Abstract
The complement system as a main column of innate immunity and the coagulation system as a main column in hemostasis undergo massive activation early after injury. Interactions between the two cascades have often been proposed but the precise molecular pathways of this interplay are still in the dark. To elucidate the mechanisms involved, the effects of various coagulation factors on complement activation and generation of anaphylatoxins were investigated and summarized in the light of the latest literature. Own in vitro findings suggest, that the coagulation factors FXa, FXIa and plasmin may cleave both C5 and C3, and robustly generate C5a and C3a (as detected by immunoblotting and ELISA). The produced anaphylatoxins were found to be biologically active as shown by a dose-dependent chemotactic response of neutrophils and HMC-1 cells, respectively. Thrombin did not only cleave C5 (Huber-Lang et al. 2006) but also in vitro-generated C3a when incubated with native C3. The plasmin-induced cleavage activity could be dose-dependently blocked by the serine protease inhibitor aprotinin and leupeptine. These findings suggest that various serine proteases belonging to the coagulation system are able to activate the complement cascade independently of the established pathways. Moreover, functional C5a and C3a are generated, both of which are known to be crucially involved in the inflammatory response.
1 Introduction
The complement system as a key sentinel of innate immunity and the coagulation system as main actor in hemostasis belong both to the “first line of defense” against injurious stimuli and invaders (Choi et al. 2006). Being descended from a common ancestor, interactions between both cascades have often been proposed, but the precise molecular pathways of this cross-talk have remained elusive. Immediately after severe trauma, massive activation of a series of cascading enzymatic reactions results in fibrin deposition as well as synchronic fibrinolysis (Lampl et al. 1994), which often causes an uncontrolled, systemic inflammatory response (SIRS) (Levi et al. 2004). Furthermore, both cascades contain series of serine-proteases with evidence of some shared activators and inhibitors, such as factor (F) XIIa, which is able to activate C1q, and thereby the classical pathway of complement. Similarly, the C1 esterase inhibitor acts not only as inhibitor of all three established complement pathways (classical: C1q/r/s, lectin: MBL, and alternative: C3b) but also of the endogenous coagulation activation path (kallikrein, FXIIa) (Davis 2004). Previously it has been shown that thrombin, generated at inflammatory sites in response to complement activation, is a physiological agonist for the PKC-dependent pathway of decay accelerating factor (DAF) regulation in terms of a negative feedback loop preventing thrombosis during inflammation (Lidington et al. 2000). The complement activation product C5a has also been reported to induce tissue factor (TF) activity in human endothelial cells (Ikeda et al. 1997) and may activate the exogenous (TF-dependent) coagulation pathway. In a recent study, a novel C5a receptor (C5aR)-TF cross-talk in neutrophils has been demonstrated (Ritis et al. 2006). Systemic inflammation is often triggered by severe trauma with subsequent extensive activation and depletion of the coagulation cascade. Findings from our laboratory suggest in accordance with other reports (Hecke et al. 1997; Ganter et al. 2007) that trauma leads not only to an early coagulopathy (DIC, disseminated intravascular coagulopathy) but also to an early hyper-activation of complement with generation of powerful anaphylatoxins, such as C3a and C5a, which may contribute to the disturbance of the coagulation system, and vice versa.
Therefore, it is tempting to speculate that these significant interactions between the coagulation and complement system may play an important role after trauma and for subsequent inflammatory reactions and complications.
2 Serine Protease Systems
2.1 Coagulation System
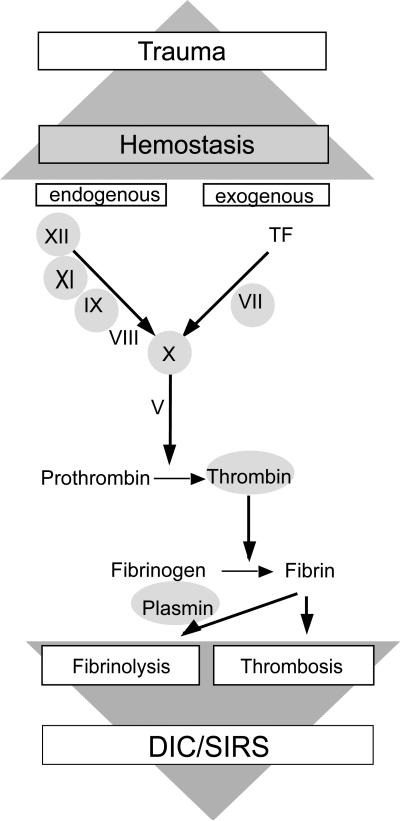
It is well known that any traumatic input rapidly activates the coagulation cascade to stop bleeding and to prevent invasion of microorganisms and the subsequent inflammatory response. Therefore, the clotting system has been considered as a crucial part of innate immunity. Most clotting factors (F) belong to the class of serine proteases (Fig. 1) with the final aim to induce fibrin polymerization in order to seal off leaking and injured vessels and also to wall off injured tissue and invading bacteria.
