Abstract
Nicotine has been shown to increase responding maintained by turning off a houselight. To examine whether this effect extends to other primary reinforcing visual stimuli, the present study assessed whether nicotine would increase responding maintained by the illumination, and not just the darkening, of a visual stimulus. One group of rats (n=4) was initially trained to press two levers, using food as a consequence, while a separate group of rats (n=4) was initially trained to press one lever. After training, all rats pressed an active lever to turn on or turn off a houselight for 10 sec, while presses on an inactive lever had no programmed consequences. A third group of rats (n=4) were never trained to press either of the two levers and did not experience any programmed consequences for pressing. Although nicotine slightly increased lever pressing on both levers in the third group, nicotine resulted in much greater increases in responding maintained by the visual stimuli in the first two groups. Nicotine selectively increased responding maintained by visual stimuli, regardless of which levers were originally trained and regardless of whether those stimuli consisted of turning on or turning off a houselight, suggesting that nicotine enhances the value of primary reinforcing visual stimuli.
Keywords: nicotine, rats, visual stimuli, fixed ratio, lever training, reinforcer-enhancer, motivating establishing operation
1. Introduction
Although about 70% of smokers report a desire to quit (Centers for Disease Control and Prevention, 2005), approximately 60–90% of those who attempt to quit relapse within one year (Carmody, 1992). Nonhuman animal models of smoking (i.e., nicotine self-administration [NSA]) have revealed what appears to be a new role for nicotine as a “reinfocer-enhancer” (Chaudhri et al., 2006). The reinforcer-enhancing role may be important in smoking maintenance and relapse. For instance, the presence of nicotine may enhance the value of a variety of stimuli in a smoker’s environment. Furthermore, when a person attempts to quit smoking he or she not only loses cigarette reinforcers, but also the nicotine-induced enhancement of stimuli in the environment.
The reinforcer-enhancer role for nicotine has been studied primarily with rats using NSA procedures. Under some conditions visual stimuli can function as primary reinforcers for rats (e.g., Barry & Symmes, 1963; Kish, 1966). Primary reinforcing visual stimuli, such as turning off a houselight, seem to be important features of NSA (Caggiula et al., 2001). When visual stimuli are absent, NSA is difficult to establish and when they are removed NSA decreases substantially (Caggiula et al., 2002). Furthermore, nicotine has been shown to increase responding maintained by primary reinforcing visual stimuli when administered contingently and noncontingently on responding (Donny et al., 2003; Raiff & Dallery, 2006, 2008).
To further support nicotine’s reinforcer-enhancing role, a recent study found that responses maintained by one reinforcing visual stimulus (i.e., turning off a houselight) were increased by pre-session injections of nicotine in rats, whereas responses maintained by a different visual stimulus (i.e., turning on a small light above the lever) were not increased by nicotine (Palmatier et al., 2007). The authors hypothesized that nicotine increases the value of “moderate”, but not “weak,” reinforcers. Unlike the Palmatier et al study, other studies have shown that nicotine increases responding maintained by turning on a conditioned reinforcing houselight (Raiff & Dallery 2006, 2008). Houselight illumination is probably a more salient visual stimulus than the small cue lights used by Palmatier and colleagues, and the salience of visual stimuli have been shown to influence their primary reinforcing function (Stewart, 1960). To further explore the generality of nicotine as a reinforcer enhancer, the current study investigated whether turning on a houselight (without prior pairings with food, cf. Raiff & Dallery, 2006, 2008) would serve as a primary reinforcer and whether nicotine would selectively increase responding maintained by turning on the light.
In the Palmatier et al. (2007) study, as well as many NSA studies (Corrigal & Coen, 1989; Donny et al., 2003), rats were initially trained to press an active lever (the lever that later resulted in nicotine and visual stimuli) using food as a consequence, while no consequences were arranged for pressing an inactive lever. Frenk and Dar (2004) posited that only responses that have been explicitly trained increase when nicotine is delivered. Although several studies suggest otherwise (e.g., Liu et al, 2006; Palmatier et al., 2006), the effects of lever training history on the reinforcer-enhancing effects of nicotine were explored further in the present study.
