Abstract
It is clear that male hamsters discriminate between the odors of individual, conspecific females, as shown by using habituation-dishabituation methods. However, it is not clear from past research whether male hamsters are able to discriminate between the odors of estrous and non-estrous females. A series of habituation-dishabituation experiments were conducted to determine whether males discriminated between different estrous cycle states using two female secretions, those from flank glands and vaginal secretions. We found that, when habituated to a female flank-gland secretion, males discriminated between this female and a second female on the test trial, whether both were in estrus, both were in diestrus, or one was in estrus and the other in diestrus. There was no difference, however, in the magnitude of their dishabituation response toward flank-gland odors of females in estrus and diestrus. These results suggest that males use flank-gland odors to gain information primarily about individuals. When tested with vaginal secretions in habituation-dishabituation tests, males only showed differences in investigation when the second female was in estrus, indicating that males use vaginal secretions to gain information primarily about reproductive state.
Keywords: chemical communication, estrous discrimination, estrus, individual recognition, Mesocricetus auratus, Syrian hamster
INTRODUCTION
In many mammalian species, unmated females show repeated estrous cycles during which females are sexually receptive on only one or a few days of the cycle. For example, female Syrian hamsters, Mesocricetus auratus, have a very regular four-day estrous cycle in which the female is only sexually receptive on one day (Lisk, 1985). During estrus, female hamsters are amicable toward males and readily assume the receptive lordosis posture, whereas on the other three days of the estrous cycle females are aggressive toward males. Consequently it would be beneficial for males to be able to determine whether females are in estrus or not. In most species tested, males discriminate and prefer the odors of estrous females to those of non-estrous females (Taylor and Dewsbury, 1990), for example, rats (Carr et al., 1965; Lydell and Doty, 1972), mice (Hayashi and Kimura, 1974; Rose and Drickamer, 1975), brown lemmings, Lemmus sibiricus, collared lemmings, Dicrostonyx groenlandicus (Huck and Banks, 1984), desert woodrat, Neotoma lepida lepida (Fleming et al., 1981), Indian desert gerbils, Meriones hurrianae (Kumari and Prakash, 1984), kangaroo rats, Dipodomys spectabilis (Randall, 1986), Columbian ground squirrels, Spermophilus columbianus (Harris and Murie, 1984), sheep (Lindsay, 1965), dogs (Beach and Gilmore, 1949), and cats (Verberne and de Boer, 1976). In contrast, male deer mice, Peromyscus maniculatus bairdi, do not seem to show a preference for odors of estrous females (Dewsbury et al., 1986).
In Syrian hamsters, results of experiments on the ability of males to discriminate between odors from females on different days of the estrous cycle are contradictory. Many studies have shown that males do not behave differently in response to odors from estrous and non-estrous females. Such negative results have been found when using the whole body of female donors but preventing the subject males from contacting those females (i.e. males were exposed only to volatile odors), either when odors of both stimulus females were presented at the same time (Johnston, 1980; Landauer and Banks, 1973; Landauer et al., 1978), or one at a time (Johnston, 1977; Kwan and Johnston, 1980). Similar results were found when using only vaginal secretions, again without the male being able to contact the stimulus (Johnston, 1974). It could be argued, however, that one problem with non-contact methods is that they disrupt the usual response of males to female vaginal secretion, which is to contact the secretion and lick it (Johnston et al., 1993). If access to the secretion is blocked, the males spend a lot of time attempting to get at the secretion, and thus it is not clear what the measure of ‘investigation time’ is measuring – trying to contact the secretion, sexual motivation, or some other motivation imposed by the method of testing.
Allowing male hamsters to contact the stimulus does not necessarily result in different results. When males were allowed to investigate two anesthetized females, one in estrus and the other in diestrus-2, no differences were observed in the duration of investigation, licking, or mounting (Landauer et al., 1978). One problem with using the whole female as the stimulus, however, is that the male can investigate sources of odor other than vaginal secretions, such as flank-glands, ear glands, or mouth/saliva odors. These other odors may contain information about sex, individual identity and reproductive state, which may influence the amount of time the males sniff the female stimulus animal (Johnston, 2003; Johnston et al., 1993; Petrulis and Johnston, 1997). In one study this problem was addressed by placing vaginal secretions of estrous or non-estrous females on the tail region of awake stimulus males. Male subjects responded similarly to awake males scented with either estrous or non-estrous vaginal secretion (Lisk et al., 1972). It is unclear, however, how relevant those results are, given that the subject males were responding to both the female and the male odors and to the behavior of the stimulus male.
There are a few studies that indicate that males do respond differently to the odors of estrous and non-estrous females. For example, when males were placed in the empty home cage of a female every day for 21–22 days, they flank marked at different rates in response to odors of females on different days of the estrous cycle, indicating that males do perceive differences in odor across the days of the cycle (Johnston, 1975, 1980). However, when stimulus females had their vaginas removed, flank-marking by males did not vary when placed in cages of females in different estrous states, suggesting that information about reproductive state was contained primarily in vaginal secretions (Johnston, 1980). In other experiments, the ultrasonic calls of males were counted during brief exposure to an estrous female and a diestrous female. Males called more frequently when the female was in estrus, but males did this only when the stimulus females were awake and not when they were anesthetized (Floody et al., 1977), suggesting that males may have been using calls of the female or other behavioral cues to determine estrous state, but not chemical cues. The context in which tests are carried out can also influence the results. For example, males preferred the soiled bedding from estrous females over that from diestrous females when testing took place in the male’s home cage but not when it took place in a neutral arena (Johnston, 1980). In another experiment in which females had their vaginas removed and thus could not produce vaginal secretions, males did not show a preference between bedding of estrous and diestrous females (Johnston, 1980). These results appear to indicate that males are able to discriminate estrous from diestrous states by odors and that vaginal secretions may be one source of such odors. However a problem still remains: when home-cage bedding is used as the stimulus, odors from several days of the female’s cycle are combined, and it is thus unclear which odors are influencing the male’s behavior.
