Abstract
CD4 help is crucial for memory CD8+ T cell development; yet the mechanisms of CD4 help and why (CD4) helpless memory CD8+ T cells elicit poor recall responses are currently not well understood. In this study we investigated these questions using an in vivo acute virus infection model. We show here that CD4 help during priming is required for memory CD8+ T cell differentiation, and that stimulation of CD40 during priming rescues the helpless defects in the absence of CD4+ T cells. The defective recall response by helpless memory cells did not correlate with the amount of cell death and were independent of TRAIL. However, helpless memory cells excessively upregulated the inhibitory receptor PD-1, and PD-1 blockade enhanced the recall response of helpless memory cells. Furthermore, providing IL-2 signaling in vivo during the recall response reduced PD-1 expression and rescued the recall response of helpless memory cells. Our study identifies molecular pathways involved in CD4 help for memory CD8+ T cell generation that are independent of TRAIL, and provide therapeutic implications that helpless memory cell function can be restored at multiple stages through various immunological interventions.
Keywords: Memory, Viral, Cytotoxic T Cells
Introduction
Memory CD8+ T cells provide protective immunity against secondary infections, and information on the signals required for their differentiation are valuable for designing effective vaccination strategies (1). Help mediated by CD4+ T cells has been shown to be crucial for memory CD8+ T cell differentiation in various models (2–6). However, there is great controversy on the timing of CD4 help required for memory CD8+ T cell differentiation. Depending on the model examined, CD4 help can be required during priming, maintenance, or recall, or in some cases can be dispensable (2–4, 7–13).
Furthermore, the molecular and cellular mechanism of how CD4+ T cells deliver help has not been resolved. One model postulates that CD4 help is mediated through the CD40L-CD40 pathway (14–16). In this model, activated CD4+ T cells upregulate CD40L and engage CD40 on dendritic cells (DCs), resulting in full maturation and “licensing” of DCs and stimulation of CD8+ T cells (17). In these initial studies, agonistic anti-CD40 mAb substituted for the lack of CD4+ T cells. However, studies investigating the role for CD40 signaling in the memory CD8+ T cell differentiation have reported contradictory results, where in several models fully functional memory cells were observed in the absence of CD40 (6, 13, 18–22). Furthermore, stimulation of CD40 during priming has been shown to impair CD8+ T cell memory in some models, further complicating the interpretation (23, 24). The second hypothesis states that IL-2 released by CD4+ T cells recognizing cognate antigen in the vicinity of CD8+ T cells mediates CD4 help. IL-2 signals during priming from a paracrine source has been shown to be critical for differentiation of memory CD8+ T cells (25, 26), although no direct evidence has been shown that the IL-2 is derived from CD4+ T cells. Another study has shown that direct ligation of CD40L on CD4+ T cells and CD40 on CD8+ T cells is required for memory CD8+ T cell development (27). However, subsequent reports using infection models and virus-like particles (VLPs) have shown that CD40−/− CD8+ T cells develop into fully functional memory cells, suggesting CD40 expression on CD8+ T cells is not required (12, 19, 20). Finally, a study using OVA-specific transgenic T cells has shown that the interaction of CD4+ T cells with DCs results in the production of CCL3 and CCL4, recruits OVA-specific CD8+ T cells through CCR5 and results in efficient priming (28). Blocking of CCL3 and CCL4 during priming impaired memory CD8+ T cell generation. However, CCR5 has been shown to be dispensable for memory differentiation in other models (12).
One of the hallmarks of helpless memory cells is the defective recall response upon secondary challenge with antigen (2–6). The mechanism behind this defective recall response is unclear. The expression of the transcription factor T-bet, which suppresses IL-7Rα expression, is increased with the absence of CD4+ T cell help, and deletion of T-bet has been shown to reverse the helpless phenotype (29). It is currently unclear why overexpression of T-bet limits the recall response in helpless memory CD8+ T cells. Using an in vivo cross priming system and an LCMV infection model, Janssen et al provided evidence that helpless memory CD8+ T cells upregulate TRAIL (tumor-necrosis factor-related apoptosis-inducing ligand) upon restimulation in vitro (30). This led to an increased cell death during recall, leading to an abortive response. Inhibition of TRAIL function through blockade or genetic deletion, as well as inhibiting cell death through the use of caspase inhibitors reversed the impaired recall response (30). However, in a separate study, TRAIL was shown to only delay the helpless defect, implicating the existence of a TRAIL-dependent and –independent mechanism for the helpless defect. Furthermore, another report has indicated that the helpless defect is completely independent of TRAIL (31). Therefore, the underlying mechanism for the helpless defect is not well understood, and furthermore, whether the function of the incorrectly “programmed” memory cells in the absence of CD4 help could be repaired after the primary response is uncertain.
Here we characterize the molecular mechanism of CD4 help in the generation of memory CD8+ T cells in vivo by non-transgenic endogenous CD8+ T cells, upon infection with acute cytopathic viral infection through a mucosal route. Intranasal infection by VV-WR represents a natural route of infection by poxviruses and results in a robust CD8+ T cell response (32). Our results indicate that CD4 help during priming and CD40 signaling is required for memory CD8+ T cell differentiation. CD40 stimulation during priming rescued the generation of CD8+ T cell memory in the absence of CD4+ T cells. The defective recall response by helpless memory cells was not associated with increased cell death, and was independent of TRAIL. We observed excessive upregulation of the inhibitory receptor programmed cell death-1 (PD-1), but not cytotoxic T lymphocyte antigen-4 (CTLA-4), on helpless memory cells during recall compared to helped cells. Blocking PD-1 in vivo enhanced the recall response of helped and helpless memory cells. Furthermore, administering IL-2 immune complexes in vivo during recall resulted in downregulation of PD-1 and rescue of the impaired recall response. Thus, our results demonstrate the involvement of multiple molecular pathways that are involved in CD4 help for memory CD8+ T cell differentiation, and interventions that modulate these pathways restore the function of helpless memory cells.
Materials and Methods
Mice and virus infections
C57BL/6 (B6) and congenic B6-Ly5.2-Cr (which are Ly5.1/CD45.1+) mice were purchased from The National Cancer Institute (Bethesda, MD). CD40−/− and TRAIL−/− mice were generously provided by Dr. Randolph J. Noelle (Dartmouth Medical School, Lebanon, NH) and Amgen (Thousand Oaks, CA), respectively, and were used with permission. The Animal Care and Use Program of Dartmouth College approved all animal experiments. For infections, mice were infected with 103 PFU of VV-WR (originally obtained from Dr. William R. Green, Dartmouth Medical School, Lebanon, NH) through the intranasal route, and loss of body weight was measured as previously described (32). MHV-68 virus (clone G2.4) was originally obtained from Prof. A. A. Nash (University of Edinburgh, Edinburgh, U.K.), and was propagated and titered as previously described (32). A recombinant vaccinia virus expressing the ORF6487–495/Db epitope of MHV-68 (rVV-ORF6) was obtained from Dr. Peter Doherty (St. Jude Children’s Research Hospital, TN). For MHV-68 infection, 400 PFU was given i.n.
Ab treatments
For depletion of CD4+ T cells, mice were treated with 500 µg of anti-CD4 (GK1.5) on days −1 and 0 of infection, followed by 250 µg on day 3 p.i. and twice weekly thereafter, resulting in < 0.1% CD4+ T cells (data not shown). Control mice were either untreated or given Rat IgG (R-IgG) (Jackson ImmunoResearch, West Grove, PA). No differences in T cell responses were observed with R-IgG injection (data not shown). The hybridoma producing anti-CD40 mAb (FGK115) was kindly provided by Dr. Noelle. 100 µg of anti-CD40 mAb was injected on days 1 and 7 p.i. 500 µg of anti-PD-1 (RMP1–14) was injected on days 0, 2, and 4 during challenge. Injection of IL-2 immune complexes has been described previously (32, 33). Briefly, a mixture of 50 µg of αIL-2 mAb (S4B6) and 1.5 µg of murine IL-2 (mIL-2; eBioscience, San Diego, CA) was injected i.p. daily, from day 0–4 post-challenge.
MHC/peptide tetramer, antibody, intracellular staining and flow cytometric analysis
Single cell suspensions of spleen and lung lymphocytes were prepared as described previously (32). MHC/peptide tetramer for the VV-WR epitope B8R20–27(TSYKFESV)/Kb or ORF6487–495(AGPHNDMEI)/Db conjugated to allophycocyanin, was obtained from the NIH Tetramer Core Facility (Emory University, Atlanta, GA). Cells were stained for 1 hour at room temperature, and were further stained with PerCP-conjugated anti-CD8α (53-6.7; BD Pharmingen, San Jose, CA) along with antibodies against surface markers (32). For PD-1 expression, PE-conjugated anti-PD-1 (J43; BD Pharmingen) was used. Staining with PE-conjugated anti-CTLA-4 (UC10-4F10-11; BD Pharmingen) and anti-Bcl-2 (BD Pharmingen) expression was performed at 4°C for 30 minutes after staining with tetramer, anti-CD8, and FITC-conjugated anti-CD45.2 (clone 104; eBioscience, San Diego, CA), followed by cell fixation with 1% formaldehyde (Ted Pella, Inc., Redding, CA) and permealization with staining buffer containing 0.5% saponin (Sigma-Aldrich, St. Louis, MO). Annexin V staining was performed after tetramer and CD45.2, CD8 staining according to the manufacturer’s protocol (BD Pharmingen). Intracellular cytokine staining was performed after restimulation of splenocytes with 1 µg/ml of peptide, 10 U/ml IL-2, and 5 µg/ml Brefeldin A for 5 hours at 37°C. IFN-γ production in control wells with no peptide were subtracted as background, as previously described (32).
