SUMMARY
Introduction
Disorders of mood and cognition overlap in the elderly and there is an emerging consensus that both groups of disorders share neurobiological substrates.
Methods
Salient peer reviewed articles focusing on late-life depression, structural neuroimaging and recent developments in positron emission tomography based in vivo protein imaging.
Results
Epidemiological and clinical evidence indicates that mood and cognition in the elderly are clinically inter-related and common neurobiological mechanisms may underlie both groups of disorders. Degenerative, vascular and related mechanisms like genetically programmed abnormal protein deposition may provide the underlying neurobiological links between these disorders.
Conclusions
Modern neuroimaging approaches such as positron emission tomography (PET) based in vivo protein binding may help further elucidate common pathophysiological mechanisms and assist in the early identification of patients at risk for developing dementia over time. These developments have important mechanistic and public health significance in the elderly.
Keywords: protein imaging, cognition, late-life depression, dementia prodrome
Historically, disorders of mood and cognition have been conceptualized and managed clinically as distinct entities. Their genetic underpinnings, pathophysiological mechanisms and clinical outcomes have been treated as very distinct with little overlap between these entities. There is, however, a growing evidence base, largely based on epidemiological and clinical observations, that suggest that mood disorders, especially in late-life and cognitive disorders overlap from both phenomenological and pathophysiological perspectives. These findings have important implications for both the diagnosis and long-term management of patients diagnosed with these disorders.
In this review, we will begin by presenting the epidemiological and clinical findings that establish a link between depression and the dementias. This will be followed by a discussion of plausible pathophysiological links between mood and cognitive disorders and of modern neuroimaging techniques aimed at identifying and estimating the protein load using in vivo approaches. We will conclude by discussing the implications of this line of enquiry for the neurobiology and the long-term management of these disorders including the concept of dementia prophylaxis.
RELATIONSHIP OF MDD TO AD
Major depressive disorder (MDD) and other clinically significant forms of ‘minor depression’ are among the most common mental disorders in the elderly (Blazer et al., 1987; Alexopoulos et al., 1988; Ruegg et al., 1988; Parmelee et al., 1989; Koenig and Blazer, 1992; Parmelee et al., 1992; Blazer, 1994). MDD has been identified both as a risk factor and a prodrome of Alzheimer’s disease (AD) in clinical and community samples (Reding et al., 1985; Kral and Emery, 1989; Kokmen et al., 1991; Jorm et al., 1991; Alexopoulos et al., 1993b; Speck et al., 1995; Devanand et al., 1996; Henderson et al., 1997; Steffens et al., 1997; Bassuk et al., 1998; Chen et al., 1999; Yaffe et al., 1999; Geerlings et al., 2000; Jorm, 2000; Lockwood et al., 2000; Visser et al., 2000; Jorm, 2001; Lockwood et al., 2002; Wilson et al., 2002; Green et al., 2003; Sweet et al., 2004; Cannon-Spoor et al., 2005; Gatz et al., 2006; Rapp et al., 2006; Steffens et al., 2006). Community based studies have identified clinical depression, including depressive symptoms as a risk factor for the subsequent development of dementia. Devanand et al. using a population based sample from northern Manhattan (NY, NY) found that after controlling for age, gender, education and initial level of cognition, depressed mood at baseline was associated with the development of AD with an odds ratio (OR) of 2.5 (Devanand et al., 1996). In the religious order study, > 600 elderly individuals were examined annually for as long as seven years (Wilson et al., 2002). Depressive symptoms at baseline predicted the subsequent development of AD in this sample and notably, with each additional symptom, the risk of AD increased by 20%. Depressive symptoms were associated with more rapid decline in episodic memory and visuospatial ability but not in semantic or working memory in this sample. The Multi Institutional Research in Alzheimer’s Genetic Epidemiology (MIRAGE) study examined the relationship between depressive symptoms and AD (Green et al., 2003). Analyzing data from a large sample of 4,046 individuals, the investigators concluded that depressive symptoms are associated with the development of AD, and that this association was stronger when depressive symptoms occurred in the year immediately prior to the onset of AD symptoms. In a study of older, educated individuals in the Netherlands, with an average follow up of 3.2 years, those with depressed mood on enrollment were more likely to develop AD, though the association was not significant among lower educated persons in the study (Geerlings et al., 2000). Not all studies found the same relationship between depression and the subsequent development of AD. A community based study in Australia found no association between baseline depression and the development of dementia 3–6 years later (Henderson et al., 1997). In a rural Pennsylvania based study, subjects with depression at the start of the study were were found to be at slightly higher risk for developing dementia (relative risk 1.27) on follow up, though the risk was not statistically significant (Chen et al., 1999). Another study of 2,812 elderly residents of New Haven, Connecticut observed that elevated levels of depression at baseline were associated with an increased risk of cognitive decline in those who were placed in the ‘medium’, as opposed to the ‘high’ category of cognitive functioning at baseline (Bassuk et al., 1998).
Using data from a clinical sample, Alexopoulos et al. observed that patients diagnosed with MDD who concurrently presented with executive function impairment were more likely to develop clinical dementia on follow up (Alexopoulos et al., 1993a). Reporting on a sample of 57 elderly patients diagnosed with MDD, they demonstrated that 43% of patients with MDD and cognitive impairment progressed to clinical dementia over time. This occurred even when the mood and cognitive symptoms improved with antidepressant treatment. Kral and coworkers reporting on a sample of elderly patients diagnosed with MDD also observed that a large proportion of patients evolved clinically into dementia over a span of 8 years (Kral and Emery, 1989).