2. Materials and Methods
2.1 Subjects
Twelve experimentally naïve male Long-Evans rats (Harlan; Indianapolis, IN), maintained at 85% (326–408 g) of their 150 day old ad libitum weights, served as subjects. Subjects were housed in individual home cages with bedding and received free access to water and post-session supplemental rodent chow (Lab Diet Rodent Diet; Formula 5001). The colony room was on a 12:12 hour light dark cycle (light from 8am–8pm).
2.2 Apparatus and Materials
Eight Med Associates® operant conditioning chambers (30.48 cm L×24.13 cm W×29.21 cm H) were used to conduct sessions. Chambers were located inside sound-attenuating boxes with fans for ventilation. Intelligence panels contained a food receptacle (5 cm×5 cm×3 cm) that was equidistant between two levers (requiring approximately 0.31 N force to register a response), each of which measured 4.5 cm× 2 cm and were located 22 cm from the chamber ceiling. Seven centimeters above each lever were three light-emitting diodes (LED; red, yellow, green; 0.8 cm in diameter, 0.7 cm apart from each other). On the wall parallel the intelligence panel was a house light (28 V), centered left to right and 1.5 cm from the ceiling. Forty-five milligram sucrose pellets (TestDiet®, Richmand, IN) were located outside the chamber, but inside the sound attenuating box. Experimental events and data collection took place on a computer in the same room using Med PC software and hardware. On drug delivery days, nicotine ([−]-Nicotine Hydrogen Tartrate Salt; Sigma, St. Louis, MO, USA), dissolved in potassium phosphate, was used. Doses are expressed as free base.
2.3 Procedure
Eight subjects were randomly assigned to one of two groups, differing with respect to lever training. One group (n = 4) was trained to press the right and left levers, hereafter the “Two-Lever” group. The second group (n = 4) was trained to press the right lever only, hereafter the “One-Lever” group. During lever training the only lights that were illuminated in the chamber were the red LEDs located above each lever, based on the lever training conditions used by Donny et al., 2003. Lever training took place on two separate days.
2.3.1 Two-Lever Training
Day one of training consisted of research assistants delivering food for approximations of pressing either the right or left lever, until one response was made on each lever. From that point forward, one food pellet was delivered for each lever press, with the restriction that the same lever could not be pressed more than three consecutive times. The fourth, and all subsequent, responses on the same lever did not have any programmed consequences, until at least one response on the alternate lever was made. The first day of training lasted 30 min or until subjects earned a minimum of 20 pellets for pressing each lever (at least 40 total pellets). There was no time limit on the second day of training and sessions ended after 37 pellets had been earned for pressing each lever (at least 74 total pellets). Subjects could earn more than the minimum number of pellets if they responded more on one lever than the other (i.e. after 3 consecutive responses on the same lever food stopped being delivered for pressing that lever and was only reinforced for pressing the other lever). Thus, a subject could make 3 reinforced responses on one lever, switch to the other lever and make a response, then switch back and make 3 more responses on the previous lever. There was no maximum number of pellets that could be earned, but sessions ended whenever a minimum of 20 or 37 pellets (depending on the day) had been earned on each side.
2.3.2 One-Lever Training
As with the Two-Lever group, the first day of training began with research assistants delivering food for approximations of lever pressing, but for pressing the right lever only. After one response was made on the right lever, all future right lever responses were followed by food pellet delivery. Responses on the left lever had no programmed consequences. The first training day ended after 30 min or after 40 pellets had been earned for pressing the right lever. There was no time limit on the second training day and sessions ended after 74 pellets had been earned for pressing the right lever.
2.3.3 Turn Lights On and Turn Lights Off
After lever training, subjects in both groups experienced the same procedures. Sessions were conducted at approximately the same time during the light cycle, seven days per week. To begin, all 6 LEDs were illuminated five seconds after the subject was placed into the chamber. A single response on either lever, or 60 sec without any response, turned off the LEDs and began the 60 min session. The right lever was designated as the active lever. All subjects experienced two phases (Turn Lights On and Off).
