBD. For example, the thrombin-directed drugs heparin and argatroban (interferes with the conversion of fibrinogen to fibrin by thrombin) have been shown to significantly reduce macroscopic and histologic damage, mucosal myeloperoxidase activity (a measure of neutrophil accumulation), and mucosal LTB4 levels in rats with TNBS-induced colitis.44,66 Similar protection against gut inflammation was recently demonstrated following intracolonic administration of the low-molecular-weight heparin CB-01-05 in rats with dinitrobenzene-induced colitis.67 Although there is some clinical evidence supporting the utility of intracolonic delivery of heparin in mild ulcerative colitis,68 the outcome of most clinical trials do not support the use of heparin in active UC,69 particularly in view of the increased risk of rectal bleeding. Inasmuch as heparin is also known to exhibit direct anti-inflammatory properties,70 it remains unclear whether the protection afforded by this antithrombin agent in human and experimental IBD reflects an action on the coagulation system.
While genetic factors have largely been dismissed as an underlying cause of the hypercoagulable state in human IBD,71 animal models have been developed to simulate some of the mutations (e.g., factor V Leiden) that were once considered to contribute to the pathophysiology of IBD. A comparison of the responses of wild type and homozygous factor V Leiden mice (which renders factor V resistant to degradation by APC) to DSS treatment revealed no differences between the extent of mucosal ulcerations, edema formation, crypt loss, fibrosis and the influx of inflammatory cells into the colon, suggesting that APC mediated factor V degradation is not critical for the inflammatory and tissue injury responses in experimental colitis.72 However, the results of another animal study9 provide evidence supporting a critical role of the protein C pathway in the colonic inflammatory responses to DSS. Colitic mice treated with murine recombinant APC exhibited reductions in disease severity, leukocyte adhesion, and histologic injury score, compared to untreated mice. The anti-inflammatory effects of APC were also demonstrated using an in vitro system of cultured human intestinal microvascular endothelial cells (HIMEC). APC-treated HIMEC exhibited a down-regulation of TNF-a induced expression of adhesion molecules (VCAM-1, ICAM-1), and chemokines (ENA-78, IL-8, MCP-1), with a corresponding attenuation of leukocyte (T-cell)-endothelial cell adhesion.9
Tissue factor has also been implicated in the gut inflammatory and tissue injury responses to experimental IBD.45 Immunoblockade of tissue factor in DSS colitic mice largely prevents the significant rise in plasma TAT complexes observed in this model. TF immunoneutralization also results in a reduction in disease severity, gross and histopathological tissue injury, and the recruitment of adherent leukocytes and platelets in DSS colitic mice. These findings, coupled with the similarly protective effects of APC treatment in DSS colitis,9 support the view that activation of different elements of the coagulation/anti-coagulation pathways contributes to the initiation, progression and/or maintenance of the inflammatory response in experimental IBD.
Conclusions
The concept of cross-talk between coagulation and inflammatory pathways is rapidly gaining support from animal studies and clinical reports. While the reciprocal relationship between coagulation and inflammation is most clearly manifested in IBD as a significantly increased risk for thromboembolism, evidence that implicates coagulation factors in the initiation and/or perpetuation of gut inflammation further supports such a linkage. It is also becoming evident that adhesive and chemical interactions between multiple cell types, including platelets, endothelial cells and leukocytes, create the interface between coagulation and inflammation. Drugs that target this interface may provide a novel therapeutic approach to lower the risk of thromboembolism and reduce the gut inflammation and resultant tissue injury in IBD.