Adoptive transfer and challenge experiments
Similar to our previous study (32), CD8+ T cells were purified (typically >95% purity) from spleens of VV-WR-infected mice (40+ days p.i.), and equal numbers of CD8+ T cells (2 to 4 × 106 cells) were injected into congenic B6-CD45.1 (B6-Ly5.2-Cr) mice intravenously (i.v.). Before transfer, cells from each group were stained to determine the percentage of CD8+ T cells and tetramer+ cells (similar frequencies were observed in all experiments; see Figure 1C), and the data was used to determine the exact number of virus-specific memory CD8+ T cells injected into each individual recipient. One day after transfer, mice were challenged i.p. with 2 × 106 PFU of VV-WR. Spleens were harvested 5 days post-challenge, and stained with VV-B8R-tetramer, anti-CD8, and anti-CD45.2, and the total number of donor-derived VV-specific CD8+ T cells was calculated. To calculate fold expansion of donor-derived VV-specific CD8+ T cells in each individual recipient, the total number of virus-specific memory CD8+ T cells 5 days post-challenge was divided by the total number of virus-specific CD8+ T cells initially transferred. Four to seven recipients per group were used in each experiment.
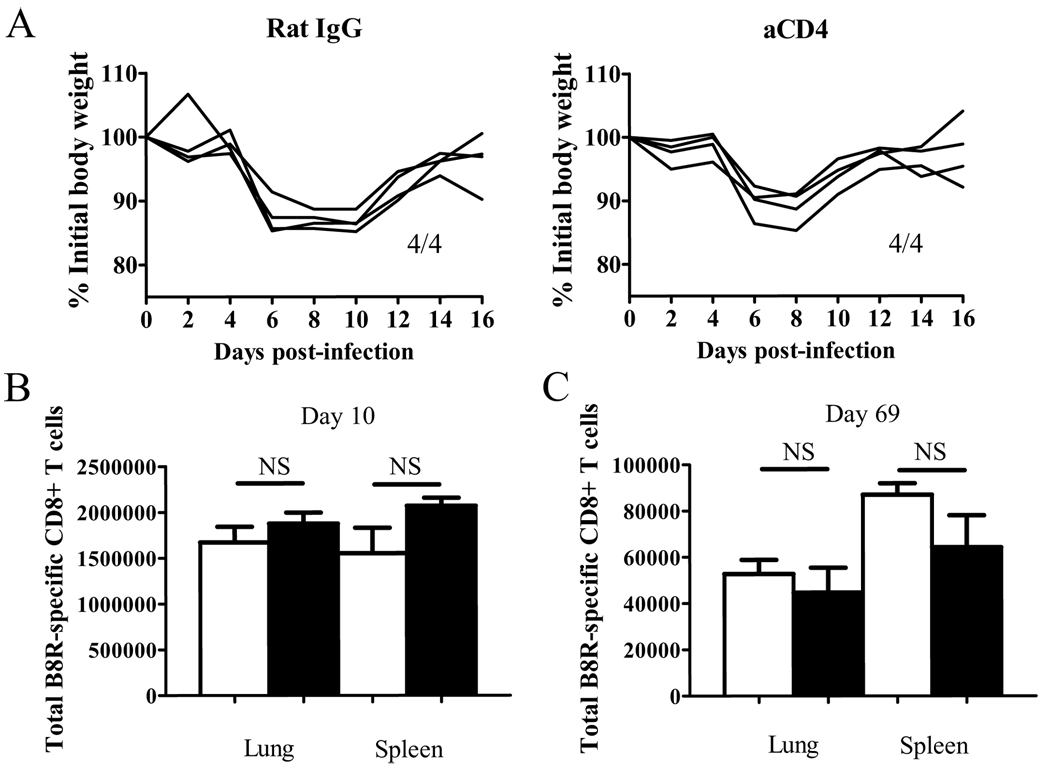
Figure 1. CD4 help does not affect the initial disease and CD8+ T cell kinetics during VV infection.
(A) Weight loss after infection was measured. Numbers indicate surviving mice. (B,C) The VV B8R-specific CD8+ T cell response was measured by tetramer staining on day 10 (B) and day 69 (C) p.i. Total numbers of CD8+ tetramer+ cells in each organ are graphed. White bars: un-depleted, black bars: CD4-depleted. Representative data from two independent experiments with 4 mice per group are shown. Error bars indicate SEM. NS: not significant.
For experiments involving MHV-68-specific memory CD8+ T cells, CD8+ T cells were purified from infected mice, and equal numbers of ORF6-specific CD8+ T cells (104 cells) as determined by tetramer staining was transferred into naïve congenic recipients. We chose to transfer equal number of tetramer+ cells because significantly higher frequencies of ORF6-specific memory CD8+ T cells were observed in CD4-depleted mice compared to untreated mice (data not shown), as opposed to VV-WR infection where similar numbers of memory cells are observed (Figure 1C). One day after transfer, recipients were challenged with 2 × 106 PFU of rVV-ORF6 i.p., and the expansion of ORF6-specific CD8+ T cells were calculated as described above using ORF6-tetramers (32).
Statistical analysis
P values were calculated using Student’s t test. p < 0.05 was considered significant.
RESULTS
CD4 help in early viral disease and the virus-specific CD8+ T cell response
Intranasal infection by VV-WR represents a natural route of infection by poxviruses and provides a model for studying CD8+ T cell responses to acute cytopathic infection through the mucosal route (32, 34). During the infection a robust CD8+ T cell response is generated, allowing us to study the generation of memory CD8+ T cells without the use of transgenic T cells, which may skew the results of our studies (35, 36). We depleted CD4+ T cells by repeated injection of anti-CD4 Ab (GK1.5) to study the effect of CD4 help on CD8+ T cell responses specific to the immunodominant epitope of VV-WR, B8R20–27 (referred to as VV-specific CD8+ T cells). Infection with a sublethal dose (103 PFU) of VV-WR through the i.n. route results in severe virulence, which can be observed by significant weight loss, followed by recovery after day 8 post-infection (p.i.) (32). We observed no role for CD4+ T cells in the initial disease caused by the infection, as CD4 depletion did not have any significant effect on the severity of the disease (Figure 1A).
In most viral infection models, CD4 help does not affect the primary virus-specific CD8+ T cell response, but in some cases help is required for maintaining the memory population. Therefore we examined the VV-specific CD8+ T cell response in the lung and the spleen using MHC/peptide tetramers. CD4+ T cells did not affect the primary virus-specific CD8+ T cell response in the lung and the spleen (Figure 1B), which occurs at day 10 in this model (32). Thus CD4+ T cells play no role in the early events that occur during this infection. At 2 months post-infection, we observed a slight decrease in the frequency of virus-specific memory CD8+ T cells in the lung of CD4-depleted mice (data not shown); however the total number of virus-specific memory CD8+ T cells in the spleen and lungs were unaffected by the absence of CD4+ T cells (Figure 1C). Overall, CD4 help does not affect the kinetics of the virus-specific CD8+ T cell response during VV-WR infection.
Helpless memory CD8+ T cells are severely impaired in function
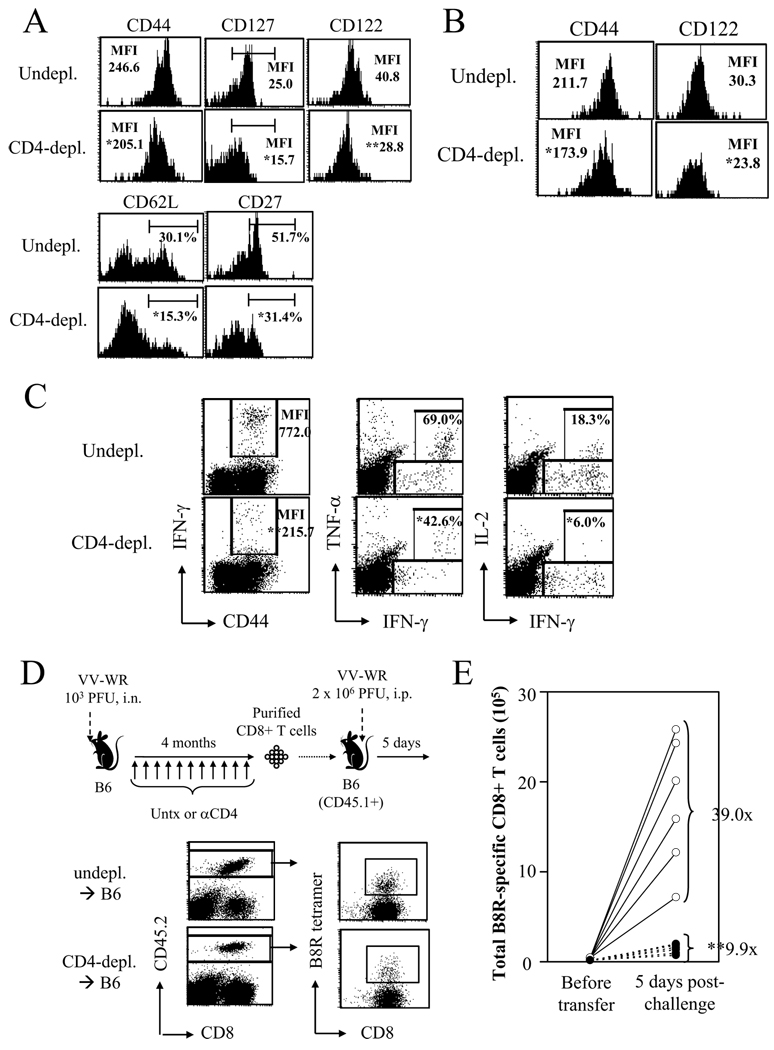
Numerous studies have provided evidence for a crucial role of CD4 help in memory CD8+ T cell differentiation (7). Thus, although the kinetics of the virus-specific memory CD8+ T cell response was unaffected by the absence of help, we suspected that the helpless memory cells may be functionally impaired. First, we investigated the phenotype of VV-specific memory CD8+ T cells in the spleen and the periphery (lungs) in the absence of CD4 help. Helpless memory cells in the spleen expressed lower levels of CD44, CD127 (IL-7Rα), CD122 (IL-2/15Rβ), and CD27 as measured by mean fluorescent intensity (MFI), and there were lower percentages of CD127hi, CD62Lhi, and CD27hi cells (Figure 2A). The helpless memory cells in the lung also expressed lower levels of CD44 and CD122 (Figure 2B). We observed minimal expression of CD25 and CD69 in the untreated and CD4-depleted groups in both organs, indicating the cells had a resting phenotype (data not shown). Second, we tested the ability of helpless memory cells to produce cytokines in response to restimulation by intracellular cytokine staining (ICCS). In the absence of CD4 help, memory cells produced lower levels of IFN-γ, as measured by MFI (Figure 2C). The frequency of IFN-γ producing CD8+ T cells was slightly reduced, but was not statistically significant (data not shown). Furthermore, lower frequencies of IFN-γ-producing memory cells produced TNF-α and IL-2 (Figure 2C).