Fig. 1.
Activation of the coagulation cascade by trauma with subsequent responses (DIC disseminated intravascular coagulopathy; SIRS systemic inflammatory response). Grey circles = serine protease
Thrombin (FIIa) represents the central serine protease cleaving fibrinogen to fibrin, the building block of a haemostatic plug. Generation of thrombin is initiated by tissue injury with corresponding vessel wall exposure to tissue factor (TF), which forms a complex with the serine protease FVIIa. The TF/FVIIa complex as part of the exogenous path activates the down-stream serine protease FX, where the exogenous and endogenous pathways of the coagulation system converge. The endogenous path involves other serine proteases, such as FXII, FXI, and FIX which are sequentially activated upon contact to negatively charged surfaces (e.g. collagen). Factor IXa and FXa assemble with their non-enzymatic protein cofactors (FVIIIa, FVa) resulting in thrombin generation and finally in conversion of fibrinogen to fibrin.
2.2 Fibrinolytic System
Fibrinolysis is a physiological regulatory process with breakdown of fibrin to limit clotting and to resolve blood clots. The fibrinolytic system is initiated when plasminogen is converted into the potent serine-protease plasmin, which leads to degradation of fibrin, fibrinogen, FV, and FVIII. Severe trauma and hemorrhage is associated with decreased plasminogen levels and enhanced plasma levels of plasmin and plasmin-anti-plasmin-complexes, indicating early fibrinolytic events (Lampl et al. 1994). It has been described that severe trauma induces fibrinolysis almost synchronically with the massive activation and subsequent depletion of the coagulation system. Hyperfibrinolysis due to excessive generation of plasmin seems to be the major cause of trauma-induced disseminated intravascular coagulopathy (DIC) as bleeding is most severe in trauma victims with low antiplasmin activity (Lampl et al. 1994). Fibrinolysis is also promoted by activated protein C (APC) which acts as a strong serine protease by interfering with important inhibitors of plasmin generation (plasmin activator inhibitor-1 [PAI-1], thrombin-activatable fibrinolysis inhibitor [TAFI]).
2.3 Complement System
There is increasing evidence that the rapid activation of the coagulation cascade after trauma is accompanied by a very early onset of an uncontrolled, progressive inflammatory response with often lethal consequences (Hierholzer and Billiar 2001). Obviously, acute blood loss and tissue trauma activate the complement cascade in humans (Hecke et al. 1997). Especially generation of the powerful anaphylatoxin C3a and C5a, and consumption of complement may play a detrimental role (Younger et al. 2001). Generally, the complement enzymes contain a single serine protease with an extremely restricted substrate specificity. The classical pathway proceeds through the sequential cleavage of C4 and C2 by active C1s and formation of the C3 convertase (C4b2a-complex). Additional binding of C3b leads to generation of the C5 convertase (C4b3b2a-complex). Both complexes develop their proteolytic activity via the serine-protease domain of C2a. Similarly, the active center of the C3- and C5-convertase of the alternative pathway (C3bBb- and (C3b)2Bb(P)-complex, respectively) resides in the serine protease domain of factor B. Activation of the lectin pathway results in subsequent activation of mannose associated serine proteases (MASP), which in turn activate C4 and C2 to assemble C4b2a. Activated MASP-1 also reveals serine protease specificity for a direct C3 cleavage (Lambris et al. 1998).
3 C3a and C5a Generation by Coagulation Factors
Recently, we have shown that authentic C5a was generated in the absence of C3 with thrombin acting as a potent C5 convertase (Huber-Lang et al. 2006). In the presence of C3, thrombin did also generate C3a dose- and time-dependently, as assessed by immunoblotting and ELISA. The produced C3a dose-dependently increased the chemotactic response of the human mast cell line-1 (HMC-1) more than threefold, indicating biological activity of the thrombin-induced C3 cleavage product.
Investigations on other central molecules of the coagulation cascade revealed that FVIII and tissue factor failed to interact with C3 and C5. This was in striking contrast to the coagulation factors FXa and FXIa. Both serine proteases as representatives of the endogenous path cleaved C3 and C5 with generation of C3a and C5a, as detected by ELISA and Western blots. Plasmin as the strongest serine protease of the fibrinolytic system was capable of cleaving both C5 and C3, respectively. The produced anaphylatoxins were biologically active as shown by a dose-dependent chemotactic response of human neutrophils to C5a and HMC-1 cells to C3a. Furthermore, the plasmin-induced cleavage activity could be blocked by the serine protease inhibitor aprotinin and leupeptine.