Acknowledgments
Supported by a grant from the National Institutes of Diabetes and Digestive and Kidney Diseases (P01 DK43785)
References
- 1.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1:1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Blanchard JF, Houston DS, et al. The incidence of deep venous thrombosis and pulmonary embolism among patients with venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost. 2001;85:430–434. [PubMed] [Google Scholar]
- 3.Solem CA, Loftus EV, Tremaine WJ, et al. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99:97–101. doi: 10.1046/j.1572-0241.2003.04026.x. [DOI] [PubMed] [Google Scholar]
- 4.Talbot RW, Heppell J, Dozois RR, et al. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140–145. doi: 10.1016/s0025-6196(12)65200-8. [DOI] [PubMed] [Google Scholar]
- 5.Vrij AA, Rijken J, van Wersch JW, et al. Coagulation and fibrinolysis in inflammatory bowel disease and in giant cell arteritis. Pathophysiol Haemost Thromb. 2003;33:75–83. doi: 10.1159/000073850. [DOI] [PubMed] [Google Scholar]
- 6.Dhillon AP, Anthony A, Sim R, et al. Mucosal capillary thrombi in rectal biopsies. Histopathology. 1992;21:127–133. doi: 10.1111/j.1365-2559.1992.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 7.Jackson LM, O’Gorman PJ, O’Connell J, et al. Thrombosis in inflammatory bowel disease: clinical setting, procoagulant profile and factor V Leiden. QJM. 1997;90:183–188. doi: 10.1093/qjmed/90.3.183. [DOI] [PubMed] [Google Scholar]
- 8.Wakefield AJ, Sawyerr AM, Dhillon AP, et al. Pathogenesis of Crohn’s disease: multifocal gastrointestinal infarction. Lancet. 1989;2:1057–1062. doi: 10.1016/s0140-6736(89)91078-7. [DOI] [PubMed] [Google Scholar]
- 9.Scaldaferri F, Sans M, Vetrano S, et al. Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease. J Clin Invest. 2007;117:1951–1960. doi: 10.1172/JCI31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Key NS. Platelet tissue factor: how did it get there and is it important? Semin Hematol. 2008;45:S16–S20. doi: 10.1053/j.seminhematol.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Klinger MH. Inflammation. In: Michelson AD, editor. Platelets. San Diego, CA: Elsevier Academic Press; 2002. pp. 459–467. [Google Scholar]
- 12.Nurden AT, Nurden P, Sanchez M, et al. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. doi: 10.2741/2947. [DOI] [PubMed] [Google Scholar]
- 13.Harries AD, Beeching NJ, Rogerson SJ, et al. The platelet count as a simple measure to distinguish inflammatory bowel disease from infective diarrhoea. J Infect. 1991;22:247–250. doi: 10.1016/s0163-4453(05)80006-4. [DOI] [PubMed] [Google Scholar]
- 14.Larsen TB, Nielsen JN, Fredholm L, et al. Platelets and anticoagulant capacity in patients with inflammatory bowel disease. Pathophysiol Haemost Thromb. 2002;32:92–96. doi: 10.1159/000065082. [DOI] [PubMed] [Google Scholar]
- 15.Collins CE, Rampton DS. Platelets dysfunction: a new dimension in inflammatory bowel disease. Gut. 1995;36:5–8. doi: 10.1136/gut.36.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papa A, Danese S, Piccirillo N, et al. Thrombopoietin serum levels in patients with inflammatory bowel disease with and without previous thromboembolic events. Hepatogastroenterology. 2003;50:132–135. [PubMed] [Google Scholar]
- 17.Collins CE, Cahill MR, Newland AC, et al. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106:840–845. doi: 10.1016/0016-5085(94)90741-2. [DOI] [PubMed] [Google Scholar]
- 18.Danese S, Katz JA, Saibeni S, et al. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52:1435–1441. doi: 10.1136/gut.52.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins CE, Rampton DS. Review article: platelets in inflammatory bowel disease--pathogenetic role and therapeutic implications. Aliment Pharmacol Ther. 1997;11:237–247. doi: 10.1046/j.1365-2036.1997.153328000.x. [DOI] [PubMed] [Google Scholar]