Figure 2. Helpless VV-specific memory CD8+ cells are impaired in function.
(A) Phenotype of VV-B8R-specific memory CD8+ cells in un-depleted and CD4-depleted mice were analyzed at day 69 p.i. by tetramer staining. Plots are gated on CD8+ tetramer+ cells. (B) Phenotype of lung-resident memory cells. (C) Cytokine production by VV-specific memory cells was examined by ICCS. Plots are gated on CD8+ cells. Numbers indicate percentage of CD8+ T cells producing IFN-γ (left), or percentage of IFN-γ+ cells that produce TNF-α (middle) or IL-2 (right). (D) The recall response of helped and helpless memory cells were analyzed by an adoptive transfer approach (see Materials and Methods for details). CD8+ T cells were purified from un-depleted or CD4-depleted VV-infected mice 4 months p.i. and were transferred into naïve congenic (CD45.1+) recipients. One day later, recipients were challenged with 2 × 106 PFU of VV-WR, i.p., and 5 days-post challenge, expansion of donor-derived VV-specific memory CD8+ T cells was analyzed by tetramer staining before and after (lower panel) challenge. (E) Results of the adoptive transfer experiment. The total number of donor-derived VV-specific CD8+ T cells analyzed by tetramer staining before transfer and 5 days post-challenge are graphed. Fold expansion of tetramer+ cells was calculated for each recipient, and the number shown indicates the average fold expansion for each group. Each circle (white: undepl. → B6; black: CD4-depl. → B6) represents individual recipients. Representative plots from 2–3 independent experiments, using 3–4 mice per groups are shown. In (D), 4 donor mice per group, and 6–7 recipients mice per group were used. * p > 0.05, ** p > 0.01.
Finally, we measured the ability of the helpless memory cells to elicit a robust recall response by utilizing an adoptive transfer approach used previously (Figure 2D) (32). CD4 help is required for the induction of neutralizing Abs; thus, directly re-challenging helped and helpless mice would result in different doses of virus infecting the host. Therefore, we purified CD8+ T cells from infected helped or helpless mice and transferred them into a naïve congenic (CD45.1+) host, which were challenged one day later with 2 × 106 PFU of VV-WR, intraperitoneally (i.p.). Before transfer, we calculated the exact number of virus-specific CD8+ T cells transferred into each host by tetramer staining (see Materials and Methods). 5 days later the recall response was analyzed in the spleen through tetramer staining, and the total number of donor-derived virus-specific CD8+ T cells was calculated. This approach allows us to accurately measure the fold expansion of virus-specific CD8+ T cells during recall in vivo in each individual animal, through dividing the total number of donor-derived virus-specific CD8+ T cells 5 days post-challenge by the total number initially transferred. VV-specific memory CD8+ T cells that received CD4 help expanded on an average of 39 fold upon challenge, while helpless memory cell expanded on average ∼10 fold (Figure 2E). Thus, VV-specific memory CD8+ T cells that developed in the absence of CD4 help were severely impaired in their ability to mount a recall response upon viral challenge (Figure 2E). Although the magnitude of reduction varied between experiments, we consistently observed reduction of the recall response by helpless memory cells in all experiments, typically two to four folds, similar to numbers reported in other models (3, 11, 22, 30, 37). The data clearly demonstrates the requirement of CD4 help for memory CD8+ T cell differentiation during acute VV infection.
During LCMV infection, lack of CD4 help or CD40L-CD40 interactions leads to viral resurgence at ~2 months post-infection, which causes deterioration of memory CD8+ T cell function (38). However, we found no evidence of viral persistence in CD4-depleted mice (data not shown), suggesting that the defects observed were a direct effect of CD4 help and not an indirect effect of CD8+ T cell exhaustion caused by viral persistence.
Role of CD40 signaling in helping memory CD8+ T cell differentiation
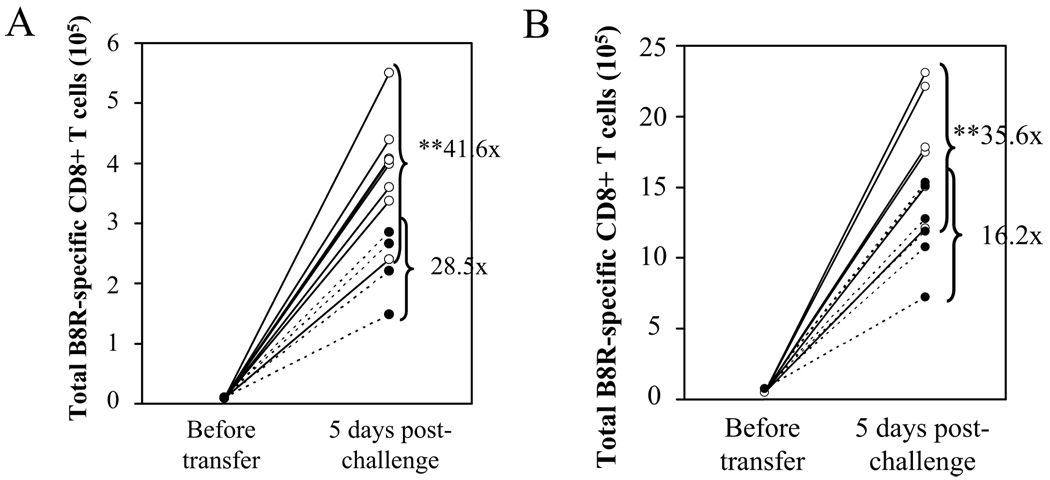
The requirement for CD4 help in memory CD8+ T cell differentiation has been documented in various infection models; yet the timing of help and the signals that mediate help have been a subject of debate (7, 8). Therefore, we utilized our adoptive transfer system to investigate the required timing and the molecular nature of CD4 help for VV-specific memory CD8+ T cells to acquire the ability to mount a robust recall response. First we tested whether CD4 help is required during priming, by injecting the anti-CD4 mAb only on days −1, 0, and 3. VV-specific memory CD8+ T cells from mice which received early CD4-depletion were defective in mounting a recall response (Figure 3A). The data supports the requirement for CD4+ T cells during priming, although it does not rule out an additional role of CD4 help during the maintenance phase. Next, due to the importance of CD40L-CD40 interactions in mediating CD4 help (14–16), we investigated the role of CD40 signaling in memory cell development by transferring VV-specific memory CD8+ T cells from infected B6 or CD40−/− mice. Upon adoptive transfer and challenge with VV-WR, memory CD8+ T cells that developed in CD40−/− mice showed a significantly reduced recall response (Figure 3B). Therefore, during VV-WR infection CD40 signaling significantly impacts memory CD8+ T cell differentiation.
Figure 3. Functional VV-specific memory cells require CD4 help during priming and CD40 signaling.
(A) Mice were infected with VV-WR, and were untreated or CD4-depleted during priming (d−1, 0, and 3). 100 days p.i., the recall response of VV-specific memory CD8+ T cells was analyzed by adoptive transfer experiments described in Figure 2. White circles: undepl. → B6; black circles: early CD4-depl. → B6. (B) B6 or CD40−/− mice were infected with VV-WR and the recall response of VV-specific memory CD8+ T cells was analyzed by adoptive transfer and challenge. White circles: B6 → B6; black circles: CD40−/− → B6. Representative data from two independent experiments are shown, using 4 donors per group and 4–8 recipients per group. Each circle represents individual recipients. Fold expansion of tetramer+ cells was calculated for each recipient, and the number shown indicated the average fold expansion for each group. ** p > 0.01.