At the beginning of the session the operant chamber was dark during the Turn Lights On phase (similar to Raiff & Dallery 2006, 2008) and lit by the houselight during the Turn Lights Off phase (similar to NSA procedures, Caggiula et al., 2002). If no response was made on the right lever, subjects remained in a dark or lit chamber, depending on the phase. Initially only one response on the right lever (i.e., fixed ratio [FR] 1) produced 10 s of the houselight turned on (Turn Lights On) or off (Turn Lights Off). Additional right or left lever responses during the stimulus presentation did not have any programmed consequences, but all responses were recorded. After five sessions of FR 1 the response requirement increased to FR 2 for five sessions, after which the requirement increased to FR 5. A minimum of 10 sessions at FR 5 were required before stability was evaluated. This FR progression was based on the nicotine plus visual stimuli self-administration schedule used by Caggiula et al. (2002).
The total number of responses per session were deemed stable by visual inspection if there were no increasing or decreasing trends (i.e., five consecutive sessions with all responses moving in the same direction) and as long as the highest or lowest number of responses did not occur during one of the last three sessions. Conditions changed upon meeting the stability criteria or after a maximum of 30 sessions, whichever occurred first.
Exposure to the Turn Lights On and Off phases was counterbalanced across subjects within a group, such that two subjects in each group were exposed to the Turn Lights On phase first. The first phase for all subjects, regardless of whether it was Turn Lights On or Off, was an ABAB design. The A conditions were baseline without drug administration. The B conditions consisted of daily subcutaneous (s.c.) administration of 0.3 mg/kg nicotine. At the beginning of the second phase (Turn Lights On for half the subjects, Turn Lights Off for the other half), stimuli were initially on a FR 1 schedule that increased to FR 5, as described earlier. The second phase for all subjects consisted of an ABABAC design. The A and B conditions were the same as before and the C conditions consisted of daily s.c. administration of potassium phosphate vehicle.
The majority of subjects in our previous research have shown nicotine-induced increases in behavior when given 0.3 mg/kg nicotine. However, similar changes have been noted at 0.56 mg/kg nicotine for some subjects (Raiff & Dallery, 2006, 2008). Subject R257 (One-Lever Group) did not show nicotine-induced increases during the first B condition when 0.3 mg/kg was administered. This subject was exposed to 0.56 mg/kg during the second B condition. Not only did this subject fail to show increases under 0.56 mg/kg, but responding ceased entirely. Therefore, after the first phase, lever training was repeated for this subject as described earlier. For all subsequent nicotine conditions in the second phase this subject received 0.1 mg/kg nicotine, which has also been shown to increase responding for one subject in a previous study (Raiff & Dallery, 2006). This dose increased responding for subject R257.
No Change
Four naïve subjects, that were never trained to press levers and never earned food in the chamber, served as a control group (hereafter “No Change”). Before each daily 60-min session, the 6 LEDs above the levers were illuminated for 60 s. Lever presses were recorded but did not have programmed consequences. An ABABC design was employed, whereby the A conditions served as baseline with no injections, the B conditions involved daily presession s.c. injections of 0.3 mg/kg nicotine, and the C condition involved daily vehicle injections. Aside from the first baseline condition, which lasted 20 days, all other conditions lasted 21 days.
3. Results
All statistical analyses reported in this section were adjusted for sphericity using Huynh-Feldt corrections (Huynh & Feldt, 1976) and were deemed statistically significant at p < 0.05. Unless otherwise noted, all values in this section are means ± standard deviations.
After subject R251 (Two-Lever group) completed the experiment, a computer program malfunction was discovered for all but the first two conditions of the Turn Lights Off phase for this subject. Thus, all other conditions for this subject during the Turn Lights Off phase were omitted from data analyses.
During the lever training procedure, the mean proportion of responses allocated to the right (future active) lever was 0.43 (±0.09) and 0.75 (±0.15) for the Two- and One-Lever groups, respectively. During all subsequent conditions of the Turn Lights On and Off phases, all subjects allocated most of their responses to the right lever (On: 0.94 ± 0.08, Off: 0.95 ± 0.09). Alternatively, responding by subjects in the No Change group was more variable and slightly less responding was allocated to the right lever on average (0.37 ± 0.35).