- 20.Webberley MJ, Hart MT, Melikian V. Thromboembolism in inflammatory bowel disease: role of platelets. Gut. 1993;34:247–251. doi: 10.1136/gut.34.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins CE, Rampton DS, Rogers J, et al. Platelet aggregation and neutrophil sequestration in the mesenteric circulation in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:1213–1217. [PubMed] [Google Scholar]
- 22.Irving PM, Macey MG, Shah U, et al. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:361–372. doi: 10.1097/00054725-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Irving PM, Pasi KJ, Rampton DS. Thrombosis and inflammatory bowel disease. Clin Gastroenterol Hepatol. 2005;3:617–628. doi: 10.1016/s1542-3565(05)00154-0. [DOI] [PubMed] [Google Scholar]
- 24.van Bodegraven AA, Schoorl M, Linskens RK, et al. Persistent activation of coagulation and fibrinolysis after treatment of active ulcerative colitis. Eur J Gastroenterol Hepatol. 2002;14:413–418. doi: 10.1097/00042737-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Hatoum OA, Miura H, Binion DG. The vascular contribution in the pathogenesis of inflammatory bowel disease. Am J Physiol Heart Circ Physiol. 2003;285:H1791–H1796. doi: 10.1152/ajpheart.00552.2003. [DOI] [PubMed] [Google Scholar]
- 26.Miehsler W, Reinisch W, Valic E, et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542–548. doi: 10.1136/gut.2003.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oldenburg B, Fijnheer R, van der Griend R, et al. Homocysteine in inflammatory bowel disease: a risk factor for thromboembolic complications? Am J Gastroenterol. 2000;95:2825–2830. doi: 10.1111/j.1572-0241.2000.03193.x. [DOI] [PubMed] [Google Scholar]
- 28.Spina L, Saibeni S, Battaglioli T, et al. Thrombosis in inflammatory bowel diseases: role of inherited thrombophilia. Am J Gastroenterol. 2005;100:2036–2041. doi: 10.1111/j.1572-0241.2005.42029.x. [DOI] [PubMed] [Google Scholar]
- 29.Stassen JM, Arnout J, Deckmyn H. The hemostatic system. Curr Med Chem. 2004;11:2245–2260. doi: 10.2174/0929867043364603. [DOI] [PubMed] [Google Scholar]
- 30.Massberg S, Enders G, Matos FC, et al. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood. 1999;94:3829–3838. [PubMed] [Google Scholar]
- 31.Strukova S. Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci. 2006;11:59–80. doi: 10.2741/1780. [DOI] [PubMed] [Google Scholar]
- 32.Mori M, Salter JW, Vowinkel T, et al. Molecular determinants of the prothrombogenic phenotype assumed by inflamed colonic venules. Am J Physiol Gastrointest Liver Physiol. 2005;288:G920–G926. doi: 10.1152/ajpgi.00371.2004. [DOI] [PubMed] [Google Scholar]
- 33.Vowinkel T, Wood KC, Stokes KY, et al. Mechanisms of platelet and leukocyte recruitment in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1054–G1060. doi: 10.1152/ajpgi.00350.2007. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K, Sugimura K, Hasegawa K, et al. Activated platelets in ulcerative colitis enhance the production of reactive oxygen species by polymorphonuclear leukocytes. Scand J Gastroenterol. 2001;36:1301–1306. doi: 10.1080/003655201317097164. [DOI] [PubMed] [Google Scholar]
- 35.Danese S, de la Motte C, Sturm A, et al. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003;124:1249–1264. doi: 10.1016/s0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 36.Gironella M, Mollà M, Salas A, et al. The role of P-selectin in experimental colitis as determined by antibody immunoblockade and genetically deficient mice. J Leukoc Biol. 2002;72:56–64. [PubMed] [Google Scholar]