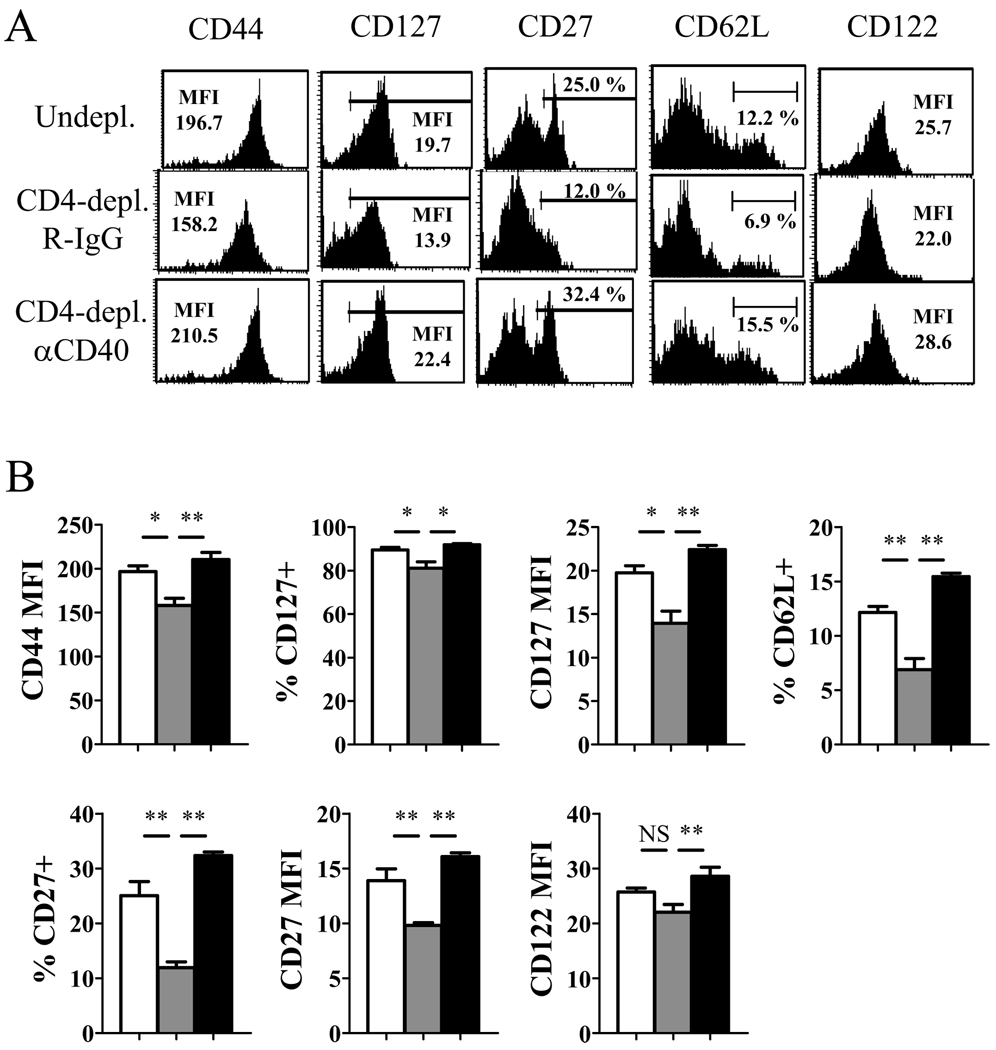
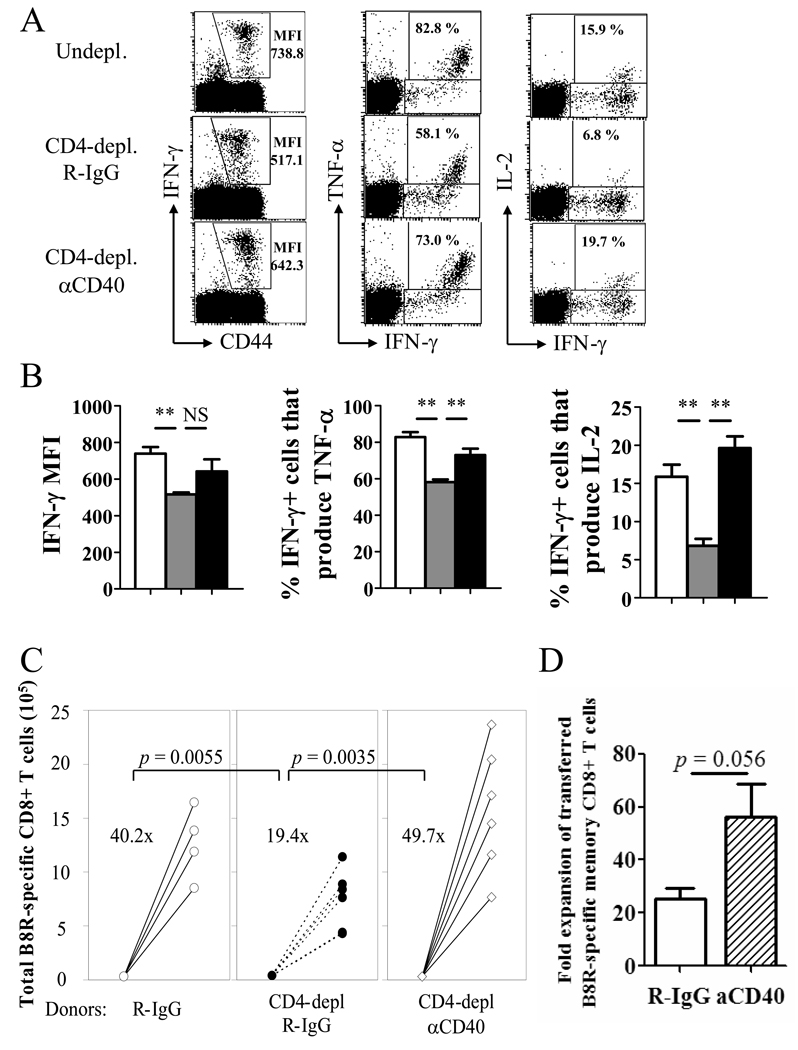
The requirement for CD4 help during priming and CD40 signaling prompted us to assess whether providing CD40 stimulation during priming in the absence of CD4+ T cells can reverse the defects of helpless memory cells. Agonistic anti-CD40 mAb was injected into CD4-depleted mice at days 1 and 7 p.i., and at 49 day post-infection, memory CD8+ T cells were analyzed for their phenotype (Figure 4A, B). The aberrant expression of CD44, CD127, CD27, and CD62L observed in CD4-depleted mice was restored by CD40 signaling during priming. Furthermore, the defects in cytokine production, particularly TNF-α and IL-2, were restored by CD40 ligation back to levels similar to memory cells that received CD4 help (Figure 5A, B). IFN-γ production was also slightly increased, although complete restoration of production of this cytokine was never observed. Finally, the defect in the secondary response by the helpless cells was reversed by administering the agonistic anti-CD40 mAb during priming (Figure 5C). The results show that CD40 stimulation during priming can rescue helpless CD8+ T cell memory differentiation, and may indicate a role for CD40 in CD4 help. However, administering CD40 stimulation during priming slightly enhanced recall responses of helped memory CD8+ T cells in undepleted mice (Figure 5D), implying that CD40 signaling may have a non-overlapping role with CD4+ T cell help.
Figure 4. Providing CD40 stimulation during priming restores phenotypic changes of helpless memory cells.
Mice were infected with VV-WR and were un-depleted, CD4-depleted/R-IgG-treated, or CD4-depleted/anti-CD40-treated (d1, 7). (A) VV-specific memory CD8+ T cells were analyzed for expression of phenotypic markers by tetramer staining on day 49 p.i. Plots are gated on CD8+ tetramer+ cells. (B) Graphed presentation of (A). White bars: un-depleted, grey bars: CD4-depleted/R-IgG-treated, black bars: CD4-depleted/anti-CD40-treated. Representative data from two independent experiments with 3–4 mice per group are shown. Error bars indicate SEM. NS: not significant. * p > 0.05, ** p > 0.01.
Figure 5. Providing CD40 stimulation during priming boosts memory cell function in the absence of CD4 help.
Mice were infected with VV-WR and were un-depleted, CD4-depleted/R-IgG-treated, or CD4-depleted/anti-CD40-treated (d1, 7). A, Cytokine production by VV-specific memory CD8+ T cells was analyzed by ICCS. Plots are gated on CD8+ cells. Numbers indicate IFN-γ MFI of CD8+ T cells producing IFN-γ (left), or percentage of IFN-γ+ cells that produce TNF-α (middle) or IL-2 (right). B, Graphed representation of (A). White bars: un-depleted, grey bars: CD4-depleted/R-IgG-treated, black bars: CD4-depleted/anti-CD40-treated. Representative data from two independent experiments with 3–4 mice per group are shown. Error bars indicate SEM. NS: not significant. * p > 0.05, ** p > 0.01. C, The recall response by VV-specific memory CD8+ T cells was analyzed by adoptive transfer and challenge. Treatments of donor mice are indicated below. Numbers indicate average fold expansion of VV-specific memory cells for each group. Each circle represents an individual recipient. D, Recall response of helped (undepleted) memory cells that received R-IgG or anti-CD40 during priming is graphed. Fold expansion of transferred (CD45.2+) memory cells is shown (error bars indicate SEM). Representative plots are shown from two independent experiments; consisting of 3–4 mice per group are shown. In C and D, 4 donor mice per group, and 4–6 recipients per group were used.