DEPRESSION AND COGNITION
Cognitive performance of depressed late-life patients has been well described in studies over the past 15 years. Deficits have been reported in domains of attention, language, episodic recall, semantic recall, nonverbal recall, visuospatial/visuoconstruction, working memory, and executive function (Abas et al., 1990; King et al., 1991; Boone et al., 1994; Boone et al., 1995; Lesser et al., 1996; Palmer et al., 1996; Kramer-Ginsberg et al., 1999; Lyness et al., 1999; Yaffe et al., 1999; Nebes et al., 2000; Palsson et al., 2000; Nebes et al., 2001b; Swainson et al., 2001; Elderkin-Thompson et al., 2003; Elderkin-Thompson et al., 2004b). Specifically, they have reduced language fluency (Wolfe et al., 1987; King et al., 1991; Boone et al., 1994; Brown et al., 1994; Palmer et al., 1996), poor processing speed and attentional ability (Boone et al., 1995; Yaffe et al., 1999; Nebes et al., 2000; Palsson et al., 2000; Nebes et al., 2001a), poor inhibition of conflicting information (Palmer et al., 1996), and impaired visuospatial skills (Boone et al., 1994; Boone et al., 1995; Lesser et al., 1996). Patients have deficits in complex tests such as the Wisconsin Card Sort (Boone et al., 1994; Brown et al., 1994; Boone et al., 1995; Lesser et al., 1996), which assesses abstract problem solving and flexibility when using environmental feedback. Recall is compromised when recalling unstructured word lists or semantically organized verbal stories (King et al., 1991; Palmer et al., 1996; Kramer-Ginsberg et al., 1999; Palsson et al., 2000; Swainson et al., 2001). The deficit appears also in recall of nonverbal designs (Lesser et al., 1996; Palmer et al., 1996; Elderkin-Thompson et al., 2004b). Researchers using the full scale Weschsler Adult Intelligence Scale III (WAIS III) and the verbal IQ and Performance IQ subscales of the WAIS III have also observed deficits (Boone et al., 1995; Palmer et al., 1996).
The magnitude of cognitive dysfunction in patients with MDD appears to be spread somewhat equally across all domains, including executive functions and memory, when compared with patients with AD where memory impairments are more striking than impairments in executive function. A meta analysis of late-life depression studies that weighted studies according to size and divided patients into hospitalized and nonhospitalized groups found that a generally uniform effect size deficit existed across all domains (Elderkin-Thompson et al., 2004a). No domain appeared selectively impaired relative to the other domains. From a neuroanatomical perspective, while the association between activation of the temporal region and recall has been well substantiated, the frontal region is also implicated in recall and attentional tasks as well as executive functions (Cabeza and Nyberg, 2000; Kramer et al., 2005; Alessio et al., 2006). Alexopoulos has suggested the use of the ‘depression-executive dysfunction syndrome’ of late-life to reflect the dysfunction of the dorsolateral neural circuits and its association with reduced physical and cognitive functioning of the patient, (Alexopoulos et al., 2002) poor response to treatment and high rates of relapse (Kalayam and Alexopoulos, 1999). Functional imaging studies of overlapping neural network functionality are consistent with the neuropsychological findings that multiple cognitive domains can become compromised at an early stage of a disease process even though the disease itself may be considered localized.
DEPRESSION AND MCI
The attentional, recall and executive deficits observed among depressed patients are also closely associated with early AD (Robbins et al., 1996), and depression is considered a major risk factor for the development of dementia (van Reekum et al., 1999; Geda et al., 2006; Lopez et al., 2006). Among patients with mild cognitive impairment (MCI) of the amnestic type, the memory deficit is an isolated deficit (Winblad et al., 2004). MCI patients who are impaired in multiple domains usually have a memory impairment, but it is of less magnitude than that observed in patients classified as amnestic MCI (Lopez et al., 2006). The multiple-domain form of MCI is the most prevalent form of MCI (Lopez et al., 2006) and is a better indicator of mild AD than memory impairment alone (Masur et al., 1994; Jacobs et al., 1995; Kluger et al., 1999; Petersen et al., 2001; Winblad et al., 2004). The neuropsychological profile of a multiple-domain MCI person appears similar to the profile of late-life depressed persons observed in the meta-analysis (Elderkin-Thompson et al., 2004a).
MECHANISMS LINKING DEPRESSION AND DEMENTIA
While the weight of the evidence indicates a significant relationship between a history of depression and the development of dementia, especially AD, the precise mechanisms that link these two disorders is not well understood. There are several plausible mechanisms. These include neuronal atrophy (degeneration), vascular pathophysiology, genetic susceptibility and amyloid deposition.
ATROPHY
MRI has been extensively applied to the study of brain structure in psychiatric diseases (Coffey et al., 1988; Krishnan et al., 1988; Morris and Rapoport, 1990; Zubenko et al., 1990; Rabins et al., 1991; Coffey et al., 1993; Fujikawa et al., 1993; Sheline et al., 1996; Alexopoulos et al., 1997; Krishnan et al., 1997; Kumar et al., 1997a, 1997b; Kumar et al., 1998; Lai et al., 2000; Kumar et al., 2002b; Steffens et al., 2002). Volumetric reductions occur in patients diagnosed with late-life depression predominantly in the prefrontal regions and hippocampus when compared with controls (Steffens et al., 2002; Ballmaier et al., 2004). A reduction in the volume of the orbitofrontal cortex has been reported in patients with late-life depression (Lai et al., 2000). Additionally, smaller volumes of the caudate nucleus and the cerebellar vermis have been reported in patients with depression when compared with controls (Krishnan, 1993). These findings formed the basis of speculation that abnormalities in the subcortical-frontal pathways may underlie the depression in some instances. Our findings indicate that smaller frontal lobe volumes in patients with MDD are circumscribed and occur in the anterior cingulate, gyrus rectus and the orbitofrontal regions (Ballmaier et al., 2004). Recently, our group (Ballmaier et al., 2007) demonstrated smaller hippocampal volumes, largely restricted to the CA1 and subiculum subfields in patients with late-life depression when compared with controls. The differences were more striking in the group with late onset depression (operationally defined as onset of the first episode occurring after age 60). Smaller hippocampal volumes were also associated with poorer performance on specific cognitive tasks in this study.