- 37.Rijcken EM, Laukoetter MG, Anthoni C, et al. Immunoblockade of PSGL-1 attenuates established experimental murine colitis by reduction of leukocyte rolling. Am J Physiol Gastrointest Liver Physiol. 2004;287:G115–G124. doi: 10.1152/ajpgi.00207.2003. [DOI] [PubMed] [Google Scholar]
- 38.Vowinkel T, Anthoni C, Wood KC, et al. CD40-CD40 ligand mediates the recruitment of leukocytes and platelets in the inflamed murine colon. Gastroenterology. 2007;132:955–965. doi: 10.1053/j.gastro.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Li N, Hu H, Lindqvist M, et al. Platelet-leukocyte cross talk in whole blood. Arterioscler Thromb Vasc Biol. 2000;20:2702–2708. doi: 10.1161/01.atv.20.12.2702. [DOI] [PubMed] [Google Scholar]
- 40.Peters MJ, Dixon G, Kotowicz KT, et al. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br J Haematol. 1999;106:391–399. doi: 10.1046/j.1365-2141.1999.01553.x. [DOI] [PubMed] [Google Scholar]
- 41.Palmantier R, Borgeat P. Transcellular metabolism of arachidonic acid in platelets and polymorphonuclear leukocytes activated by physiological agonists: enhancement of leukotriene B4 synthesis. Adv Exp Med Biol. 1991;314:73–89. doi: 10.1007/978-1-4684-6024-7_4. [DOI] [PubMed] [Google Scholar]
- 42.Herd CM, Page CP. Pulmonary immune cells in health and disease: platelets. Eur Respir J. 1994;7:1145–1160. [PubMed] [Google Scholar]
- 43.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onomura M, Tsukada H, Fukuda K, et al. Effect of argatroban on trinitrobenzene sulfonic acid-induced colitis. J Gastroenterol Hepatol. 2000;15:931–938. doi: 10.1046/j.1440-1746.2000.02279.x. [DOI] [PubMed] [Google Scholar]
- 45.Anthoni C, Russell J, Wood KC, et al. Tissue factor: a mediator of inflammatory cell recruitment, tissue injury, and thrombus formation in experimental colitis. J Exp Med. 2007;204:1595–1601. doi: 10.1084/jem.20062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida H, Russell J, Stokes KY, et al. Role of the protein C pathway in the extraintestinal thrombosis associated with murine colitis. Gastroenterology. 2008;135:882–888. doi: 10.1053/j.gastro.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida H, Russell J, Granger DN. Thrombin mediates the extraintestinal thrombosis associated with experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G904–G908. doi: 10.1152/ajpgi.90400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 49.Li W, Zheng X, Gu J, et al. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost. 2005;3:1351–1359. doi: 10.1111/j.1538-7836.2005.01385.x. [DOI] [PubMed] [Google Scholar]
- 50.Rumbaut RE, Randhawa JK, Smith CW, et al. Mouse cremaster venules are predisposed to light/dye-induced thrombosis independent of wall shear rate, CD18, ICAM-1, or P-selectin. Microcirculation. 2004;11:239–247. doi: 10.1080/10739680490425949. [DOI] [PubMed] [Google Scholar]
- 51.Rumbaut RE, Bellera RV, Randhawa JK, et al. Endotoxin enhances microvascular thrombosis in mouse cremaster venules via a TLR4-dependent, neutrophil-independent mechanism. Am J Physiol Heart Circ Physiol. 2006;290:H1671–H1679. doi: 10.1152/ajpheart.00305.2005. [DOI] [PubMed] [Google Scholar]
- 52.Ollivier V, Parry GC, Cobb RR, et al. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271:20828–20835. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- 53.Osnes LT, Foss KB, Joø GB, et al. Acetylsalicylic acid and sodium salicylate inhibit LPS-induced NF-kappa B/c-Rel nuclear translocation, and synthesis of tissue factor (TF) and tumor necrosis factor alfa (TNF-alpha) in human monocytes. Thromb Haemost. 1996;76:970–976. [PubMed] [Google Scholar]
- 54.Szotowski B, Antoniak S, Poller W, et al. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida H, Russell J, Granger DN. TNF-alpha play an important role in extra-intestinal thrombus formation associated with inflammatory bowel disease. FASEB J. 2009 (in press) [Google Scholar]