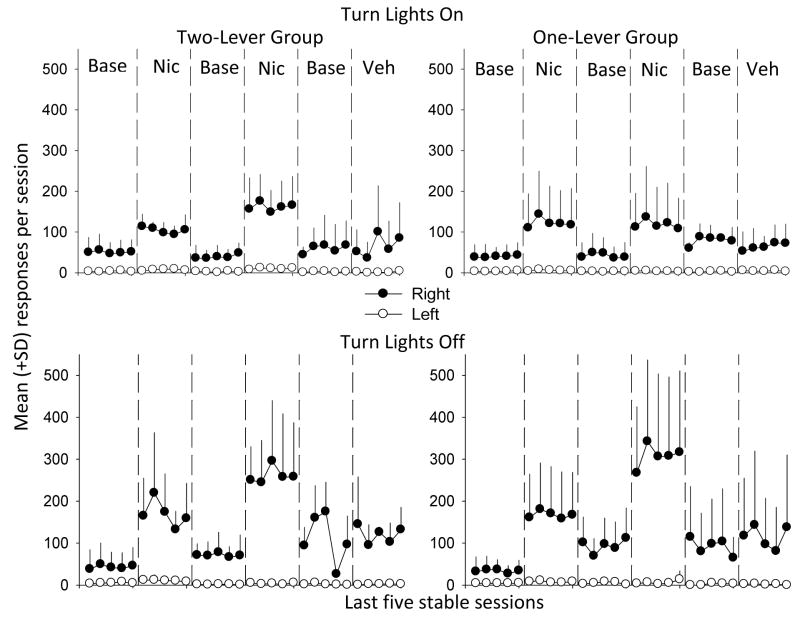
A 2 × 2 × 6 repeated-measure ANOVAs was conducted with lever training group (One-versus Two-Lever), lever (active versus inactive) and condition (three baseline, two nicotine, one vehicle) for the Turn Lights On phase. The top row of Figure 1 shows that a greater total number of responses were allocated to the right lever than to the left lever during the Turn Lights On phase (Right = 81.3 ± 64, Left = 4.4 ± 4.6; F [1,188] = 425.6). There was a significant effect of condition (F (5, 188) = 23.8), as well as a significant lever × condition interaction (F (5,188) = 23.6). To further explore the significant effect of condition, an ANOVA compared conditions for each lever, and Bonferroni post-hoc analyses were conducted when significant differences were found. Nicotine increased responses on the right lever and left lever, but to a greater extent than on the right lever (Right = 126.7 ± 68.8, Left = 6.6 ± 5.8), relative to baseline (Right = 51.7 ± 28.7, Left = 2.83 ± 2.8) and vehicle conditions (Right = 65.2 ± 49.1, Left = 2.8 ± 2.7). There were no significant differences between groups (One versus Two levers) and there were no significant group × lever, or group × lever × condition interactions.
Figure 1.
Mean (+ standard deviations) total responses per 60 min session during the Turn Lights On (top row) and Turn Lights Off phases (bottom row) for the Two-Lever (left column) and One-Lever (right column) training groups. Filled circles show responses on the right, active, lever and open circles show responses on the left, inactive, lever. Base = Baseline, Nic = Nicotine, Veh = Vehicle.
Another 2 × 2 × 6 repeated-measure ANOVAs was conducted for the Turn Lights Off phase. The bottom row of Figure 1 shows that a greater total number of responses were also allocated to the right lever than to the left lever during this phase (Right = 135.1 ± 121.0, Left = 4.8 ± 6.1; F [1,188] = 375.0). Again, there was a significant effect of condition (F (5, 188) = 33.2) and a significant lever × condition interaction (F (5, 188) = 34.2). As before, to further explore the significant effect of condition, an ANOVA compared conditions for each lever and Bonferroni post-hoc analyses were conducted when significant effects were observed. Nicotine reliably increased right (226.6 ± 115.5), but not left, responses relative to baseline conditions (74.0 ± 50.6). Responding on the right lever during the vehicle condition was significantly higher than during the first baseline condition (mean difference +79.5 responses), and significantly lower than during the second nicotine condition (mean difference −166.9 responses). Responding on the left lever during the vehicle condition was only significantly lower than the first nicotine condition (mean difference −7.4 responses). There were no significant differences between groups (One versus Two levers) and there were no significant group × lever, or group × lever × condition interactions. Finally, to compare phase, lever, and condition, regardless of group assignment, another ANOVA was conducted and there was a significant lever × phase interaction (F (1, 378) = 52.9), whereby a greater number of responses occurred on the active lever during the Turn Lights Off than during the Turn Lights On phase.