The helpless defect is independent of TRAIL
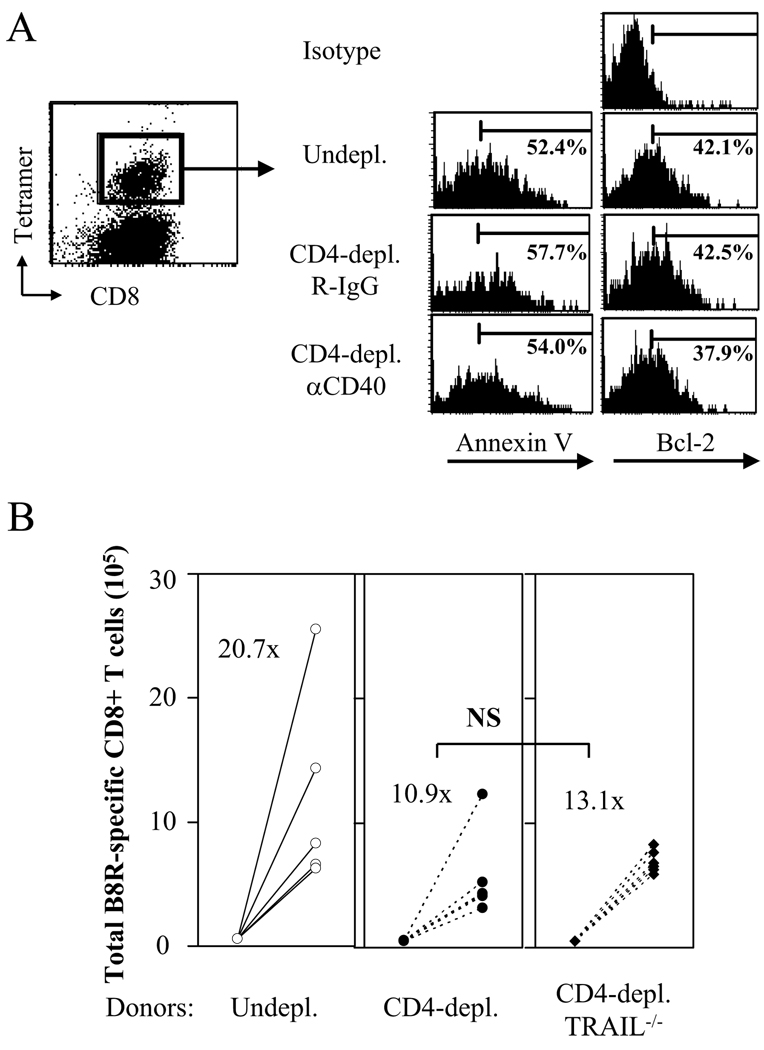
Although our data demonstrates that CD4 help is required for VV-specific memory CD8+ T cells to acquire the ability to mount a robust recall response, the molecular mechanism of why helpless memory cells elicit defective recall responses remains elusive. Evidence provided by Janssen et al. suggested that the upregulation of TRAIL by helpless memory cells upon recall leads to increased cell death and an impaired recall response (30). Therefore, we hypothesized that the absence of CD4 help leads to increased cell death of memory CD8+ T cells during recall. VV-specific memory CD8+ T cells from un-depleted, CD4-depleted/R-IgG treated, or CD4-depleted/anti-CD40 treated mice were challenged with VV-WR upon adoptive transfer into naïve recipients (as described in Figure 5C), and 5 days later, donor tetramer-positive cells were analyzed for cell death by Annexin V staining. No differences in the percentage of virus-specific CD8+ T cells undergoing cell death were observed among the three groups (Figure 6A). Furthermore, we found no difference in the expression of the anti-apoptotic molecule Bcl-2 by tetramer+ cells (Figure 6A). Thus, the data implies that the reason for the impaired recall response by helpless memory cells is not due to increased cell death. We next examined whether TRAIL deficiency could reverse the defective recall response by helpless memory cells, as previously shown (30, 37). As shown in Figure 6B, VV-specific memory CD8+ T cells from CD4-depleted mice were impaired in their recall response, and this defect was not restored by TRAIL deficiency. Recently, TRAIL has been shown to possess anti-viral function against dengue virus (39); however, we observed no defects by TRAIL−/− mice in the ability to control i.n. VV-WR infection (data not shown). Altogether, the results imply that the impaired recall response by helpless memory cells in VV-WR infection is not due to increased cell death and is independent of TRAIL.
Figure 6. The defective recall response by helpless memory cells is independent of TRAIL.
(A) VV-specific memory CD8+ T cells from un-depleted, CD4-depleted/R-IgG-treated, or CD4-depleted/anti-CD40 treated mice were adoptively transferred into naïve recipients and challenged as shown in Figure 5C. 5 days post-challenge, donor-derived VV-specific CD8+ T cells were stained with Annexin V or by intracellular Bcl-2. Representative plots from two independent experiments with 6 mice per group are shown. Left: representative plot (gated on donor cells) showing gating on tetramer+ cells. Right: gated on CD45.2+ CD8+ tetramer+ cells. (B) The recall response of VV-specific memory CD8+ T cells from un-depleted B6, CD4-depleted B6, or CD4-depleted TRAIL−/− mice was analyzed by adoptive transfer and challenge as shown in Figure 2D. Numbers indicate average fold expansion of VV-specific memory cells for each group. Each circle represents an individual recipient. Representative data from two independent experiments using 4 donor mice per group, 4–6 recipient mice per group are shown.
Excessive upregulation of PD-1 on helpless memory cells impairs the recall response
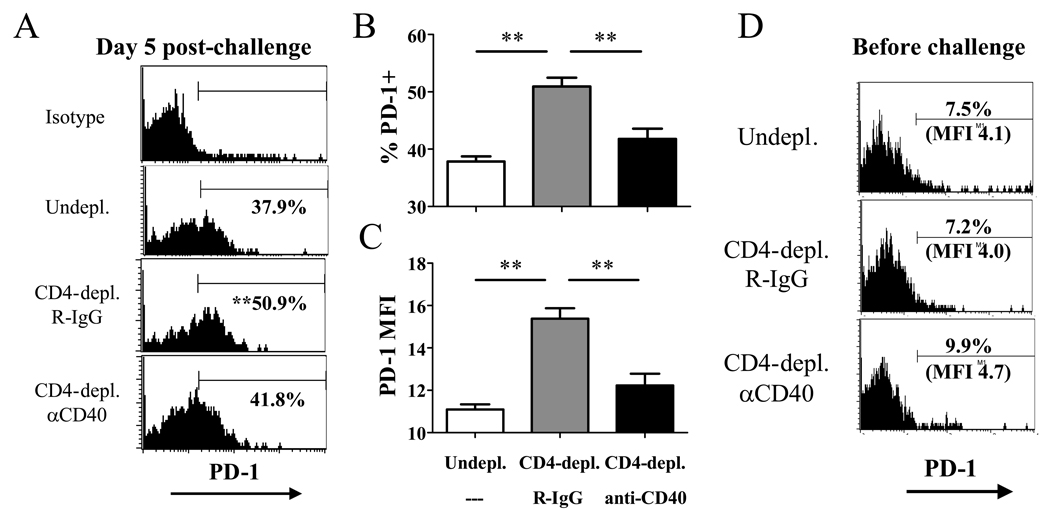
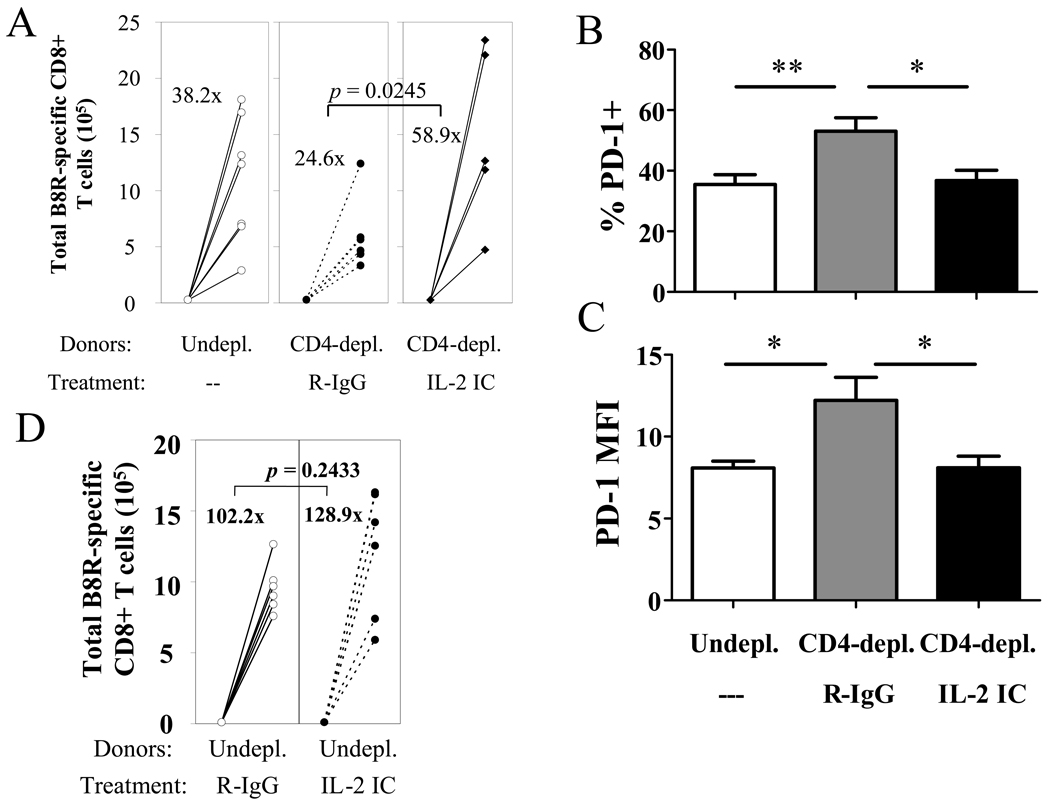
Data suggesting that the defective recall response of helpless memory CD8+ T cells is independent of TRAIL-mediated cell death prompted us to investigate other pathways that may mediate the helpless defect. We hypothesized that during recall, helpless memory cells express higher levels of receptors that inhibit T cell proliferation, reducing the magnitude of the recall response. We explored two immunoregulatory receptors in the B7-CD28 superfamily, CTLA-4 and PD-1, which are known for their potent inhibitory role in the T cell response. VV-specific memory CD8+ T cells from un-depleted, CD4-depleted/R-IgG treated, or CD4-depleted/anti-CD40 treated mice were challenged upon transfer, and 5 days later, were assessed for the expression these molecules. The expression of CTLA-4 during recall did not correlate with the ability of the memory cells to mount a recall response (data not shown), indicating that the molecule does not account for the difference in proliferative capacity between helped and helpless memory cells. Next, we examined the expression of PD-1 on memory CD8+ T cells during the recall response. The expression of PD-1, both in terms of percentage of VV-specific CD8+ T cells expressing the receptor and the MFI, was significantly increased on memory CD8+ T cells that did not receive CD4 help (Figure 7A–C). Furthermore, restoring the recall response in CD4-depleted mice by administering agonistic anti-CD40 mAb resulted in the downregulation of PD-1 expression during the recall response. Thus, expression of PD-1 during the recall response correlated with CD4 help and CD40 signaling, and the proliferative capacity of helped vs. helpless memory cells during recall. PD-1 expression was not observed on resting memory cells before recall in all groups (Figure 7D).