Huck et al. (1989) hypothesized that one reason for the negative results in studies of the discrimination of days of the estrous cycle was that so much odorous material was used (either vaginal secretions or females) that there were ceiling effects (Huck et al., 1989). These authors thus used a 4-choice olfactometer and either diluted the odors coming from four anesthetized females in different estrous states or used these odors without dilution. When odors were diluted, males investigated odors from diestrous-2 females more than those of estrous females, but when odors were not diluted, there were no differences in investigation (Huck et al., 1989). These results seem to indicate that males can discriminate different states of the estrous cycle by odors, even though the preference for diestrous odors over estrous odors was not in the expected direction.
A drawback of the negative results described above is that the lack of a preference does not necessarily prove an inability to discriminate between two stimuli; such results only show that there was no preference. Consequently, all the studies showing no preferences by male Syrian hamsters for odors of females on different days of the estrous cycle do not prove that males cannot discriminate between estrous and non-estrous females, especially when other studies provide evidence that male hamsters can indeed discriminate between odors from females in different estrous states.
The aim of the present study was to determine whether male Syrian hamsters can discriminate between the odors of estrous and non-estrous females using methods that avoid some of the pitfalls of previous studies. First, we used isolated secretions (vaginal secretions and flank-gland secretions) instead of the whole female as stimulus. Second, we allowed males to contact the stimulus. Third, we used the habituation-dishabituation design, a technique that is known to provide evidence for the ability to discriminate between different odor samples (Brown et al., 1990; Johnston, 1993; Johnston et al., 1993; Johnston and Jernigan, 1994; Schellinck and Brown, 1994).
MATERIALS AND METHODS
Animals
All animals were born and raised in captivity at Cornell University, Ithaca, NY. Hamsters were weaned at 30 days of age and housed singly in solid bottom polycarbonate cages (45 ×24 × 14.5 cm) with sani-chip bedding material and constant access to food (Prolab 1000, Agway, Syracuse, NY) and water. The colony was maintained on a 14L:10D light-dark schedule with lights off between 09:00 and 19:00 hours (Eastern Standard Time). Experiments were run within the first three hours of the dark phase.
Subjects were males and odor donors were females. The number of animals used and their ages are provided below. Male subjects and female scent donors were unrelated and unfamiliar with each other. The estrous cycles of all females were determined several days before a trial. To confirm that a female was in estrus on a specific day, a dominant male hamster was placed inside the female’s home cage. If lordosis was elicited, the female was in estrus. No mounting occurred during estrous checking. If no lordosis was observed, the female was retested on the following days until lordosis was elicited. Given the reliable 4-day estrous cycle in Syrian hamsters (Lisk, 1985), once the day of estrus was determined, we could always determine the consequent estrous and diestrous-2 days (the estrous cycle in Syrian hamsters consists of the following phases: estrus, diestrus-1, diestrus-2, and proestrus). These ‘days’ refer to the female’s state during the dark phase of the daily light cycle, during which hamsters are active in captivity (Gattermann et al., 2008).
All research was conducted with approval from Cornell University’s Institutional Animal Care and Use Committee (protocol #1993-0120).
Habituation-Dishabituation Procedure
Subject males were initially tested in four consecutive habituation trials with the odors (flank-gland secretions in experiment 1 or vaginal secretions in experiment 2) of one donor female in a given estrous state (either estrus or diestrus-2). Because the male is exposed to the same odors in these four trials, he will become habituated to those odors and investigation time will decrease significantly in the fourth habituation trial compared to the first habituation trial. After the four habituation trials, subject males were exposed to the odors of a second donor female, in either the same or the other estrous state. This was the dishabituation trial, and if the subject experiences the odors of the second donor as different from those of the first donor, we would expect an increase in investigation in the dishabituation trial compared to the fourth habituation trial. Since males are exposed to different females in the habituation and test trials, they should tend to show dishabituation in all tests trials. However, as the estrous states of the two stimulus females can be either the same or different, the males responses should also vary depending on this variable if they do indeed discriminate between different reproductive states based on the specific odor being used (flank gland or vaginal secretion). In particular, if males experience estrous odors as more salient than non-estrous odors, we expected that males would show a greater dishabituation response toward odors of estrous females than toward odors of non-estrous females. That is, we expected that the degree by which males discriminate between two unfamiliar females depends on the relative attractivity of those two females, estrous females being more attractive than non-estrous females (Lai et al., 1996). In our habituation-dishabituation tests, the increase in investigation toward the second female was expected to be lower when a male was tested with two non-estrous females, or when a male habituated to an estrous female is dishabituated with a non-estrous female, because in both cases the odors of the second female are less attractive. Conversely, the increase in investigation was expected to be higher when a male was tested with two estrous females, or when a male habituated to a non-estrous female was dishabituated with an estrous female. This pattern was expected to occur only when a particular odor carries information about the estrous cycle. Vaginal secretions differ in their chemical composition across the estrous cycle (O’Connell et al., 1981), so we expected that males would use vaginal secretions to determine the estrous state of a female. The chemistry of flank-gland secretions across the estrous cycle has not been analyzed but it may not differ across the estrous cycle; if so, males should not show differences in discrimination depending on estrous state.