VASCULAR
MRI determined high intensity lesions are more frequently observed in patients with late-life MDD when compared with controls. The putative link between high intensity lesions and MRI lesions has been interpreted as support for a vascular basis to depression in late-life (Krishnan et al., 1997; Kumar et al., 2002a). The high intensity lesions may account for some, though not all of the cognitive deficits observed in patients with late-life MDD (Lesser et al., 1996; Salloway et al., 1996; O’Brien et al., 1998; Kramer-Ginsberg et al., 1999; Nebes et al., 2001b). It is important to remember that while AD is not typically conceptualized as a vascular dementia, mixed vascular-degenerative dementias are increasingly recognized both clinically and neuropathologically. Vascular mechanisms clearly contribute to late-life depression and preliminary work in our laboratory indicates that smaller frontal lobe volumes (degeneration) and high intensity lesions (putatively linked to vascular disease) detected using MRI are complementary, albeit autonomous pathways to MDD (Kumar et al., 2000, 2002a). These pathways are comparable to mechanisms and pathways that have been implicated in AD and mixed dementia and suggest that neurodegeneration and vascular compromise may represent pathophysiological mechanisms that transcend traditional diagnostic boundaries.
THE ROLE OF AMYLOID AND TAU
In a preliminary, but important, report on the neuropathological correlates of cognitive changes in patients initially diagnosed as having late-life MDD, Sweet et al. examined post mortem brain tissue in nine patients diagnosed as having late-onset major depression and one patient diagnosed with bipolar disorder (Sweet et al., 2004). This sample of patients had MDD without clinical evidence of dementia during the initial assessment and participated in research protocols for patients with late-life major depression. The follow-up period varied from 7 to 87 months. Eight patients evolved clinically into dementia over time and the remaining two did not develop dementia. Post mortem examination of brain tissue showed neuropathological hallmarks of AD in six subjects. Diffuse Lewy body pathology was found in one subject and rare diffuse amyloid plaques restricted to the middle frontal gyrus in another subject. Three patients with AD pathology also displayed evidence of vascular disease and the other three demonstrated Lewy body pathology (dementia with Lewy bodies being the secondary diagnosis). In another recent neuropathological study, increased numbers of plaques and tangles in the hippocampal region were identified in post mortem tissue in AD patients with prior episodes of major depression (Rapp et al., 2006). The density of plaques and tangles were even greater in patients in whom there was concurrent depression and dementia at the time of the initial AD diagnosis (Rapp et al., 2006).
NEURODEGENERATIVE CHANGES IN AGING AND AD
Neuropathological, neuroimaging, and clinical research support the idea that the dementing process leading to AD begins years before a clinical diagnosis of probable AD can be confirmed (McKhann et al., 1984). Post-mortem studies of non-demented older people (Price and Morris, 1999) indicate that tangle density in healthy aging correlates with age, but that some cases demonstrate widely distributed neuritic and diffuse plaques throughout neocortex and limbic structures. This preclinical AD group also shows increased tangles. Braak and Braak have shown that neurofibrillary tangle density increases in some individuals, presumably those who will eventually develop AD, very early in adult life, perhaps even by the fourth decade (Braak and Braak, 1991). The diffuse amyloid deposits in middle-aged non-demented subjects are consistent with an early stage of AD pathology and suggest that the pathological process progresses gradually, taking 20 to 30 years for the clinical manifestation of dementia (Arai et al., 1999). Our studies (Small et al., 1995, 2000), confirmed by others (Reiman et al., 1996, 2001), indicate lower regional brain metabolism in middle-aged and older persons with a genetic risk (APOE-4), lending further support for a prolonged presymptomatic AD stage.
IN VIVO SP AND NFT MEASURES
Previous feasibility analysis demonstrated the possibility of brain receptor imaging with PET (Small et al., 2006a). Using receptor-binding concepts, the feasibility of imaging Aβ (and tau) aggregates (SPs and NFTs, respectively) in the living brain of AD patients can be established by analogous analysis. This assumes that the molecular imaging probe binds to the aggregate site(s) in a saturable and specific manner, similar to neuroreceptor binding.
A feature of the pathogenesis of AD is the pathological aggregation of the β-amyloid peptide into fibrillary SPs and the hyperphosphorylation of the tau protein into NFTs. The prospect of in vivo visualization of these neuropathological lesions has driven several groups [e.g. Pittsburgh (Klunk et al., 2003b, 2004), UCLA (Shoghi-Jadid et al., 2002), University of Pennsylvania (Kung et al., 2003)] to search for imaging biomarkers of these pathologies. The ideal AD imaging biomarker should be specific for the intended molecular targets (e.g. amyloid and tau aggregates or both), clear well from non-specific binding areas (i.e. have low general lipid binding, like white matter), and yield a reliable signal to noise ratio for amyloid/tau to non-specific sites. All this assumes that the probe binds to the aggregate site(s) in a saturable and specific manner, similar to neuroreceptor binding, although it is now apparent that amyloid and tau aggregates are complex conglomerates that contain multiple binding sites with different affinities for probes (e.g. [18F]FDDNP binds at sites different from thioflavin probes in general). Similar to receptor binding, binding of Aβ (and tau) aggregates should be displaceable in vivo (Kepe et al., 2006).