4 Complement: New Activation Paths
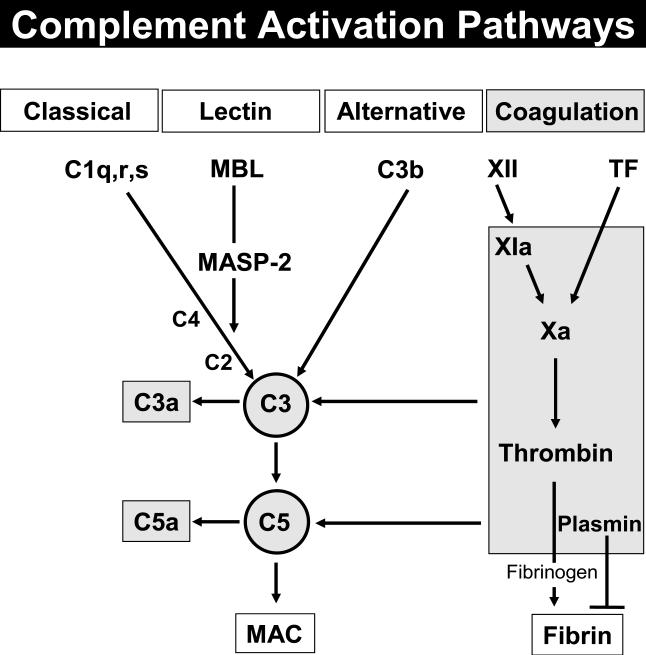
It has been shown by our lab that phagocytic cells are capable of generating biologically active C5a from C5 independently of the plasma complement system (Huber-Lang et al. 2002). The actual findings indicate the existence of additional complement activation paths in the plasma (Huber-Lang et al. 2006) besides the three established pathways of complement activation (Fig. 2).
Fig. 2.
Complement activation pathways (MAC membrane attack complex; MBL mannose binding lectin; MASP-2 mannose associated serine protease-2; TF tissue factor.
5 Interaction Between the Coagulation and Complement Cascade After Trauma
Many experimental and clinical studies have focused on the extreme coagulation challenge after severe trauma and during hemorrhagic shock (Kaplan et al. 1981). When reflecting a more philosophical consideration that “the end is in the beginning”, the very early and rapid activation of both, the coagulation system and the complement system seem to be crucially involved in the initiation and progression of the systemic inflammatory response and also in the deathly escalation of posttraumatic organ failure (Hierholzer et al. 2001). During experimental hemorrhagic shock, pretreatment of rats with carboxypeptidase N inhibitor (blocker of C5a clearance) turned out lethally (Younger et al. 2001). Furthermore, pre-shock depletion of complement significantly improved post-resuscitation blood pressure. One in vitro study claimed that C5a induces TF activity on endothelial cells (Ikeda et al. 1997) and may thereby activate the exogenous coagulation pathway. Recently, a C5a-induced “switch” in mast cells from a pro-fibrinolytic (t-PA release) to a pro-thrombotic phenotype (PAI-1 release) has been reported (Wojta et al. 2004) thus modifying the balance between pro- and anti-coagulation. To ameliorate the inflammatory response, various immune modulators have been examined, some of which revealed regulatory effects on both the complement and the coagulation system (Weiler and Linhardt 1991; Campbell et al. 2001). The C1-esterase-inhibitor, which curbs not only complement components of all three pathways (C1r/s, mannose-associated serine protease-2 [MASP-2], C3b) but also the endogenous coagulation cascade (kallikrein, XIIa), evinced a cross-talk between both systems (Jansen et al. 1998; Davis 2004). Similarly, the application of the soluble complement receptor 1 (sCR1) revealed some protective effects during hemorrhagic shock by blocking the endothelial dysfunction and the post-shock vasoconstriction (Fruchterman et al. 1998). In addition, sCR1 has been shown to decrease rolling, adherence, and influx of neutrophils in the mesenteric circulation and gut after hemorrhagic shock (Spain et al. 1999). A nexus between complement and coagulation may also be suggested by the successful use of recombinant activated protein C (APC) during sepsis (Bernard et al. 2001; Rezende et al. 2004). The beneficial effects of APC during systemic inflammation seem not only to be based on the interaction with the fibrinolytic system but also on anti-inflammatory effects.
6 Conclusion
The findings suggest that various serine proteases belonging to the coagulation system are able to activate the complement cascade independently of the so far established pathways. Moreover, functional active C5a and C3a are generated, both of which are known to be crucially involved in the inflammatory response. Consequently, a misdirected interaction of the coagulation cascade and complement system may play a crucial role in hemorrhagic shock and the subsequent systemic inflammatory response.
Acknowledgement
We thank Sonja Albers and Barbara Acker for outstanding laboratory assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG HU 823/2-2, HU 823/2-3, European Shock Society/Novo Nordisk Grant 2005) and NIH AI068730.