Figure 7. Helpless memory cells express excess levels of PD-1 during recall compared to helped memory cells.
VV-specific memory CD8+ T cells from un-depleted, CD4-depleted/R-IgG-treated, or CD4-depleted/anti-CD40 treated mice were adoptively transferred into naïve recipients and challenged as shown in Figure 5C. 5 days post-challenge, donor-derived VV-specific CD8+ T cells were stained for PD-1 expression. (A) Representative plots gated on CD45.2+ CD8+ tetramer+ cells are shown, and numbers indicate the average percentage of VV-specific CD8+ T cells expressing PD-1. (B) Graphed presentation of (A). (C) MFI of PD-1 expression by donor-derived VV-specific CD8+ T cells. Representative data from two independent experiments with 6 recipients per group are shown. (D) PD-1 expression on resting VV-specific helped and helpless memory CD8+ T cells at 49 days post-infection. Representative data from two independent experiments with 3–4 mice per group are shown. Plots are gated on CD8+ tetramer+ cells. Error bars indicate SEM. * p > 0.05, ** p > 0.01.
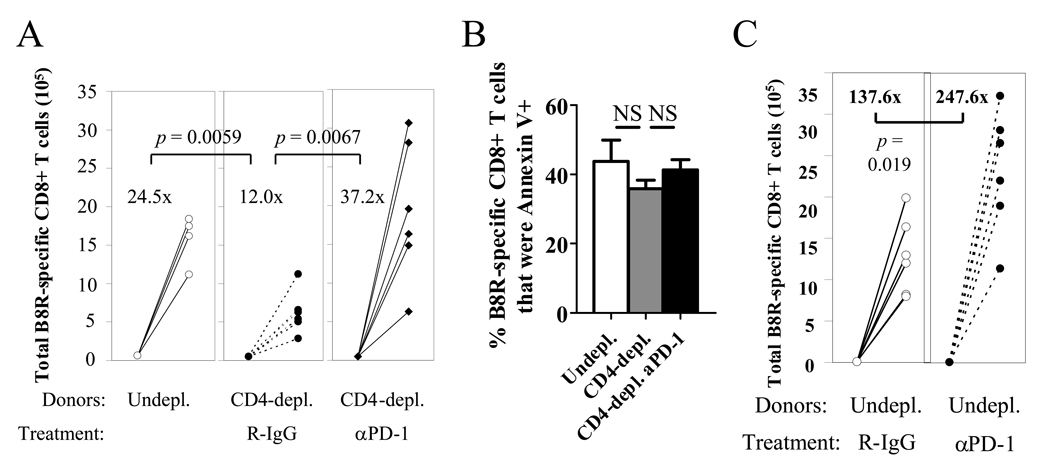
Because the expression of PD-1 was higher on helpless memory cells during recall compared to helped cells, we hypothesized that the level of PD-1 expression may play a role in blunted recall responses in the absence of CD4 help. To investigate the functional significance of PD-1 expression, we inhibited PD-1 on helpless memory cells during the recall response by Ab blockade. Treatment with anti-PD-1 during recall significantly enhanced the recall response of helpless memory cells, to the levels of helped memory cells (Figure 8A). Interestingly, treatment with anti-PD-1 had no effect on Annexin V staining (Figure 8B) or Bcl-2 expression (data not shown) on the virus-specific CD8+ T cells, implying that PD-1 inhibits the recall response without affecting cell survival. PD-1 blockade also enhanced recall responses of helped memory cells (Figure 8C), which is in line with our observation of PD-1 upregulation on helped memory cells during the recall response (Figure 7A, second panel). These data indicate that PD-1 signaling acts as an important brake for recall responses by VV-specific memory CD8+ T cells. Furthermore, it supports our hypothesis that the blunted recall response by helpless cells may be caused by excessive upregulation of PD-1 by helpless memory cells during recall compared to helped cells.
Figure 8. Excessive PD-1 expression on helpless memory cells impairs the recall response.
(A) Helpless VV-specific memory CD8+ T cells were adoptively transferred and challenged, and were treated with R-IgG or anti-PD-1. 5 days post-challenge, expansion of donor-derived VV-specific CD8+ T cells was analyzed as described in Figure 2D. Numbers indicate average fold expansion of VV-specific memory cells for each group. (B) Donor-derived tetramer+ cells at 5 days post-challenge in the experiment described in (A) were stained with Annexin V. Error bars indicate SEM. (C) Effect of anti-PD-1 on recall response of helped VV-specific memory cells. Representative data from two independent experiments, with 4–6 recipient mice per group are shown. Error bars indicate SEM. * p > 0.05, ** p > 0.01.
IL-2 signaling in vivo rescues the recall response of helpless memory cells
Previous studies by Carter et al. have shown that PD-1 inhibits T cell proliferation and IL-2 production, and this inhibition can be overcome by augmenting IL-2 in vitro (40). Interestingly, helpless memory cells exhibit defective proliferation upon recall and are unable to produce IL-2 upon restimulation, and both these defects are restored by CD40 signaling during priming (Figure 5A, B). Thus, the ability to mount a robust recall response correlates with their ability to produce IL-2. We therefore tested whether supplying IL-2 signaling in vivo could rescue the defective recall response by helpless memory cells. IL-2 signaling was administered by daily injections of immune complexes, combination of IL-2 and anti-IL-2 mAb (S4B6), which have been shown to deliver a strong IL-2 signal to T cells (25, 33). We have previously shown that injection of the IL-2 immune complex could restore defective recall responses by memory CD8+ T cells which failed to receive CD28 costimulation (32). Delivering IL-2 signaling during recall revived the blunted recall response by helpless memory CD8+ T cells (Figure 9A). Furthermore, treatment with the IL-2 immune complex resulted in a significant downregulation of PD-1 expression on responding helpless cells (Figure 9B, C). Furthermore, augmenting IL-2 signaling to helped memory cells did not enhance recall responses by helped memory cells (Figure 9D). In summary, these data imply that IL-2 signaling allows the memory cells to override inhibition by PD-1 likely through downregulation of the receptor.
Figure 9. IL-2 signaling in vivo rescues the recall response of helpless memory cells.
(A) Helpless VV-specific memory CD8+ T cells were adoptively transferred and challenged, and were treated with R-IgG or the IL-2 immune complex. 5 days post-challenge, transferred memory cells were analyzed for expansion as described in Figure 2D. Numbers indicate average fold expansion of VV-specific memory cells for each group. (B,C) Donor-derived tetramer+ cells at 5 days post-challenge were stained for PD-1 expression. Percentage of VV-specific CD8+ T cells expressing PD-1 (B), and MFI of PD-1 expression (C) by donor-derived VV-specific CD8+ T cells is graphed. Error bars indicate SEM. (D) Effect of IL-2 immune complex on recall response of helped VV-specific memory cells. Representative data from two independent experiments, with 4–6 recipient mice per group are shown. * p > 0.05, ** p > 0.01.