Testing Procedure
We carried out two experiments, differing in the stimulus odor used (flank-gland secretion in experiment 1, vaginal secretion in experiment 2). The stimulus odor was presented on the top of a flat rectangular glass plate (8 × 16 cm) placed on the floor within the subject’s cage. Experimenters wore clean latex gloves to avoid the transfer of human scents. Each glass plate was divided in half by a line drawn on the bottom side using a blue marker. One half of the glass plate was scented on the top side with female secretion (either flank-gland or vaginal secretion), and the other half was left unscented. The glass plate was then placed on the floor of the subject’s cage for three minutes, odor side up, parallel to the front wall of the cage. Time spent investigating both halves of the glass plate was recorded using the free software Stopwatch+ (http://www.cbn-atl.org/research/stopwatch.shtml). At the end of a trial, the glass plate was removed and a 12-minute, inter-trial interval started. We used a different, clean glass plate for each trial.
Experiment 1. Flank-Gland Secretions
There were four conditions with 10 males in each. The only difference among conditions was the estrous states of the two stimulus females: (1) condition E-E, the male was habituated to the flank-gland secretions of a female in estrus, and then tested in the dishabituation trial with the flank-gland secretions of a different estrous female; (2) condition Di-Di, the first female donor was in diestrus-2, and the female donor used in the dishabituation trial was also in diestrus-2; (3) condition Di-E, the first female donor was in diestrus-2 and the second female donor in estrus; (4) condition E-Di, the first female donor was in estrus and the second female donor in diestrus-2.
For each trial, a flank gland region of the donor female was rubbed 10 times against one half of the glass plate. Male average age was 255.08 ± 10.18 d (range, 117 –375 d).
Experiment 2. Vaginal Secretions
We used the same four conditions (N = 10 per condition) as in experiment 1 (i.e. E-E, Di-Di, Di-E, and E-Di), the only difference being that instead of using flank-gland secretions from the female donors, we used vaginal secretions. We used different males than those used in experiment 1. To collect vaginal secretions, the experimenter pressed with a thumb firmly down and forward on the abdomen just above the vagina. When pressing, the vaginal secretion was expressed. Vaginal secretion samples were always collected immediately before testing; no samples were stored or frozen for later use. To apply secretion to the glass plate, the experimenter used a cotton swab. We scented one half of the glass plate with vaginal secretion covering an area approximately 1.5 cm in diameter using the cotton swab. Male average age was 306.48 ± 13.76 d (range, 128 – 462 d).
Statistical Analyses
Data from experiments 1 and 2 were analyzed separately. In experiment 1, we tried to use each subject male in only one condition. However, four males were used in two different conditions. In those statistical analyses in which data from different conditions were pooled or analyzed together, data from those four males were not included to maintain the assumption of independence (inclusion of such data, however, did not affect the result of any statistical analysis). The remaining 32 males used in experiment 1 were only used in one condition. Of the 36 males used in experiment 1, eight males had mated once prior to testing, whereas the rest were sexually naïve. In experiment 2, ten subject males were tested in all four conditions, randomizing the testing order (due to low availability of animals). Three of these males had mated once prior to testing, whereas the other seven males were sexually naïve. We did not detect any order effects (p > 0.58). Trials with the same male were separated at least by a week.
For each of the four conditions (E-E, Di-Di, Di-E, and E-Di), we used paired-sample t-tests to compare the investigation time between the first and the fourth habituation trials to determine whether habituation to the odors of the first stimulus female took place. We also used paired-sample t-tests to compare the time that males investigated the fourth habituation trial and the dishabituation trial. To compare the dishabituation response across conditions, we calculated a dishabituation response variable by subtracting the investigation time in the fourth habituation trial from the investigation time in the dishabituation trial. We compared this response variable across the four conditions using ANOVA and Tukey post-hoc tests if necessary in experiment 1, and a repeated-measures ANOVA and paired-sample t-tests as post-hoc tests in experiment 2. Finally, to determine whether males investigated the odors of females in estrus or diestrus-2 differently in their first encounter with those odors, we compared investigation of the estrous odor and diestrous-2 odor during the first habituation trial. We pooled data from conditions E-E and E-Di to get investigation time of the estrous odor, and data from conditions Di-Di and Di-E to get investigation time of diestrous-2 odor. We used an independent t-test in experiment 1 and a paired-sample t -test in experiment 2. All values are shown as mean ± sem. All statistical analyses were conducted using SPSS 14.0 for Windows.