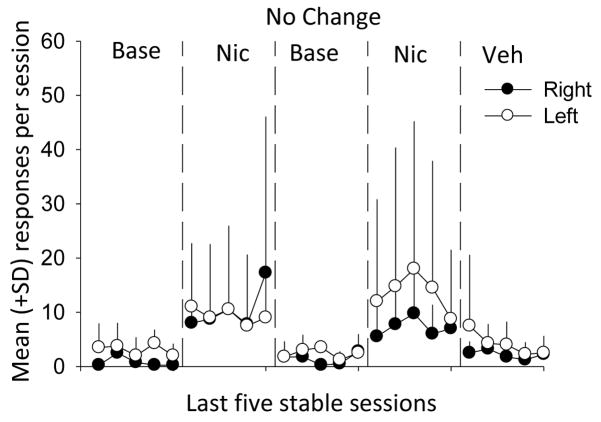
Figure 2 shows that the number of responses per session was substantially lower for subjects in the No Change group than for subjects in the Turn Lights On and Off phases (note different y-axis scales for Figures 1 and 2). An ANOVA was conducted to compare right and left lever presses across conditions to determine whether there was a lever × condition interaction. The mean number of responses were significantly higher during nicotine conditions (10.2 ± 12.8) than during baseline (1.9 ± 2.2) and vehicle (3.2 ± 4.3) conditions (F(4,95) = 6.64).
Figure 2.
Mean (+ standard deviations) of total responses per 60 min session during the No Change group. Filled circles show responses on the right lever and open circles show responses on the left lever. Base = Baseline, Nic = Nicotine, Veh = Vehicle. Note the differences in the y-axes between Figures 1 and 2.
4. Discussion
The visual stimuli used in the current study, turning on and turning off a houselight, maintained lever pressing. Regardless of whether subjects were initially trained to press one or both levers, subjects eventually showed a preference for the right, active, lever. During lever training, subjects in the Two-Lever training group allocated their responses equally across the two levers, until food was removed and stimulus changes were presented contingent on right lever responses only. These findings strongly suggest that both visual stimuli used in the current study served as primary reinforcers.
The current results provide additional support for the reinforcer-enhancing account of nicotine. Responding maintained by primary reinforcing visual stimuli increased when nicotine was administered before experimental sessions. Increases on the inactive lever, even for subjects that were initially trained to press the inactive lever with food as a consequence (Two-Lever group) were not consistent. When increases were seen on the inactive lever and in the No Change group, the magnitude of the increases was small compared to the large increases on the active lever. These small increases may be due to general locomotor increasing effects of nicotine (Kosowski & Liljequist, 2005), but such effects cannot fully account for the large increases that occurred on the active lever.
In the current study nicotine increased responding maintained by both types of stimulus change (turning lights on and off). Palmatier et al (2007) had one group of subjects turning off a houselight and another group of subjects turning on a stimulus light above the lever. Nicotine only increased responding maintained by turning off the houselight in their study, whereas responding maintained by both types of stimuli increased in the current study. A few differences may account for this disparity. First, the current study involved turning on the houselight from a dark chamber, whereas Palmatier et al. had subjects turning on a small stimulus light. Second, the current study maintained a greater number of responses per session during the Turn Lights On phase than were seen in the lights on group in the Palmatier et al study. Stimulus intensity has been found to affect the primary reinforcing function of visual stimulus reinforcers on FR 5 schedules of reinforcement (Stewart, 1960), with lights of stronger intensity maintaining a greater number of responses. Additionally, in the current study sessions were not only conducted during the light cycle but subjects were also fed during the light cycle. Palmatier et al. conducted sessions and fed subjects during the dark cycle. Feeding conditions (in the light or in the dark) have been shown to influence responding maintained by turning on lights (Roberts, Marx, Collier, 1958).