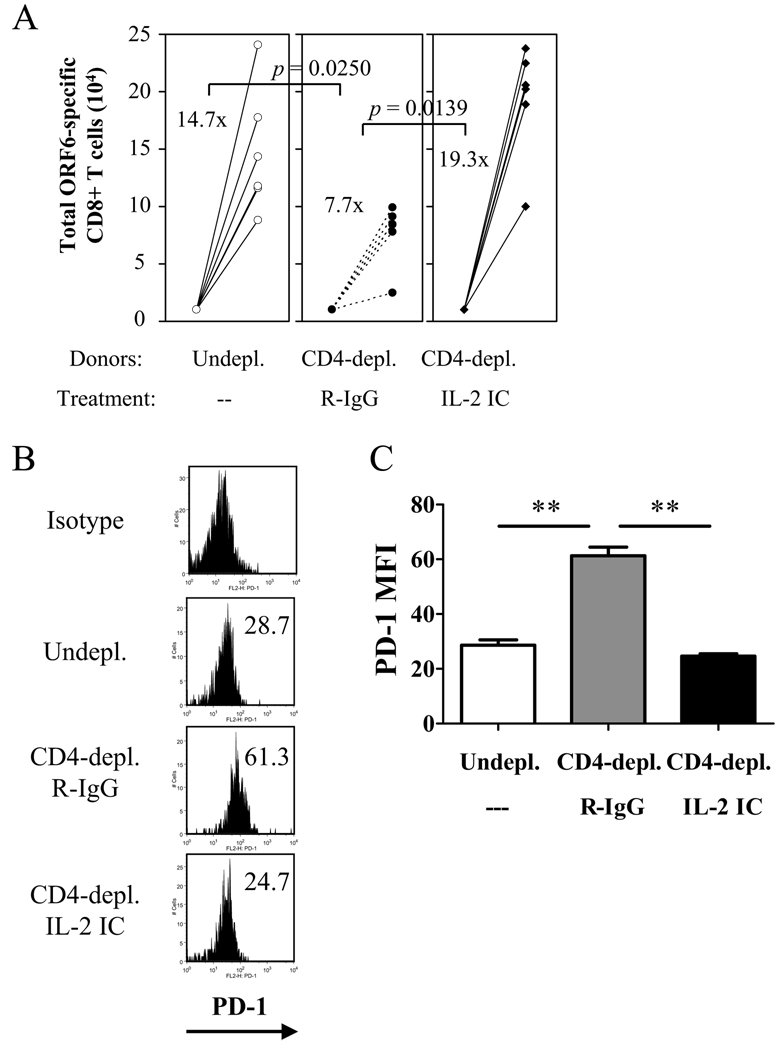
We further investigated whether the upregulation of PD-1 by helpless memory CD8+ T cells during the recall response and the restoration by IL-2 signaling is a unique feature of VV-WR infection, or can also be seen in other infection models. The murine gammaherpesvirus-68 (MHV-68) causes a latent infection characterized by a low level viral persistence. MHV-68-specific CD8+ T cells elicit robust recall responses upon challenge with recombinant VV (rVV) expressing MHV-68 antigens (41). In the absence of CD40-mediated CD4 help, CD8+ T cell-mediated immune surveillance of the persistent virus is lost (42, 43). We measured the ability of helpless memory CD8+ T cells specific for the MHV-68 ORF6487–495/Db epitope upon transfer into naïve recipients and rVV-ORF6 challenge. Similar to VV-specific memory CD8+ T cells, helpless MHV-68-specific memory CD8+ T cells showed a severely impaired recall response (Figure 10A), and the helpless cells expressed significantly higher levels of PD-1 during the recall response (Figure 10B, C). Furthermore, administering the IL-2 immune complex during the recall response resulted in the downregulation of PD-1 and the restoration of the recall response (Figure 10A–C). Thus, the observation made in the VV-WR model that helpless memory CD8+ T cells express higher levels of PD-1, is not a unique feature of VV-WR infection but is also seen in other models where CD4 help plays a critical role for memory CD8+ T cell differentiation.
Figure 10. PD-1 upregulation by MHV-68-specific helpless memory CD8+ T cells and restoration by IL-2 in vivo .
(A) Helpless MHV-68-ORF6-specific memory CD8+ T cells were adoptively transferred and challenged (see Materials and Methods for details), and were treated with R-IgG or the IL-2 immune complex. 5 days post-challenge, transferred memory cells were analyzed for expansion as described in Figure 2D. Numbers indicate average fold expansion of MHV-68-specific memory cells for each group. (B) Donor-derived ORF6 tetramer+ cells at 5 days post-challenge were stained for PD-1 expression. Representative plots gated on CD45.2+ CD8+ cells are shown. Numbers indicate average MFI. (C) MFI of PD-1 expression by donor-derived VV-specific CD8+ T cells is graphed. Representative data from two independent experiments, with 4–6 recipient mice per group are shown. Error bars indicate SEM. ** p > 0.01.
Discussion
Help by CD4+ T cells has been shown to be pivotal for memory CD8+ T cell differentiation in most infection models studies (7). However, the discrepancies in mechanisms of CD4 help reported among the model studied are perplexing. One such controversy is the involvement of the CD40 pathway. Fully functional memory CD8+ T cells develop in the absence of CD40 during Listeria monocytogenes infection and following immunization with VLPs (6, 19, 21). In contrast, CD40 on non-T cells is required for full differentiation of memory CD8+ T cells following influenza infection (20), and loss of CD8-mediated control of murine gammaherpesvirus-68 (MHV-68) infection occurs in the absence of CD40-mediated CD4 help (42–44). The requirement for CD40 in our model may suggest that during infection through the i.n. route, there is a general requirement for CD40. This may be due to priming by distinct populations of DCs in the lung (45) or the unique lung environment (46). Outliers to this generalization exist, as recall CD8+ T cell responses after priming with systemic Listeria infection in Balb/c mice have been shown to be dependent on CD40L (22), suggesting that the location of priming does not completely account for the requirement for CD40.
In our model, administering agonistic CD40 during priming restored the phenotype, cytokine production, and the recall response in the absence of CD4+ T cells (Figure 4 and Figure 5). The results directly contrast with recent work suggesting that early CD40 ligation results in the deterioration of T cell memory (23, 24). On the contrary, CD40 stimulation has been shown to replace CD4 help and restore CD8-mediated control of MHV-68 (43). Furthermore, immunization with CD40 stimulation in combination with a TLR agonist results in the generation of functional CD8+ T cell memory to a tumor/self antigen, which is otherwise poorly immunogenic (47), showing that CD40 could provide beneficial signals for memory generation.
The requirement for CD4 help during priming and CD40 signaling is in direct contrast with a recent study using immunization with VLPs, which did not require CD4 help during priming and was CD40-independent (12). The discrepancy is particularly interesting, since both models use i.p. infection of VV during recall, thus the only difference is the method used to prime the CD8+ T cell response. There are notable differences between the VLP immunization and the VV-WR infection used in our model, which may explain the disparities observed. Such differences include route of priming (subcutaneous immunization vs. i.n. infection), duration of antigen (non-replicating VLPs vs. live virus infection), and degree of inflammation. Although the immunization using VLPs included a TLR agonist, inflammation induced by live virus infection exceeds the immunization both in degree and duration. Inflammation has been shown to negatively regulate generation of memory precursors and memory CD8+ T cells (48, 49), and the effects of CD40 signaling may shift the balance in favor of generating potent memory under inflammatory conditions. Similar requirements for early CD4 help have been reported upon systemic VV infection (11).
In our experiments, helpless memory cells did not exhibit higher levels of cell death during recall, and the defect was independent of TRAIL (Figure 6). During LCMV infection, TRAIL only mediates the helpless defects observed early (~60 days), but at later time points (~90 days) the impairment occured in a TRAIL-independent manner (37). Furthermore, TRAIL deficiency did not restore the function of helpless memory cells during Listeria infection or responses to help-dependent antigens (31). Our recall experiments were performed at 36 days p.i., thus indicating the helpless defect in our model may be completely TRAIL-independent. Our data provides evidence that the TRAIL-independent impairment is mediated through the PD-1 pathway. PD-1 is a potent negative costimulatory molecule that inhibits T and B cell responses (50). The molecule is highly expressed on exhausted T cells during chronic viral infections, and blocking this pathway reverses functional exhaustion (51). Upon resolution of acute VV infection, no PD-1 expression was observed on resting memory cells, whether or not they received CD4 help (Figure 7D). Upon recall, both helped and helpless memory cells upregulated PD-1; however, helpless memory cells expressed significantly higher levels of PD-1 compared to helped cells (Figure 7A–C). The upregulation of PD-1 is likely due to a lack of CD40 signaling and CD4 help, since administering agonistic anti-CD40 mAb during priming reversed PD-1 expression. This upregulation was selective, since expression of CTLA-4 was not affected. Furthermore, the excessive upregulation of PD-1 by helpless memory cells during the recall response compared to helped cells was not a unique characteristic of VV-WR infection and was also observed in helpless MHV-68-specific memory CD8+ T cells (Figure 10B, C). Blocking PD-1 during the recall response completely restored the response by the helpless memory cells (Figure 8A). It is worth noting that in our experiments, CD8+ T cells are purified and transferred into naïve recipients, where the cells are challenged. Thus, memory CD8+ T cells from both groups (helped and helpless) are challenged in the same environment, and the only difference is the transferred CD8+ T cells themselves. Therefore, the most likely explanation is that the helpless cells are “imprinted” along the differentiation pathway through CD40 signaling during priming, to upregulate PD-1 during recall. CD4 help has been shown to influence epigenetic remodeling of the IFN-γ and IL-2 promoter and enhancer regions (52), which links the inability of helpless memory cells to rapidly produce these cytokines. We hypothesize that one of the important roles for CD4 help through CD40 during priming may be the epigenetic imprinting of the PD-1 locus, in which upon recall is tightly regulated, enabling these cells to rapidly proliferate and mount a robust recall response. The lack of CD4 help could result in loosening of this tight regulation, causing excessive upregulation of PD-1 compared to helped cells and inhibiting the recall response. Our future experiments will test this hypothesis.