- 56.Clegg CH, Rulffes JT, Haugen HS, et al. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int Immunol. 1997;9:1111–1122. doi: 10.1093/intimm/9.8.1111. [DOI] [PubMed] [Google Scholar]
- 57.Slupsky JR, Kalbas M, Willuweit A, et al. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80:1008–1014. [PubMed] [Google Scholar]
- 58.Prasad KS, Andre P, Yan Y, et al. The platelet CD40L/GP IIb-IIIa axis in atherothrombotic disease. Curr Opin Hematol. 2003;10:356–361. doi: 10.1097/00062752-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 59.André P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 60.Russell J, Stokes KY, Granger DN. Role of CD40-CD40L signaling in the extra-intestinal thrombosis associated with colonic inflammation. FASEB J. 2008;22:924–8. [Google Scholar]
- 61.Ostrovsky L, Woodman RC, Payne D, et al. Antithrombin III prevents and rapidly reverses leukocyte recruitment in ischemia/reperfusion. Circulation. 1997;96:2302–2310. doi: 10.1161/01.cir.96.7.2302. [DOI] [PubMed] [Google Scholar]
- 62.Sharma L, Melis E, Hickey MJ, et al. The cytoplasmic domain of tissue factor contributes to leukocyte recruitment and death in endotoxemia. Am J Pathol. 2004;165:331–340. doi: 10.1016/S0002-9440(10)63300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnold CS, Parker C, Upshaw R, et al. The antithrombotic and anti-inflammatory effects of BCX-3607, a small molecule tissue factor/factor VIIa inhibitor. Thromb Res. 2006;117:343–349. doi: 10.1016/j.thromres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47:305–314. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Levi M, Dörffler-Melly J, Reitsma P, et al. Aggravation of endotoxin-induced disseminated intravascular coagulation and cytokine activation in heterozygous protein-C-deficient mice. Blood. 2003;101:4823–4827. doi: 10.1182/blood-2002-10-3254. [DOI] [PubMed] [Google Scholar]
- 66.Fries W, Pagiaro E, Canova E, et al. The effect of heparin on trinitrobenzene sulphonic acid-induced colitis in the rat. Aliment Pharmacol Ther. 1998;12:229–236. doi: 10.1046/j.1365-2036.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 67.Celasco G, Moro L, Bozzella R, et al. Efficacy of Intracolonic Administration of Low-Molecular-Weight Heparin CB-01-05, Compared to Other Low-Molecular-Weight Heparins and Unfractionated Heparin, in Experimentally Induced Colitis in Rat. Dig Dis Sci. 2008;53:3170–3175. doi: 10.1007/s10620-008-0299-6. [DOI] [PubMed] [Google Scholar]
- 68.Pastorelli L, Saibeni S, Spina L, et al. Oral, colonic-release low-molecular-weight heparin: an initial open study of Parnaparin-MMX for the treatment of mild-to-moderate left-sided ulcerative colitis. Aliment Pharmacol Ther. 2008;28:581–588. doi: 10.1111/j.1365-2036.2008.03757.x. [DOI] [PubMed] [Google Scholar]
- 69.Chande N, McDonald JW, Macdonald JK. Unfractionated or low-molecular weight heparin for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2008;2:CD006774. doi: 10.1002/14651858.CD006774.pub2. [DOI] [PubMed] [Google Scholar]
- 70.Papa A, Danese S, Gasbarrini A, et al. Review article: potential therapeutic applications and mechanisms of action of heparin in inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1403–1409. doi: 10.1046/j.1365-2036.2000.00860.x. [DOI] [PubMed] [Google Scholar]
- 71.Twig G, Zandman-Goddard G, Szyper-Kravitz M, et al. Systemic thromboembolism in inflammatory bowel disease: mechanisms and clinical applications. Ann N Y Acad Sci. 2005;1051:166–173. doi: 10.1196/annals.1361.058. [DOI] [PubMed] [Google Scholar]
- 72.Spek CA, ten Kate FJ, te Velde AA. Factor V Leiden and the etiology of inflammatory bowel. Thromb Haemost. 2007;98:670–673. doi: 10.1160/th07-02-0129. [DOI] [PubMed] [Google Scholar]