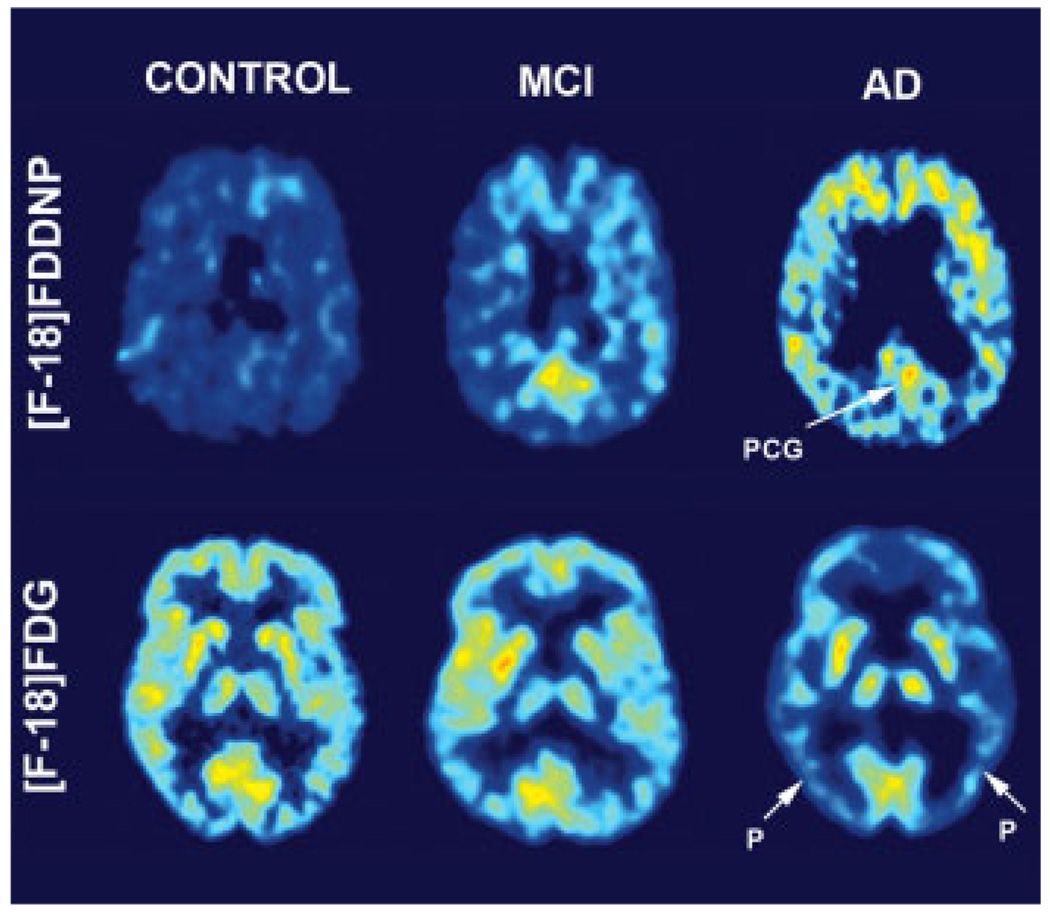
Two ligands emerged as primary candidates for imaging protein aggregates in the living brain. These are 1,1-dicyano-2-[6-(dimethylamino)-2-naphthalenyl]-propene ([18F]FDDNP) and N-methyl-[11C]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole (Pittsburgh compound B or PIB). PET studies using both ligands are able to discriminate between AD patients and controls, potentially track conversion from mild cognitive impairment to dementia (see Figure 1) and discriminate patients diagnosed with AD from Prion dementia (Small et al., 2006b; Mintun et al., 2006; Boxer et al., 2007; Rabinovici et al., 2007).
Figure 1.
Provides representative examples of PET images (FDDNP) binding and glucose metabolism in subjects diagnosed with MCI (mild cognitive impairment), AD (Alzheimer disease), and Healthy Control. Bright areas show increased glucose utilization and FDDNP binding. Note decrease in glucose metabolism and increase in FDDNP binding from MCI to AD. P, Parietotemporal Cortex; PCG, Posterior Cingulate Cortex.
Results in living patients with [18F]FDDNP confirm the in vitro analysis of feasibility about in vivo detection of brain pathologies in dementia patients. Analysis of binding data in all neocortical areas of AD patients offers an understanding of the progressive nature of the disease in agreement with the Braak model of brain neuropathological changes (Braskie et al., unpublished observation). Excellent correlations with glucose metabolic rates (FDG-PET) in the same subjects are also observed. Initial neuropathological processes occur in the medial temporal lobe, expanding later to the rest of the temporal lobe, the parietal lobe and finally engulfing the whole neocortex. Brain pathology accumulation in the medial/lateral temporal lobe, and not the average Logan standard uptake volume (SUV) throughout the cortex, is emerging as a key tool to identify early brain pathology in agreement with earlier reports (Shoghi-Jadid et al., 2002). The sensitivity of [18F]FDDNP to both NFTs and SPs offers an opportunity to follow the neuropathological evolution of the disease, initiated by intraneuronal NFT formation in the transentorhinal cortex, entorhinal cortex and hippocampus. [18F]FDDNP also has permitted the visualization of tauopathies in living patients. Frontal lobe dementia patients present prominent frontal and temporal signals compared with controls, suggesting [18F]FDDNP utility in differentiating FTD from AD (Small et al., 2002; Boxer et al., 2007).