RESULTS
Experiment 1. Flank-Gland Secretions
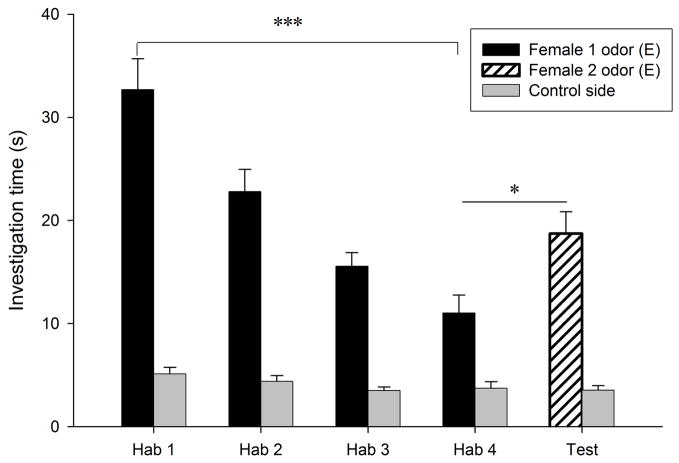
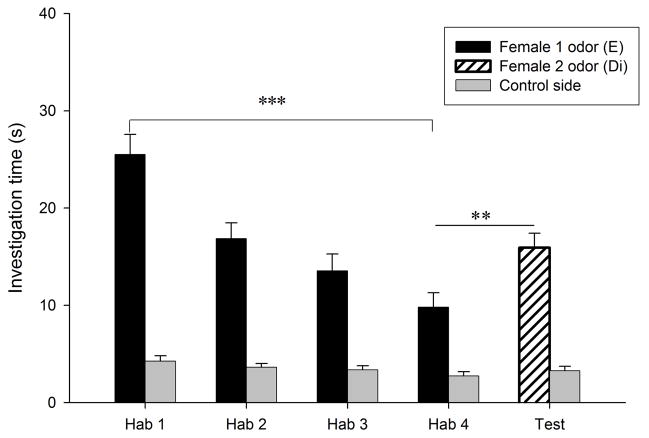
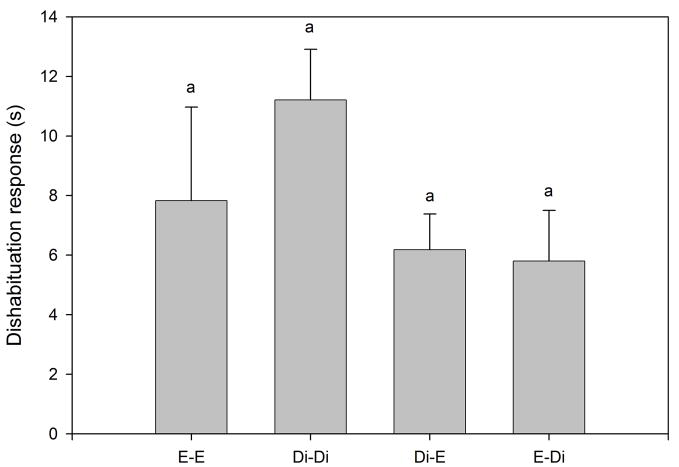
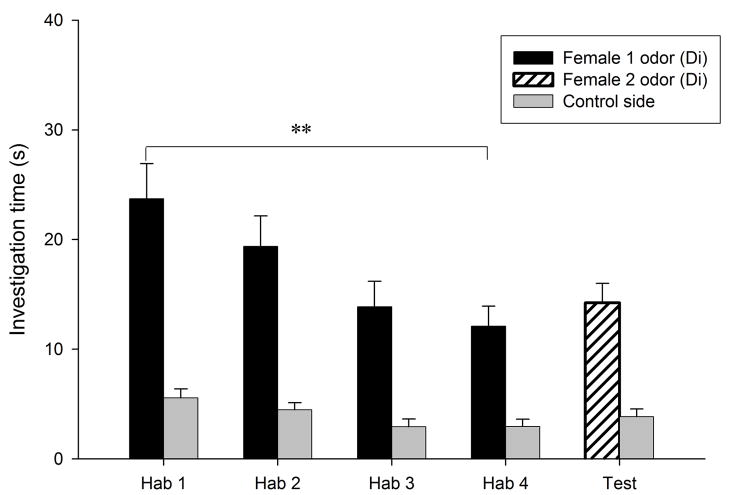
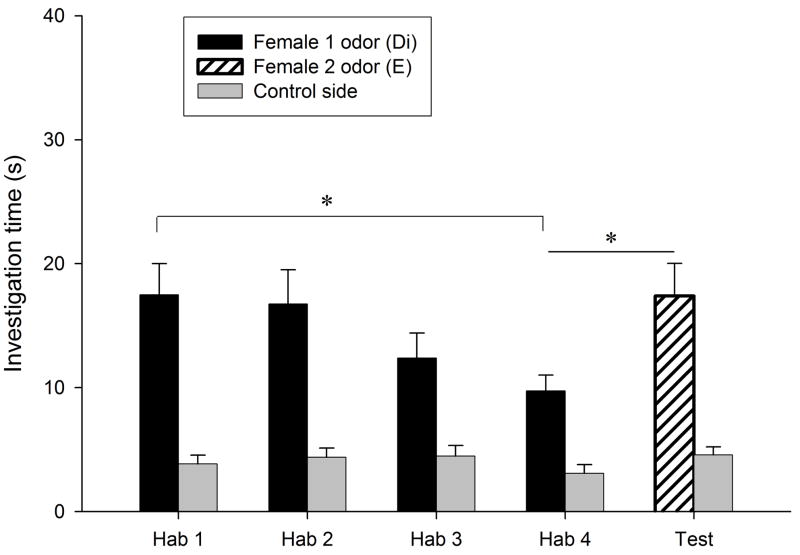
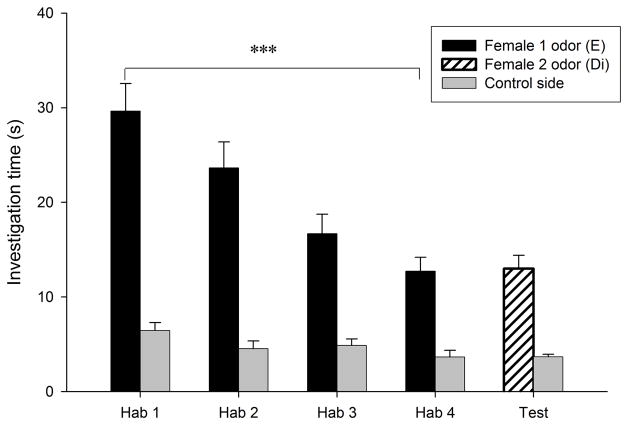
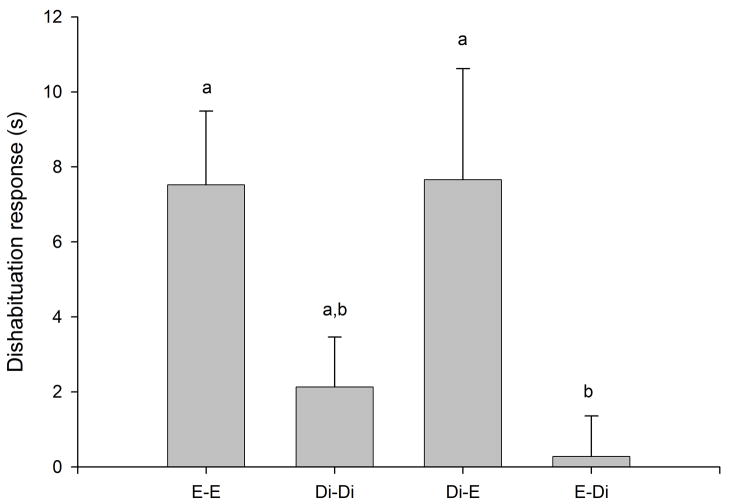
Habituation to the flank-gland secretions of the first donor female occurred in all conditions, as indicated by the significant reduction in investigation time between the first and the fourth trials (P < 0.0005 for the four conditions; Fig. 1). Dishabituation to the flank-gland secretion of the second female also was shown in all four conditions (condition E- E, t9 = −2.74, P = 0.023, Fig. 1a; condition Di-Di, t9 = −4.79, P = 0.001, Fig. 1b; condition Di-E, t9 = −5.79, P < 0.0005, Fig. 1c; condition E-Di, t9 = −3.95, P = 0.003, Fig. 1d). These results indicate that males in all four conditions discriminated between flank-gland secretions from the first and second donor females, independently of their day of the estrous cycle. The dishabituation response was not significantly different across the four conditions (F3,35 = 1.43, P = 0.25, Fig. 2).
Figure 1.
Time spent by males investigating flank-gland secretions of two different donor females in four conditions. The male was habituated to the odors of the first female and then tested in a dishabituation trial with the odors of a second female: (a) both female donors were in estrus; (b) both female donors were in diestrus-2; (c) the first female was in diestrus-2 and the second female in estrus; (d) the first female was in estrus and the second female in diestrus-2. E, estrus; Di, diestrus-2; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 2.
Dishabituation response (increase in investigation time between the fourth habituation trial and the dishabituation trial) in the four conditions (E-E, Di-Di, Di-E, and E-Di) when males were exposed to flank-gland secretions of two female donors. Di, female in diestrus-2; E, female in estrus. The same letter over the bars indicates no statistically significant differences.
In addition, males pseem able to detect estrous state from flank-gland secretions, since during the first trial males investigated secretions from estrous females (29.48 ± 1.99 s) longer than secretions from diestrous-2 females (22.81 ± 1.65 s; t31 = 2.6, P = 0.014).