Raiff and Dallery (2006, 2008) also reported nicotine-induced increases in responding maintained by turning on lights. In their experiments, however, light illumination had been paired with a primary reinforcer (sucrose pellets), and thus the visual stimulus likely served as a conditioned reinforcer. Results of the Turn Lights On procedure in the current study suggest that nicotine may increase responding maintained by these stimuli without prior pairing with food. It is possible the pairings with food may have conferred additional reinforcing value to the lights. Indeed, Raiff and Dallery (2008) showed that before pairings the lights engendered very low response rates, and after pairing responding increased substantially. In addition, the magnitudes of the nicotine-induced increases were greater in the previous experiments (about 5.5 and 4.2 responses per minute compared to about 1.2 in the current study).
Thus, whether the lights served as primary or conditioned reinforcers, the results are consistent with the hypothesis that the reinforcer-enhancing effects of nicotine are limited to visual stimuli that serve as reinforcers (Palmatier et al., 2007). If the reinforcer-enhancer concept is to serve a practical function in preventing smoking relapse and developing more effective cessation techniques, it will be necessary to continue to explore the generality and limitations of nicotine as a reinforcer-enhancer (or more technically, as a motivating establishing operation, see Raiff & Dallery, 2008; Michael, 2000). For example, it will be important to determine whether the same outcomes apply to different stimulus modalities (e.g., auditory, olfactory) and different species (e.g., humans).
Acknowledgments
We would like to thank Matthew Capprioti, Rachel Cassidy, Jeb Jones, Matthew Locey, Julie Marusich, Gabe Mazur, Steven Meredith and Kathryn Saulsgiver for their assistance in conducting this research. We also thank Marc Branch, Timothy Hackenberg, Neil Rowland, and Adriaan Bruijnzeel for providing feedback on this study and an early version of the manuscript. This study was used in partial fulfillment for the requirements of the doctoral degree at the University of Florida for the first author, Bethany R. Raiff. This research was supported by US Public Health grants R03DA019467 and F31DA021439.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barry H, Symmes D. Reinforcing effects of illumination change in different phase of the rat’s diurnal cycle. J Comp Physiol Psych. 1963;56:117–119. [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Carmody TP. Preventing relapse in the treatment of nicotine addiction: current issues and future directions. J Psych Drugs. 1992;24:131–158. doi: 10.1080/02791072.1992.10471634. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United States, 1997–2001. Morb Mort Weekly Rep. 2005;54:625–628. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5425a1.htm. [PubMed]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–66. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib S, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Frenk H, Dar R. Reward potentiation or behavioral activation? A comment on Donny et al. Psychopharmacology. 2004;171:472–473. doi: 10.1007/s00213-003-1622-8. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. J Educ Stat. 1976;1:69–82. [Google Scholar]
- Kish GB. Studies of sensory reinforcement. In: Honig WK, editor. Operant Behavior. Appleton-Century-Crofts; New York: 1966. pp. 109–159. [Google Scholar]
- Kosowski AR, Liljequist S. Behavioural sensitization to nicotine precedes the onset of nicotine-conditioned locomotor stimulation. Beh Brain Research. 2005;156:11–17. doi: 10.1016/j.bbr.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN. Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology. 2006;184:417–25. doi: 10.1007/s00213-005-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J. Implications and refinements of the establishing operation concept. J of App Beh Anal. 2000;33:401–410. doi: 10.1901/jaba.2000.33-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Effects of acute and chronic nicotine on responses maintained by primary and conditioned reinforcers in rats. Exp Clin Psychopharmacol. 2006;14:296–305. doi: 10.1037/1064-1297.14.3.296. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. The generality of nicotine as a reinforcer-enhancer: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology. 2008;201:305–314. doi: 10.1007/s00213-008-1282-9. [DOI] [PubMed] [Google Scholar]
- Roberts CL, Marx MH, Collier G. Light onset and light offset as reinforcers for the albino rat. J Comp Physiol Psych. 1958;51:575–579. doi: 10.1037/h0042974. [DOI] [PubMed] [Google Scholar]
- Stewart J. Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psych. 1960;53:187–193. doi: 10.1037/h0047315. [DOI] [PubMed] [Google Scholar]