References
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Campbell W, Okada N, Okada H. Caboxypeptidase R is an inactivator of complement-derived inflammatory peptides and an inhibitor of fibrinolysis. Immunol Rev. 2001;180:162–167. doi: 10.1034/j.1600-065x.2001.1800114.x. [DOI] [PubMed] [Google Scholar]
- Choi G, Schultz MJ, Levi M, van der Poll T. The relationship between inflammation and the coagulation system. Swiss Med Wkly. 2006;136:139–144. doi: 10.4414/smw.2006.11059. [DOI] [PubMed] [Google Scholar]
- Davis AE., III Biological effects of C1 inhibitor. Drug News Pespect. 2004;17:439–446. doi: 10.1358/dnp.2004.17.7.863703. [DOI] [PubMed] [Google Scholar]
- Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Complement inhibition prevents gut ischemia and endothelial cell dysfunction after hemorrhage/resuscitation. Surgery. 1998;124:782–791. doi: 10.1067/msy.1998.91489. [DOI] [PubMed] [Google Scholar]
- Ganter MT, Brohi K, Cohen MJ, Shaffer LA, Walsh MC, Stahl GL, Pittet JF. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28:29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg. 2001;386:302–308. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecke F, Schmidt U, Kola A, Bautsch W, Klos A, Kohl J. Circulating complement proteins in multiple trauma patients – correlation with injury severity, development of sepsis, and outcome. Crit Care Med. 1997;25:2015–2024. doi: 10.1097/00003246-199712000-00019. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M, Younkin EM, Sarma VJ, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA. Generation of C5a by phagocytic cells. Am J Pathol. 2002;161:1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997;77:394–398. [PubMed] [Google Scholar]
- Jansen PM, Eisele B, de Jong IW, Chang A, Delvos U, Taylor FB, Jr, Hack CE. Effect of C1 inhibitor on inflammatory and physiologic response patterns in primates suffering from lethal septic shock. J Immunol. 1998;160:475–484. [PubMed] [Google Scholar]
- Kaplan AP, Ghebrehiwet B, Silverberg M, Sealey JE. The intrinsic coagulationn-kinin pathway, complement cascades, plasma renin-angiotensin system, and their interrelationships. Crit Rev Immunol. 1981;3:75–93. [PubMed] [Google Scholar]
- Lambris JD, Sahu A, Wetsel R. The chemistry and biology of C3, C4, and C5. In: Volanakis JE, Frank M, editors. The Human Complement System in Health and Disease. Marcel Dekker; New York: 1998. pp. 83–118. [Google Scholar]
- Lampl L, Helm M, Specht A, Bock KH, Hartel W, Seifried E. Blood coagulation parameters as prognostic factors in multiple trauma: can clinical values be an early diagnostic aid? Zentralblatt für Chirurgie. 1994;119:683–689. [PubMed] [Google Scholar]
- Levi M, van der Poll T, Büller HB. Relation between inflammation and coagulation. Circulation. 2004;109:2698–704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- Lidington EA, Haskard DO, Mason JC. Induction of decay-accelerating factor by thrombin through a protease-activated receptor 1 and protein kinase C-dependent pathway protects vascular endothelial cells from complement-mediated injury. Blood. 2000;96:2784–2792. [PubMed] [Google Scholar]
- Rezende SM, Simmonds RE, Lane DA. Coagulation, inflammation, and apoptosis: different roles for protein S and the protein S-C4b binding protein complex. Blood. 2004;103:1192–1201. doi: 10.1182/blood-2003-05-1551. [DOI] [PubMed] [Google Scholar]
- Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel C5a receptor-tissue factor crosstalk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- Spain DA, Fruchtermann TM, Matheson PJ, Wilson MA, Martin AW, Garrison RN. Complement activation mediates intestinal injury after resuscitation from hemorrhagic shock. J Trauma. 1999;46:224–233. doi: 10.1097/00005373-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Weiler JM, Linhardt RJ. Antithrombin III regulates complement activity in vitro. J Immunol. 1991;146:3889–3894. [PubMed] [Google Scholar]
- Wojta J, Huber K, Valent P. New aspects in thrombotic research: complement induced switch in mast cells from a profibrinolytic to a prothrombotic phenotype. Pathophysiol Haemost Thromb. 2004;33:438–441. doi: 10.1159/000083842. [DOI] [PubMed] [Google Scholar]
- Younger JG, Sasaki N, Waite MD, Murray HN, Saleh EF, Ravage ZA, Hirschl RB, Ward PA, Till GO. Detrimental effects of complement activation in hemorrhagic shock. J Appl Physiol. 2001;90:441–446. doi: 10.1152/jappl.2001.90.2.441. [DOI] [PubMed] [Google Scholar]