Experiment 2. Vaginal Secretions
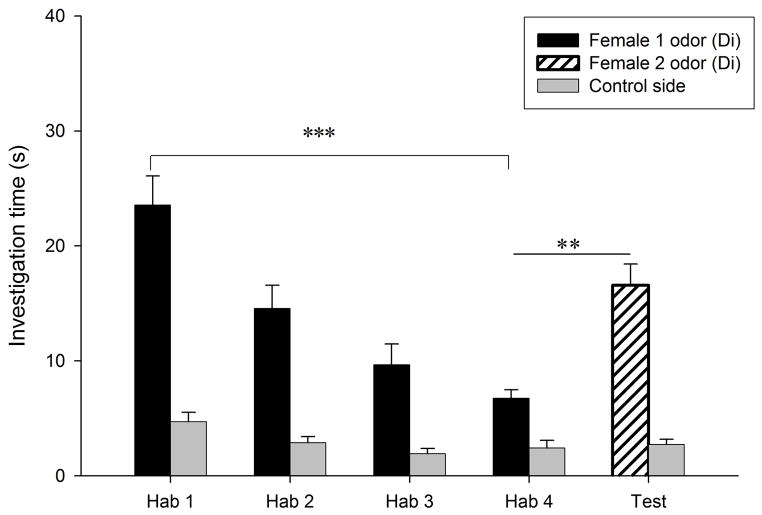
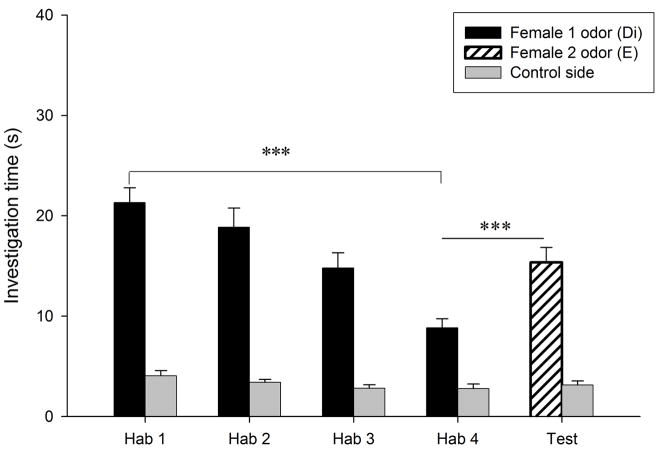
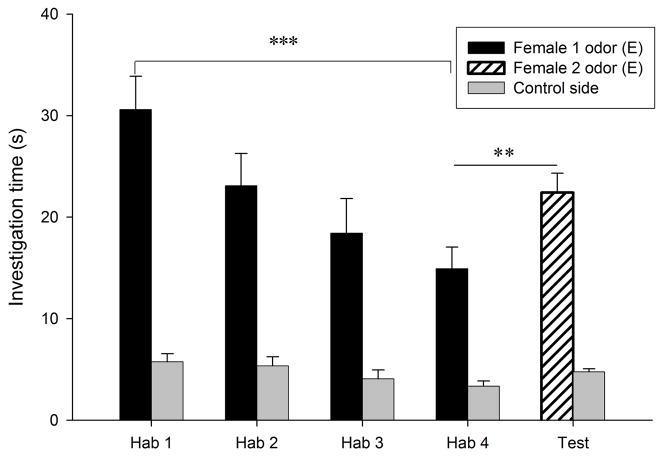
Habituation to the vaginal secretion of the first donor female occurred in all conditions (P < 0.018; Fig. 3a–d). Dishabituation to the vaginal secretion of a second female, however, only occurred when that second female was in estrus (condition E- E, t9 = −3.82, P = 0.004, Fig. 3a; condition Di-E, t9 = −2.59, P = 0.029, Fig. 3c). When the second female was in diestrus-2, males did not show a dishabituation response (condition Di-Di, t9 = −1.6, P = 0.14, Fig. 3b; condition E-Di, t9 = −0.26, P = 0.8, Fig. 3d). Consequently, there was a significant difference among conditions in the dishabituation response (F3,39 = 3.64, P = 0.022; Fig. 4), with the lowest increase in investigation occurring when the first female was in estrus and the second female was in diestrus-2 (condition E-Di, 0.28 ± 1.08 s), and large increases in investigation when the second female was in estrus (E-E and Di-E). The level of investigation in the dishabituation trial in condition E-Di was significantly lower than when the second female was in estrus (condition E-E, 7.52 ± 1.97 s, P = 0.018; condition Di-E, 7.66 ± 2.96 s, P = 0.047). These results suggest that males are more interested in vaginal secretions of estrous females than those of non-estrous females, and also that males are able to detect the estrous state of a female from her vaginal secretion. Supporting this conclusion, we found that during the first trial males investigated the vaginal secretions of estrous females (30.12 ± 2.67 s) longer than those of diestrous-2 females (20.9 ± 2.11 s, t9 = 4.94, P = 0.001).
Figure 3.
Time spent by males investigating vaginal secretions of two different donor females in four conditions. The male was habituated to the odors of the first female and then tested in a dishabituation trial with the odors of a second female: (a) both female donors were in estrus; (b) both female donors were in diestrus-2; (c) the first female was in diestrus-2 and the second female in estrus; (d) the first female was in estrus and the second female in diestrus-2. E, estrus; Di, diestrus-2; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 4.
