Abstract
We report a case of multiple myeloma with gastric involvement occurring in a patient who underwent an upper gastrointestinal series (UGIS), CT and MRI. UGIS depicted a luminal protruding mass, while contrast-enhanced CT demonstrated marked thickening of the gastric wall, with subtle contrast enhancement. At T1- and T2-weighted MR imaging, the mass showed iso- and intermediate signal intensity, respectively. After the administration of contrast material, subtle homogeneous enhancement was apparent.
Keywords: Stomach, neoplasms; Stomach, radiography; Stomach, magnetic resonance imaging; Stomach, CT
In multiple myeloma, involvement of the gastrointestinal tract (gastric myeloma) is rare. Although more than 100 cases of gastric myeloma have been reported in the literature, only the UGIS and CT findings have, to our knowledge, been described (1-3). In this article, we report the findings in a case of gastric myeloma in which the patient underwent not only UGIS and CT, but also MR imaging studies.
CASE REPORT
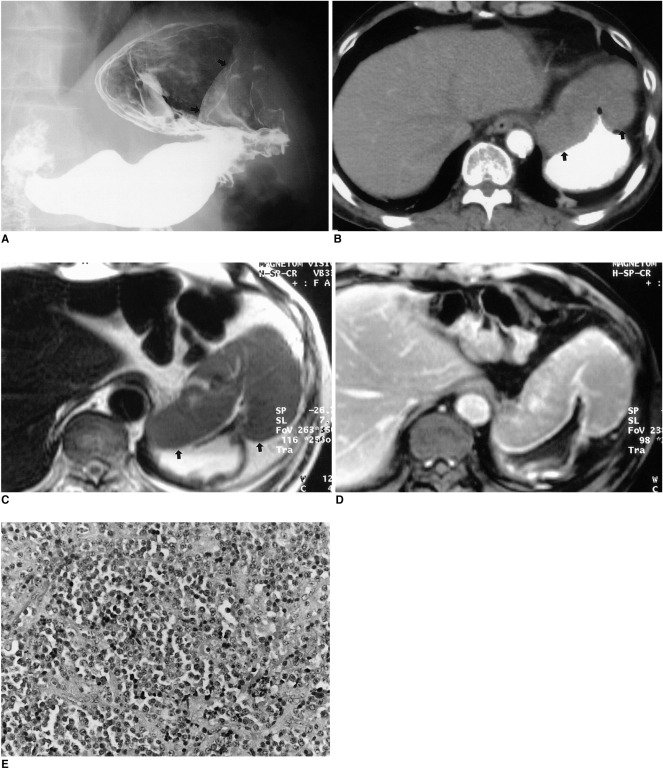
A 61-year-old man presented with epigastric pain and weight loss. Two years previously, a mass involving the left thigh had been diagnosed after wide excision as multiple myeloma and the patient had undergone chemotherapy. After 6 months, the disappearance of serumal monoclonal protein and a free light chain in the urine indicated complete remission. UGIS demonstrated a luminal protruding mass in the anterior wall of the gastric cardia and along the greater curvature of the body (Fig. 1A), while contrast-enhanced CT revealed marked thickening of the gastric wall, with subtle contrast enhancement (Fig. 1B). There was no evidence of perigastric infiltration or lymphadenopathy. MR imaging depicted a well-defined, homogeneous mass with prominent gastric wall thickening, but no necrotic portion. As demonstrated by T1-weighted imaging sequences, the signal intensity of the mass was less than that of the liver, though T2-weighting showed it as slightly higher, and homogeneous (Fig. 1C). After the administration of contrast material, the observed homogeneous enhancement was similar to that of hepatic parenchyma (Fig. 1D).
Fig. 1.
Multiple myeloma with gastric involvement in a 61-year-old man.
A. Upper gastrointestinal series shows a large, protruding, luminal mass (arrows) with a well-defined margin in the anterior wall of the gastric cardia.
B. Contrast-enhanced CT scan demonstrates marked gastric wall thickening with subtle contrast enhancement (arrows).
C. Axial T2-weighted image shows homogeneous signal intensity slightly higher than that of the liver (arrows).
D. Axial gadolinium-enhanced MR image obtained 3 minutes after the administration of contrast material depicts homogeneous enhancement, similar to that of hepatic parenchyma.