Another intriguing finding is that supplying IL-2 in vivo through the use of immune complexes restores recall response by helpless memory cells (Figure 9A and Figure 10A). The restoration of the recall response coincided with the downregulation of PD-1 (Figure 9 and Figure 10), suggesting this may be one of the mechanisms in which IL-2 restores the response by helpless memory cells. The PD-L1-PD-1 interaction results in the inhibition of proliferation of both CD4+ and CD8+ T cells, and addition of exogenous IL-2 has been shown to overcome this inhibition and restore proliferation of polyclonal T cells in vitro (40). An interesting similarity with the study by Carter et al. (40), our previous study (32), and our current report is that the intrinsic ability of CD8+ T cells to produce IL-2 correlates with their proliferative capacity (Figure 5). Furthermore, in all three studies, providing IL-2 signaling in vitro or in vivo restores the impaired proliferation by these cells (Figure 9 and Figure 10) (32, 40). Thus, one reason helpless memory cells may be defective is their inability to produce IL-2, through the epigenetic alteration of their promoter (52). To our interest, a recent study reported a correlation between reduced CD27 expression on helpless memory cells and its inability to produce IL-2 and mount a recall response (53). We also observed reduced CD27 expression on VV-specific helpless memory cells (Figure 2), and its expression was restored by CD40 stimulation during priming, along with IL-2 production and recall potential (Figure 4). Thus, reduced CD27 expression, impaired IL-2 production, and excessive PD-1 expression during recall, may act in concert to restrict secondary expansion of helpless memory cells.
Our data reveals a previously uncharacterized mechanism for why memory CD8+ T cell differentiation is impaired in the absence of CD4 help. Furthermore, we provide evidence that the helpless defects are reversible at multiple stages through various interventions (CD40 stimulation during priming, and PD-1 blockade or IL-2 signaling during recall). The information may be valuable especially in designing vaccination in settings where CD4+ T cells are unavailable or dysfunctional, such as in the context of chronic HIV infection.
Acknowledgements
We thank Dr. Randolph J. Noelle for insight and comments.
Footnotes
This work was supported by National Institute of Health Grant CA103642.
Contributor Information
Shinichiro Fuse, Department of Microbiology and Immunology, Dartmouth Medical School, Lebanon, NH 03756, USA..
Ching-Yi Tsai, Department of Microbiology and Immunology, Dartmouth Medical School, Lebanon, NH 03756, USA..
Michael J. Molloy, Department of Microbiology and Immunology, Dartmouth Medical School, Lebanon, NH 03756, USA.
S. Rameeza Allie, Department of Microbiology and Immunology, Dartmouth Medical School, Lebanon, NH 03756, USA..
Weijun Zhang, Department of Microbiology and Immunology, Dartmouth Medical School, Lebanon, NH 03756, USA..
Hideo Yagita, Department of Immunology, Juntendo University School of Medicine, Tokyo, Japan.
Edward J. Usherwood, Department of Microbiology and Immunology, Dartmouth Medical School, Lebanon, NH 03756, USA..
References
- 1.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 2.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 3.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 4.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172:2834–2844. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann MF, Schwarz K, Wolint P, Meijerink E, Martin S, Manolova V, Oxenius A. Cutting edge: distinct roles for T help and CD40/CD40 ligand in regulating differentiation of proliferation-competent memory CD8+ T cells. J Immunol. 2004;173:2217–2221. doi: 10.4049/jimmunol.173.4.2217. [DOI] [PubMed] [Google Scholar]
- 7.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 8.Khanolkar A, Badovinac VP, Harty JT. CD8 T cell memory development: CD4 T cell help is appreciated. Immunol Res. 2007;39:94–104. doi: 10.1007/s12026-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 9.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 11.Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol. 2007;179:8243–8251. doi: 10.4049/jimmunol.179.12.8243. [DOI] [PubMed] [Google Scholar]
- 12.Agnellini P, Wiesel M, Schwarz K, Wolint P, Bachmann MF, Oxenius A. Kinetic and mechanistic requirements for helping CD8 T cells. J Immunol. 2008;180:1517–1525. doi: 10.4049/jimmunol.180.3.1517. [DOI] [PubMed] [Google Scholar]
- 13.Marzo AL, Vezys V, Klonowski KD, Lee SJ, Muralimohan G, Moore M, Tough DF, Lefrancois L. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173:969–975. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 14.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 15.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 16.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 17.Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, Belz GT. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 18.Borrow P, Tishon A, Lee S, Xu J, Grewal IS, Oldstone MB, Flavell RA. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun JC, Bevan MJ. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol. 2004;172:3385–3389. doi: 10.4049/jimmunol.172.6.3385. [DOI] [PubMed] [Google Scholar]
- 20.Lee BO, Hartson L, Randall TD. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient environment. J Exp Med. 2003;198:1759–1764. doi: 10.1084/jem.20031440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montfort MJ, Bouwer HG, Wagner CR, Hinrichs DJ. The development of functional CD8 T cell memory after Listeria monocytogenes infection is not dependent on CD40. J Immunol. 2004;173:4084–4090. doi: 10.4049/jimmunol.173.6.4084. [DOI] [PubMed] [Google Scholar]
- 22.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH, Welniak LA, Redelman D, Murphy WJ. IFN-gamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nat Med. 2007;13:354–360. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]
- 24.Bartholdy C, Kauffmann SO, Christensen JP, Thomsen AR. Agonistic anti-CD40 antibody profoundly suppresses the immune response to infection with lymphocytic choriomeningitis virus. J Immunol. 2007;178:1662–1670. doi: 10.4049/jimmunol.178.3.1662. [DOI] [PubMed] [Google Scholar]
- 25.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 27.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 28.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 29.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 31.Sacks JA, Bevan MJ. TRAIL Deficiency Does Not Rescue Impaired CD8+ T Cell Memory Generated in the Absence of CD4+ T Cell Help. J Immunol. 2008;180:4570–4576. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuse S, Zhang W, Usherwood EJ. Control of Memory CD8+ T Cell Differentiation by CD80/CD86-CD28 Costimulation and Restoration by IL-2 during the Recall Response. J Immunol. 2008;180:1148–1157. doi: 10.4049/jimmunol.180.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 34.Reading PC, Smith GL. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J Gen Virol. 2003;84:1973–1983. doi: 10.1099/vir.0.19285-0. [DOI] [PubMed] [Google Scholar]
- 35.Marzo AL, Klonowski KD, Bon AL, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8(+) T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badovinac VP, Messingham KA, Griffith TS, Harty JT. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 38.Bachmann MF, Hunziker L, Zinkernagel RM, Storni T, Kopf M. Maintenance of memory CTL responses by T helper cells and CD40-CD40 ligand: antibodies provide the key. Eur J Immunol. 2004;34:317–326. doi: 10.1002/eji.200324717. [DOI] [PubMed] [Google Scholar]
- 39.Warke RV, Martin KJ, Giaya K, Shaw SK, Rothman AL, Bosch I. TRAIL is a novel antiviral protein against dengue virus. J Virol. 2008;82:555–564. doi: 10.1128/JVI.01694-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Belz GT, Stevenson PG, Castrucci MR, Altman JD, Doherty PC. Postexposure vaccination massively increases the prevalence of gamma-herpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2725–2730. doi: 10.1073/pnas.040575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarawar SR, Lee BJ, Reiter SK, Schoenberger SP. Stimulation via CD40 can substitute for CD4 T cell function in preventing reactivation of a latent herpesvirus. Proc Natl Acad Sci U S A. 2001;98:6325–6329. doi: 10.1073/pnas.101136898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks JW, Hamilton-Easton AM, Christensen JP, Cardin RD, Hardy CL, Doherty PC. Requirement for CD40 ligand, CD4(+) T cells, and B cells in an infectious mononucleosis-like syndrome [In Process Citation] J Virol. 1999;73:9650–9654. doi: 10.1128/jvi.73.11.9650-9654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 47.Ahonen CL, Wasiuk A, Fuse S, Turk MJ, Ernstoff MS, Suriawinata AA, Gorham JD, Kedl RM, Usherwood EJ, Noelle RJ. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared to unitary adjuvants as cancer vaccines. Blood. 2008;111:3116–3125. doi: 10.1182/blood-2007-09-114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 49.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 52.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 53.Matter MS, Claus C, Ochsenbein AF. CD4(+) T cell help improves CD8(+) T cell memory by retained CD27 expression. Eur J Immunol. 2008;8:1847–1856. doi: 10.1002/eji.200737824. [DOI] [PubMed] [Google Scholar]