PIB retention also differentiates AD patients from controls (Klunk et al., 2003a, 2004). Healthy control subjects show little PIB retention in cortical areas, while patients diagnosed with AD show PIB retention in frontal, temporal and parietal regions. In the cerebellum and the white matter, areas without known amyloid distribution, retention in controls and AD patients were comparable with PIB. However, the pattern is somewhat distinct from the pattern of distribution of protein aggregates in the brain that typically begins in the entorhinal areas and progress to involve the other limbic and neocortical areas.
The early success with the use of [18F]FDDNP (Shoghi-Jadid et al., 2002) and PIB (Klunk et al., 2004) offers an unprecedented opportunity to follow the neuropathological evolution of AD in living subjects. These in vivo probes can also be applied to examine the biology of related disorders such as depression where there is considerable overlap in neurobiological substrates. Post mortem studies in patients with late-life depression are limited and point to focal pathological changes that may contribute to the pathophysiology of the disorder (Rajkowska, 2000). Post mortem tissue from well characterized samples will help better elucidate the pathways to depression, especially if the post mortem data can be integrated with antemortem neuroimaging findings.
NEUROSCIENTIFIC IMPLICATIONS
Depression in the elderly is characterized by multiple cognitive aberrations perhaps most striking in the domains of executive functions, attention and memory. Executive function impairment is associated with poorer clinical response to antidepressants and a more chronic course of illness. Long-term response to antidepressants may be modest in patients where the mood disturbance is a prodrome of dementia. Specific cognitive profiles may help identify depressed patients at risk for developing dementia over time. Vascular, degenerative, genetically mediated abnormal protein deposition and other biological processes may serve as common neurobiological processes to both depression and dementia in the elderly. Neuroimaging provides a critical link between the phenotype and its underlying neurobiological underpinnings. Protein imaging in vivo may help in ascertaining the protein load in critical brain regions in patients diagnosed with late-life depression without clinical evidence of dementia at baseline. Comparable to patients currently diagnosed with MCI, higher protein binding at baseline may predict the subsequent conversion to AD over time. The relationship of regional binding of both FDDNP and PIB to specific cognitive domains such as language, memory and executive functioning in patients with MDD will provide insights into brain-protein-behavior relationships in this patient group. If higher protein binding is associated conversion to AD, this finding will have profound implications for pharmachotherapy in patients with MDD. Patients diagnosed with MDD are treated with a combination of antidepressants and psychosocial approaches. Currently, there is no evidence base to recommend the prophylactic use of cognitive enhancers in patients diagnosed with late-life MDD. However, if PET imaging helps in identifying a subgroup of patients ‘at risk’ for developing dementia over time, it will help us reconceptualize management approaches to patients diagnosed with MDD. The approaches have broad implications and include more precise psychosocial approaches such as family education on the dementia spectrum, disease progression and recommendations about driving and conservatorship. As more effective compounds become available over time, the early identification of patients at risk for developing specific neurodegenerative disorders will become critical and widespread. A combination of judicious clinical assessment combined with precise neuroimaging measures is likely to have major clinical and public health impact.
KEY POINTS.
Mood and cognition in the elderly are interrelated
Depression in late-life is both a risk factor and a prodrome of dementia
Plaques and tangles may be imaged in vivo using positron emission tomography (PET)
ACKNOWLEDGEMENTS
CONFLICT OF INTEREST
The University of California, Los Angeles (UCLA), owns a U.S. patent, Methods for Labeling β-Amyloid Plaques and Neurofibrillary Tangles (6,274,119), that uses the approach outlined in this article and has been licensed to Siemens. The FDDNP synthesis was performed at the UCLA Cyclotron Laboratory under Dr Satyamurthy’s direction. Drs Small, Huang, Cole, Satyamurthy, and Barrio, who are among the inventors, report receiving royalties and will receive royalties on future sales. Dr Small reports receiving consulting fees, lecture fees, or both from Abbott, Brainstorming, Dakim, Eisai, Forest, the Memory Fitness Institute, Myriad Genetics, Novartis, Ortho-McNeil, Pfizer, Radica, and Siemens, stock options from Dakim, and a grant from GlaxoSmithKline; Dr Kepe, consulting fees from Siemens; Dr Huang, lecture fees from GlaxoSmithKline; Dr Satyamurthy, consulting fees from PETNet Pharmaceuticals and Siemens; Dr Barrio, consulting fees and lecture fees from Nihon Medi-Physics, Bristol-Myers Squibb, PETNet Pharmaceuticals, and Siemens; and Dr Kumar, one-time consultant to Bristol-Myers Squibb.
Financial support of the NIH through grants MH 074545:01, MH 063764:04, AG13308, P50AG16570, MH/AG58156, MH52453, AG10123, M01-RR00865, P01AG025831, and the Department of Energy DOE contract DE-FC03-87-ER60615.
Footnotes
No other potential conflict of interest relevant to this article was reported.