E. Photograph of endoscopic biopsy specimen shows dense and monotonous infiltration by plasma cells (original magnification, ×200; hematoxylin-eosin staining). Note the presence of a monomorphic population of plasma cells with variable atypia.
Fiberoptic gastroscopy depicted a large, protruding, luminal mass and multiple biopsies were performed. Histopathologic examination revealed dense and monotonous infiltration by both mature and immature plasma cells (Fig. 1E), which at immunohistochemistry stained positive for IgG light chains.
DISCUSSION
The extraskeletal spread of multiple myeloma occurs more frequently than is currently recognized, but clinical involvement of the gut is rarely reported. Where it does occur, the small bowel is most commonly involved, followed by the stomach, colon, and esophagus. Patients with gastric myeloma have presented with nonspecific gastrointestinal symptoms such as epigastric pain, weight loss, and upper gastrointestinal bleeding (1-4). Pathologically, gastric myeloma is thought to originate from lymphoid follicles in the submucosa, or from plasma cells in the submucosa or mucosal lamina propria (5). Early gastric myeloma is confined to the mucosa or submucosa of the gastric wall. The most advanced form of gastric myeloma infiltrates the deep muscular layer or serosa, and to avoid misdiagnosis, deep endoscopic biopsy may be required (3).
Gastric myeloma can manifest in various forms: nodular, infiltrative, ulcerative, and polypoid (5), among which the nodular type is most common. In our case the infiltrative form occurred, manifesting as diffuse thickening of the anterior wall of the gastric cardia and along the greater curvature of the body.
The radiologic findings of gastric myeloma have been reported in the literature (2, 3). UGIS has indicated the presence of infiltrative and polypoid mass lesions, though to differentiate on the basis of those findings between gastric myeloma and a gastric stromal tumor, lymphoma, neuroendocrine tumor, or adenocarcinoma is difficult. Yoon et al. (3) describe the CT findings in two patients with gastric myeloma: in both, homogeneous gastric wall thickening was apparent, and contrast enhancement was poor and similar in degree to that of paraspinal muscle. T1-weighted MR imaging sequences indicated that the signal intensity of the mass was less than that of the liver, while T2 weighting showed it as slightly higher. The administration of contrast material revealed no necrotic portion, and the homogeneous enhancement observed was similar to that of hepatic parenchyma. Although these findings simulate those of lymphoma, neuroendocrine tumor and gastric stromal tumor (6), differentiation is based on the fact that in lymphoma the nodes are usually bulkier, while a neuroendocrine or stromal tumor usually has a necrotic portion.
In conclusion, gastric myeloma is an uncommon entity that may mimic other malignant gastric tumors and tends to present as a homogeneous solid mass without a necrotic portion. Correct diagnosis depends upon a correctly interpreted biopsy combined with the immunohistochemical study of immunoglobin.
References
- 1.Kinoshita Y, Watanabe M, Takahashi H, et al. A case of gastric plasmacytoma: genetic analysis and immunofixation electrophoresis. Am J Gastroenterol. 1991;86:349–353. [PubMed] [Google Scholar]
- 2.Spagnoli I, Gattoni F, Mazzoni R, Uslenghi C. Primary gastrointestinal plasmacytoma: report of three cases. Diagn Imaging. 1983;52:23–27. [PubMed] [Google Scholar]
- 3.Yoon SE, Ha HK, Lee YS, et al. Upper gastrointestinal series and CT findings of primary gastric plasmacytoma: report of two cases. AJR. 1999;173:1266–1268. doi: 10.2214/ajr.173.5.10541102. [DOI] [PubMed] [Google Scholar]
- 4.Pimental RR, Van Stolk R. Gastric plasmacytoma: a rare cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1993;88:1963–1964. [PubMed] [Google Scholar]
- 5.Remingio PA, Klaum A. Extramedullary plasmacytoma of the stomach. Cancer. 1971;27:562–568. doi: 10.1002/1097-0142(197103)27:3<562::aid-cncr2820270308>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Marcos HB, Semelka RC. Stomach diseases: MR evaluation using combined T2-weighted single-shot echo train spin-echo and gadolinium-enhanced spoiled gradient-echo sequences. J Magn Reson Imaging. 1999;10:950–960. doi: 10.1002/(sici)1522-2586(199912)10:6<950::aid-jmri7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]