Dishabituation response (increase in investigation time between the fourth habituation trial and the dishabituation trial) in the four conditions (E-E, Di-Di, Di-E, and E-Di) when males were exposed to vaginal secretions of two female donors. Di, female in diestrus-2; E, female in estrus. Different letters indicate P < 0.05.
DISCUSSION
Our data indicate that male Syrian hamsters behave differently to the odors of estrous and non-estrous females, especially in response to vaginal secretions. When considering the first exposure to the stimuli, males investigated estrous odors longer than non-estrous odors. This was so when males investigated vaginal secretions or flank-gland secretions. Also, when males were first habituated to vaginal secretions of one female, they showed a higher response to the odors of the second female when she was in estrus (i.e. conditions Di-E and E-E).
When exposed to female flank-gland odors, males showed discrimination between individual females in all conditions (i.e. independently of the relative estrous state of the two females). Even though the dishabituation response did not differ significantly across the four conditions, on the first trial males were more interested in the flank-gland secretions of estrous females than in those of non-estrous females, indicating that males are able to discriminate between estrous and non-estrous females by investigating flank-gland secretions. We do not know how flank-gland secretions vary chemically across the estrous cycle. Alternatively, it is possible that during self-grooming females spread vaginal secretions over their bodies, including the flank glands, and that consequently the flank-gland secretions we collected were a composite of flank-gland and vaginal secretions. However, this seems unlikely, given that odors collected from different places on the body do not contain the same types of information (Johnston, 2003), and also, if this were true, we would expect the same pattern of dishabituation in flank-gland and vaginal secretion tests. Either way, odors from the flank-gland area do not seem to be important in the response of males to females in relation to their estrous states, as shown by the similar dishabituation responses in the four test conditions.