REFERENCES
- Abas MA, Sahakian BJ, Levy R. Neuropsychological deficits and CT scan changes in elderly depressives. Psychol Med. 1990;20(3):507–520. doi: 10.1017/s0033291700017025. [DOI] [PubMed] [Google Scholar]
- Alessio A, Bonilha L, Rorden C, et al. Memory And language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav. 2006;8(3):593–600. doi: 10.1016/j.yebeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the ‘depression-executive dysfunction syndrome’ of late life. Am J Geriatr Psychiatry. 2002;10(1):98–106. [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with ‘reversible dementia’: a controlled study. Am J Psychiatry. 1993a;150(11):1693–1699. doi: 10.1176/ajp.150.11.1693. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Young RC, Meyers BS. Geriatric depression: age of onset and dementia. Biol Psychiatry. 1993b;34(3):141–145. doi: 10.1016/0006-3223(93)90383-o. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Young RC, Meyers BS, et al. Late-onset depression. Psychiatr Clin North Am. 1988;11(1):101–115. [PubMed] [Google Scholar]
- Arai T, Ikeda K, Akiyama H, et al. A high incidence of apolipoprotein e epsilon4 allele in middle-aged non-demented subjects with cerebral amyloid beta protein deposits. Acta Neuropathol (Berl) 1999;97(1):82–84. doi: 10.1007/s004010050958. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Narr KL, Toga AW, et al. Hippocampal morphology distinguishes late-onset from early-onset elderly depression. Am J Psychiatry. 2007 doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161(1):99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Arch Gen Psychiatry. 1998;55(12):1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- Blazer D, Hughes DC, George LK. The epidemiology of depression in an elderly community population. Gerontologist. 1987;27(3):281–287. doi: 10.1093/geront/27.3.281. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Dysthymia in community and clinical samples of older adults. Am J Psychiatry. 1994;151(11):1567–1569. doi: 10.1176/ajp.151.11.1567. [DOI] [PubMed] [Google Scholar]
- Boone KB, Lesser IB, Miller BL, et al. Cognitive functioning in older depressed outpatients: relationship of presence and severity of depression to neuropsychological test scores. Neuropsychology. 1995;9:390–398. [Google Scholar]
- Boone KB, Lesser I, Miller B, et al. Cognitive functioning in a mildly to moderately depressed geriatric sample: relationship to chronological age. J Neuropsychiatry Clin Neurosci. 1994;6(3):267–272. doi: 10.1176/jnp.6.3.267. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Rabinovici GD, Kepe V, et al. Amyloid imaging in distinguishing atypical prion disease from alzheimer disease. Neurology. 2007;69(3):283–290. doi: 10.1212/01.wnl.0000265815.38958.b6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of alzheimer-related changes. Acta Neuropathol (Berl) 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brown RG, Scott LC, Bench CJ, Dolan RJ. Cognitive function in depression: its relationship to the presence and severity of intellectual decline. Psychol Med. 1994;24(4):829–847. doi: 10.1017/s0033291700028932. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition ii: an empirical review of 275 PET and FMRI studies. J Cogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cannon-Spoor HE, Levy JA, Zubenko GS, et al. Effects of previous major depressive illness on cognition in alzheimer disease patients. Am J Geriatr Psychiatry. 2005;13(4):312–318. doi: 10.1176/appi.ajgp.13.4.312. [DOI] [PubMed] [Google Scholar]
- Chen P, Ganguli M, Mulsant BH, Dekosky ST. The temporal relationship between depressive symptoms and dementia: a community-based prospective study. Arch Gen Psychiatry. 1999;56(3):261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Figiel GS, Djang WT, et al. Leukoencephalopathy in elderly depressed patients referred for ECT. Biol Psychiatry. 1988;24(2):143–161. doi: 10.1016/0006-3223(88)90270-3. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Weiner RD, et al. Quantitative cerebral anatomy in depression. a controlled Magnetic Resonance Imaging study. Arch Gen Psychiatry. 1993;50(1):7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53(2):175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Boone KB, Hwang S, Kumar A. Neurocognitive profiles in elderly patients with frontotemporal degeneration or major depressive disorder. J Int Neuropsychol Soc. 2004a;10(5):753–771. doi: 10.1017/S1355617704105067. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Kumar A, Bilker WB, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol. 2003;18(5):529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Kumar A, Mintz J, et al. Executive dysfunction and visuospatial ability among depressed elders in a community setting. Arch Clin Neuropsychol. 2004b;19(5):597–611. doi: 10.1016/j.acn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Yamawaki S, Touhouda Y. Incidence of silent cerebral infarction in patients with major depression. Stroke. 1993;24(11):1631–1634. doi: 10.1161/01.str.24.11.1631. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer’s disease. Arch Gen Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein e genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63(3):435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Schoevers RA, Beekman AT, et al. Depression and risk of cognitive decline and Alzheimer’s disease. results of two prospective community-based studies in The Netherlands. Br J Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer’s disease: The Mirage Study. Arch Neurol. 2003;60(5):753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- Henderson AS, Korten AE, Jacomb PA, et al. The course of depression in the elderly: a longitudinal community-based study in Australia. Psychol Med. 1997;27(1):119–129. doi: 10.1017/s0033291796004199. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Sano M, Dooneief G, et al. Neuropsychological detection and characterization of preclinical Alzheimer’s disease. Neurology. 1995;45(5):957–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000;46(4):219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- Jorm A. History of depression as a risk factor for dementia: an updated review. Aust NZ J Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Jorm A, Van Duijn CM, Chandra V, et al. the Eurodem Risk Factors Research Group. Psychiatric history and related exposures as risk factors for Alzheimer’s disease: a collaborative re-analysis of case-control studies. Int J Epidemiol. 1991;20(Suppl 2):S58–S61. doi: 10.1093/ije/20.supplement_2.s43. [DOI] [PubMed] [Google Scholar]
- Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56(8):713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- Kepe V, Huang SC, Small GW, et al. Visualizing pathology deposits in the living brain of patients with Alzheimer’s disease. Methods Enzymol. 2006;412:144–160. doi: 10.1016/S0076-6879(06)12010-8. [DOI] [PubMed] [Google Scholar]
- King DA, Caine ED, Conwell Y, Cox C. The neuropsychology of depression in the elderly: a comparative study of normal aging and Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1991;3(2):163–168. doi: 10.1176/jnp.3.2.163. [DOI] [PubMed] [Google Scholar]
- Kluger A, Ferris SH, Golomb J, et al. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol. 1999;12(4):168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, et al. Imaging the pathology of Alzheimer’s disease: amyloid-imaging with Positron Emission Tomography. Neuroimaging Clin N Am. 2003a;13(4):781–789. doi: 10.1016/s1052-5149(03)00092-3. Ix. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Wang Y, Huang GF, et al. The binding of 2-(4’-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J Neurosci. 2003b;23(6):2086–2092. doi: 10.1523/JNEUROSCI.23-06-02086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig HG, Blazer DG. Epidemiology of geriatric affective disorders. Clin Geriatr Med. 1992;8(2):235–251. [PubMed] [Google Scholar]
- Kokmen E, Beard CM, Chandra V, et al. Clinical risk factors for Alzheimer’s disease: a population-based case-control study. Neurology. 1991;41(9):1393–1397. doi: 10.1212/wnl.41.9.1393. [DOI] [PubMed] [Google Scholar]
- Kral VA, Emery OB. Long-term follow-up of depressive pseudodementia of the aged. Can J Psychiatry. 1989;34(5):445–446. doi: 10.1177/070674378903400515. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Rosen HJ, Du AT, et al. Dissociations in hippo-campal and frontal contributions to episodic memory performance. Neuropsychology. 2005;19(6):799–805. doi: 10.1037/0894-4105.19.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Ginsberg E, Greenwald BS, Krishnan KR, et al. Neuropsychological functioning and MRI signal hyperintensities in geriatric depression. Am J Psychiatry. 1999;156(3):438–444. doi: 10.1176/ajp.156.3.438. [DOI] [PubMed] [Google Scholar]
- Krishnan KR. Neuroanatomic substrates of depression in the elderly. J Geriatr Psychiatry Neurol. 1993;6(1):39–58. doi: 10.1177/002383099300600107. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Goli V, Ellinwood EH, et al. Leukoencephalo-pathy in patients diagnosed as major depressive. Biol Psychiatry. 1988;23(5):519–522. doi: 10.1016/0006-3223(88)90025-x. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154(4):497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bilker W, Jin Z, Udupa J. Atrophy and high intensity lesions: Complementary Neurobiological Mechanisms In Late-Life Major Depression. Neuropsychopharmacology. 2000;22(3):264–274. doi: 10.1016/S0893-133X(99)00124-4. [DOI] [PubMed] [Google Scholar]
- Kumar A, Jin Z, Bilker W, et al. Late-onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci USA. 1998;95(13):7654–7658. doi: 10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Miller D, Ewbank D, et al. Quantitative anatomic measures and comorbid medical illness in late- life major depression. Am J Geriatr Psychiatry. 1997a;5(1):15–25. [PubMed] [Google Scholar]
- Kumar A, Mintz J, Bilker W, Gottlieb G. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychophar-macology. 2002a;26(2):229–236. doi: 10.1016/S0893-133X(01)00331-1. [DOI] [PubMed] [Google Scholar]
- Kumar A, Schweizer E, Jin Z, et al. Neuroanatomical substrates of late-life minor depression. a quantitative magnetic resonance imaging study. Arch Neurol. 1997b;54(5):613–617. doi: 10.1001/archneur.1997.00550170085018. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gupta RK, Rathore RK, et al. Multiparametric quantitation of the perilesional region in patients with healed or healing solitary cysticercus granuloma. Neuroimage. 2002b;15(4):1015–1020. doi: 10.1006/nimg.2001.1036. [DOI] [PubMed] [Google Scholar]
- Kung HF, Kung MP, Zhuang ZP, et al. Iodinated tracers for imaging amyloid plaques in the brain. Mol Imaging Biol. 2003;5(6):418–426. doi: 10.1016/j.mibio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Lai T, Payne ME, Byrum CE, et al. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48(10):971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- Lesser IM, Boone KB, Mehringer CM, et al. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153(10):1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG. Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiatry. 2000;8(3):201–208. [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, Van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159(7):1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Jagust WJ, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77(2):159–165. doi: 10.1136/jnnp.2004.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyness JM, King DA, Cox C, et al. The importance of subsyndromal depression in older primary care patients: prevalence and associated functional disability. J Am Geriatr Soc. 1999;47(6):647–652. doi: 10.1111/j.1532-5415.1999.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Masur DM, Sliwinski M, Lipton RB, et al. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44(8):1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- Mckhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report Of The NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force On Alzheimer’ disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, et al. [11c]Pib In A Nondemented Population: Potential Antecedent Marker Of Alzheimer’ disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Morris P, Rapoport S. Neuroimaging and affective disorder in late life: a review. Can J Psychiatry. 1990;35(4):347–354. doi: 10.1177/070674379003500415. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Houck PR, et al. Dual-task performance in depressed geriatric patients. Psychiatry Res. 2001a;102(2):139–151. doi: 10.1016/s0165-1781(01)00244-x. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med. 2000;30(3):679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Vora IJ, Meltzer CC, et al. Relationship of deep white matter hyperintensities and apolipoprotein e genotype to depressive symptoms in older adults without clinical depression. Am J Psychiatry. 2001b;158(6):878–884. doi: 10.1176/appi.ajp.158.6.878. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Ames D, Chiu E, et al. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow up study. BMJ. 1998;317(7164):982–984. doi: 10.1136/bmj.317.7164.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BW, Boone KB, Lesser IM, et al. Neuropsychological deficits among older depressed patients with predominantly psychological or vegetative symptoms. J Affect Disord. 1996;41(1):17–24. doi: 10.1016/0165-0327(96)00059-6. [DOI] [PubMed] [Google Scholar]
- Palsson S, Johansson B, Berg S, Skoog I. A population study on the influence of depression on neuropsychological functioning in 85-year-olds. Acta Psychiatr Scand. 2000;101(3):185–193. [PubMed] [Google Scholar]
- Parmelee PA, Katz IR, Lawton MP. Depression among institutionalized aged: assessment and prevalence estimation. J Gerontol. 1989;44(1):M22–M29. doi: 10.1093/geronj/44.1.m22. [DOI] [PubMed] [Google Scholar]
- Parmelee PA, Katz IR, Lawton MP. Incidence of depression in long-term care settings. J Gerontol. 1992;47(6):M189–M196. doi: 10.1093/geronj/47.6.m189. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, et al. Current concepts in Mild Cognitive Impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and ‘preclinical’ Alzheimer’s disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Furst AJ, O’Neil JP, et al. 11c-Pib pet imaging in Alzheimer’s disease and frontotemporal lobar degeneration. Neurology. 2007;68(15):1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- Rabins PV, Pearlson GD, Aylward E, et al. Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry. 1991;148(5):617–620. doi: 10.1176/ajp.148.5.617. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48(8):766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer’s disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63(2):161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- Reding M, Haycox J, Blass J. Depression in patients referred to a dementia clinic. a three-year prospective study. Arch Neurol. 1985;42(9):894–896. doi: 10.1001/archneur.1985.04060080080019. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, et al. Declining brain activity in cognitively normal apolipoprotein e epsilon 4 heterozygotes: a foundation for using Positron Emission Tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98(6):3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s Disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 2001;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Elliott R, Sahakian BJ. Neuropsychology—dementia and affective disorders. Br Med Bull. 1996;52(3):627–643. doi: 10.1093/oxfordjournals.bmb.a011572. [DOI] [PubMed] [Google Scholar]
- Ruegg RG, Zisook S, Swerdlow NR. Depression in the aged. an overview. Psychiatr Clin North Am. 1988;11(1):83–99. [PubMed] [Google Scholar]
- Salloway S, Malloy P, Kohn R, et al. MRI and neuropsychological differences in early and late-onset geriatric depression. Neurol. 1996;46(6):1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93(9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoghi-Jadid K, Small GW, Agdeppa ED, et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2002;10(1):24–35. [PubMed] [Google Scholar]
- Small GW, Mazziota JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer’s disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- Small GW, Agdeppa ED, Kepe V, et al. In vivo brain imaging of tangle burden in humans. J Mol Neurosci. 2002;19(3):323–327. doi: 10.1385/jmn:19:3:321. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97(11):6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Kepe V, Barrio JR. Seeing is believing: neuroi-maging adds to our understanding of cerebral pathology. Curr Opin Psychiatry. 2006a;19(6):564–569. doi: 10.1097/01.yco.0000245747.53008.e2. [DOI] [PubMed] [Google Scholar]
- Small GW, Kepe V, Ercoli LM, et al. Pet of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006b;355(25):2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- Speck CE, Kukull WA, Brenner DE, et al. History of depression as a risk factor for Alzheimer’s disease. Epidemiology. 1995;6(4):366–369. doi: 10.1097/00001648-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Otey E, Alexopoulos GS, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006;63(2):130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Payne ME, Greenberg DL, et al. Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatry. 2002;10(1):62–71. [PubMed] [Google Scholar]
- Steffens DC, Plassman BL, Helms MJ, et al. A twin study of late-onset depression and apolipoprotein e epsilon 4 as risk factors for Alzheimer’s disease. Biol Psychiatry. 1997;41(8):851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, et al. Early detection and differential diagnosis of alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12(4):265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Hamilton RL, Butters MA, et al. Neuropathologic correlates of late-onset major depression. Neuropsychopharmacology. 2004;29(12):2242–2250. doi: 10.1038/sj.npp.1300554. [DOI] [PubMed] [Google Scholar]
- Van Reekum R, Simard M, Clarke D, et al. Late-life depression as a possible predictor of dementia: cross-sectional and short-term follow-up results. Am J Geriatr Psychiatry. 1999;7(2):151–159. [PubMed] [Google Scholar]
- Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48(5):479–484. doi: 10.1111/j.1532-5415.2000.tb04992.x. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Mendes De Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairmenTamp;Ndash;–beyond controversies, towards a consensus: report of The International Working Group On Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Granholm E, Butters N, et al. Verbal memory deficits associated with major affective disorders: a comparison of unipolar and bipolar patients. J Affect Disord. 1987;13(1):83–92. doi: 10.1016/0165-0327(87)90077-2. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Gore R, et al. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56(5):425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Sullivan P, Nelson JP, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47(10):1107–1111. doi: 10.1001/archneur.1990.00530100075016. [DOI] [PubMed] [Google Scholar]