When males were exposed only to vaginal secretions, they did not show a dishabituation response if the second female was not in estrus. A priori, there are two possible explanations for this result. First, males may be unable to discriminate between vaginal secretions from two females. However, since males can discriminate between two females when the first vaginal-secretion donor is in diestrus and the second donor is in estrus, or when both donors are in estrus, we can conclude that males have the capacity to discriminate between two females just by investigating vaginal secretions. Also, previous experiments show that male hamsters can discriminate between two estrous females on basis of vaginal secretions (Johnston et al., 1993). There remains the possibility that males are unable to discriminate between the vaginal secretions of two females when both females are in diestrus-2 (i.e. it is possible that vaginal secretions contain individual information during estrus but not during diestrus-2). The chemical composition of the vaginal secretion does change throughout the estrous cycle (O’Connell et al., 1981). For example, the molecule dimethyl disulfide, a component of the vaginal secretion considered to be a sexual attractant (however, see Petrulis and Johnston, 1995; Singer et al., 1976) has a high concentration when females are in estrus and the lowest concentration when females are in diestrus-2 (O’Connell et al., 1981). However, given that hamsters have been shown to discriminate between very similar odors and that vaginal secretions are mixtures of many compounds (Singer et al., 1976), we find it unlikely that males do not have the capacity to discriminate between the vaginal secretions of two different diestrous-2 females. A second and more likely explanation is that males do not show dishabituation when the second donor is of a lower value to the male. After all, a male exhibits his capacity to discriminate between two females by showing more interest in the novel stimulus of the second female. If that second female is much less interesting than the first female, an increase in investigation may not occur. Our results suggest that females in estrus are more interesting to males than diestrous-2 females, and that a lack of dishabituation in response to non-estrous females may simply show a lack of interest or attraction toward those females. An experiment with the flank mark odors of the same female in diestrous (habituation) and then in estrous (dishabituation) would give also interesting information to clarify this question We believe that our results offer new information to the growing literature on discrimination functionality and mechanisms (Johnston and Bullock, 2001; Tang-Martinez, 2001; Todrank and Heth, 2001). A future extension of this study would imply running experiments in which odors of the same female collected during both estrus and diestrus would be used as stimuli for the habituation and dishabituation trials. This type of design would render individual information of the donor equal between the habituation and dishabituation trials and thus would more clearly indicate the relative male interest for estrous and diestrous odors for each one of the female’s odor sources.
Acknowledgments
This work was supported by NIHM grant NIMH 5 R01 MHO58001-08 to R. E. Johnston.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beach FA, Gilmore RW. Response of male dogs to urine from females in heat. J Mammal. 1949;30:391–392. [Google Scholar]
- Brown RE, Roser B, Singh PB. The MHC and individual odors in rats. In: Macdonald DW, Müller-Schwarze D, Natynczuk SE, editors. Chemical Signals in Vertebrates. Oxford University Press; New York: 1990. pp. 228–243. [Google Scholar]
- Carr WJ, Loeb LS, Dissinger ML. Responses of rats to sex odors. J Comp Physiol Psychol. 1965;59:370–377. doi: 10.1037/h0022036. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA, Ferguson B, Hodges AW, Taylor SA. Tests of preferences of deer mice (Peromyscus maniculatus bairdi) for individuals and their odors as a function of sex and estrous condition. J Comp Psychol. 1986;100:117–127. [Google Scholar]
- Fleming AS, Chee P, Vaccarino F. Sexual behaviour and its olfactory control in the desert woodrat (Neotoma lepida lepida) Anim Behav. 1981;29:727–745. [Google Scholar]
- Floody OR, Pfaff DW, Lewis CD. Communication among hamsters by high-frequency acoustic signals. II Determinants of calling by females and males. J Comp Physiol Psychol. 1977;91:807–819. [Google Scholar]
- Gattermann R, Johnston RE, Yigit N, Fritzsche P, Larimer S, Özkurt S, Neumann K, Song Z, Colak E, Johnston J, McPhee ME. Golden hamsters are nocturnal in captivity but diurnal in nature. Biology Letters. 2008;4:253–255. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MA, Murie JO. Discrimination of oestrous status by scent in Columbian ground squirrels. Anim Behav. 1984;32:939–940. [Google Scholar]
- Hayashi S, Kimura T. Sex-attractant emitted by female mice. Physiol Behav. 1974;13:563–567. doi: 10.1016/0031-9384(74)90287-x. [DOI] [PubMed] [Google Scholar]
- Huck UW, Banks EM. Social olfaction in male brown lemmings (Lemmus sibiricus = trimucronatus) and collared lemmings (Dicrostonyx groenlandicus): I. Discrimination of species, sex, and estrous condition. J Comp Psychol. 1984;98:54–59. [PubMed] [Google Scholar]
- Huck UW, Lisk RD, Kim S, Evans AB. Olfactory discrimination of estrous condition by the male golden hamster (Mesocricetus auratus) Behav Neural Biol. 1989;51:1–10. doi: 10.1016/s0163-1047(89)90608-0. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Sexual attraction function of golden hamster vaginal secretion. Behav Biol. 1974;12:111–117. doi: 10.1016/s0091-6773(74)91101-8. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Scent marking by male golden hamsters (Mesocricetus auratus). I Effects of odors and social encounters. Z Tierpsychol. 1975;37:75–98. doi: 10.1111/j.1439-0310.1975.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Sex pheromones in golden hamsters. In: Müller-Schwarze D, Mozell MM, editors. Chemical Signals in Vertebrates. Plenum Press; New York: 1977. pp. 225–249. [Google Scholar]
- Johnston RE. Responses of male golden hamsters to odors of females in different reproductive states. J Comp Physiol Psychol. 1980;94:894–904. [Google Scholar]
- Johnston RE. Memory for individual scent in hamsters (Mesocricetus auratus) as assessed by habituation methods. J Comp Psychol. 1993;107:201–207. doi: 10.1037/0735-7036.107.2.201. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Chemical communication in rodents: from pheromones to individual recognition. J Mammal. 2003;84:1141–1162. [Google Scholar]
- Johnston RE, Bullock TA. Individual recognition by use of odours in golden hamsters: the nature of individual representations. Anim Behav. 2001;61:545–557. [Google Scholar]
- Johnston RE, Derzie A, Chiang G, Jernigan P, Lee HC. Individual scent signatures in golden hamsters: Evidence for specialization of function. Anim Behav. 1993;45:1061–1070. [Google Scholar]
- Johnston RE, Jernigan P. Golden hamsters recognize individuals, not just individual scents. Anim Behav. 1994;48:129–136. [Google Scholar]
- Kumari S, Prakash I. Perception of oestrous odours by male Meriones hurrianae. Anim Behav. 1984;32:927–929. [Google Scholar]
- Kwan M, Johnston RE. The role of vaginal secretion in hamsters sexual behavior: Male’s responses to normal and vaginectomized females and their odors. J Comp Physiol Psychol. 1980;94:905–913. doi: 10.1037/h0077814. [DOI] [PubMed] [Google Scholar]
- Lai SC, Vasilieva NY, Johnston RE. Odors providing sexual information in Djungarian hamsters: evidence for an across-odor code. Horm Behav. 1996;30:26–36. doi: 10.1006/hbeh.1996.0005. [DOI] [PubMed] [Google Scholar]
- Landauer MR, Banks EM. Olfactory preferences of male and female golden hamsters. Bulletin of the Ecological Society of America. 1973;54:44. [Google Scholar]
- Landauer MR, Banks EM, Carter CS. Sexual and olfactory preferences of naive and experienced male hamsters. Anim Behav. 1978;26:611–621. [Google Scholar]
- Lindsay DR. The importance of olfactory stimuli in the mating behaviour of the ram. Anim Behav. 1965;13:75–78. [Google Scholar]
- Lisk RD. The estrous cycle. In: Siegel HI, editor. The Hamster: Reproduction and Behavior. Plenum Press; New York: 1985. pp. 23–51. [Google Scholar]
- Lisk RD, Zeiss J, Ciaccio LA. The influence of olfaction on sexual behavior in the male golden hamster (Mesocricetus auratus) J Exp Zool. 1972;181:69–78. doi: 10.1002/jez.1401810108. [DOI] [PubMed] [Google Scholar]
- Lydell K, Doty RL. Male rat odor preferences for female urine as a function of sexual experience, urine age, and urine source. Horm Behav. 1972;3:205–212. doi: 10.1016/0018-506x(72)90033-5. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Singer AG, Stern FL, Jesmajian S, Agosta WC. Cyclic variations in the concentration of sex attractant pheromone in hamster vaginal discharge. Behav Neural Biol. 1981;31:457–464. doi: 10.1016/s0163-1047(81)91533-8. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. A reevaluation of dimethyl disulfide as a sex attractant in golden hamsters. Physiol Behav. 1995;57:779–784. doi: 10.1016/0031-9384(94)00332-7. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. Causes of scent marking in female golden hamsters (Mesocricetus auratus): Specific signals or classes of information? J Comp Psychol. 1997;111:25–36. doi: 10.1037/0735-7036.111.1.25. [DOI] [PubMed] [Google Scholar]
- Randall JA. Preference for estrous female urine by male kangaroo rats (Dipodomys spectabilis) J Mammal. 1986;67:736–739. [Google Scholar]
- Rose E, Drickamer LC. Castration, sexual experience, and female urine odor preferences in adult BDF1 male mice. Bull Psychon Soc. 1975;5:84–86. [Google Scholar]
- Schellinck HM, Brown RE. Methodological questions in the study of the rat’s ability to discriminate between the odours of individual conspecifics. Adv Biosci. 1994;93:427–436. [Google Scholar]
- Singer AG, Agosta WC, O’Connell RJ, Pfaffmann C, Bowen DV, Field FH. Dimethyl disulfide: an attractant pheromone in hamster vaginal secretion. Science. 1976;191:948–950. doi: 10.1126/science.1251205. [DOI] [PubMed] [Google Scholar]
- Tang-Martinez Z. The mechanisms of kin discrimination and the evolution of kin recognition in vertebrates: a critical re-evaluation. Behavioural Processes. 2001;53:21–40. doi: 10.1016/s0376-6357(00)00148-0. [DOI] [PubMed] [Google Scholar]
- Taylor SA, Dewsbury DA. Male preferences for females of different reproductive conditions: a critical review. In: Macdonald DW, Müller-Schwarze D, Natynczuk SE, editors. Chemical Signals in Vertebrates. Oxford University Press; Oxford: 1990. pp. 184–198. [Google Scholar]
- Todrank J, Heth G. Rethinking cross-fostering designs for studying kin recognition mechanisms. Anim Behav. 2001;61:503–505. [Google Scholar]
- Verberne G, de Boer J. Chemocommunication among domestic cats, mediated by the olfactory and vomeronasal senses. I Chemocommunication Z Tierpsychol. 1976;42:86–109. [PubMed] [Google Scholar]