Abstract
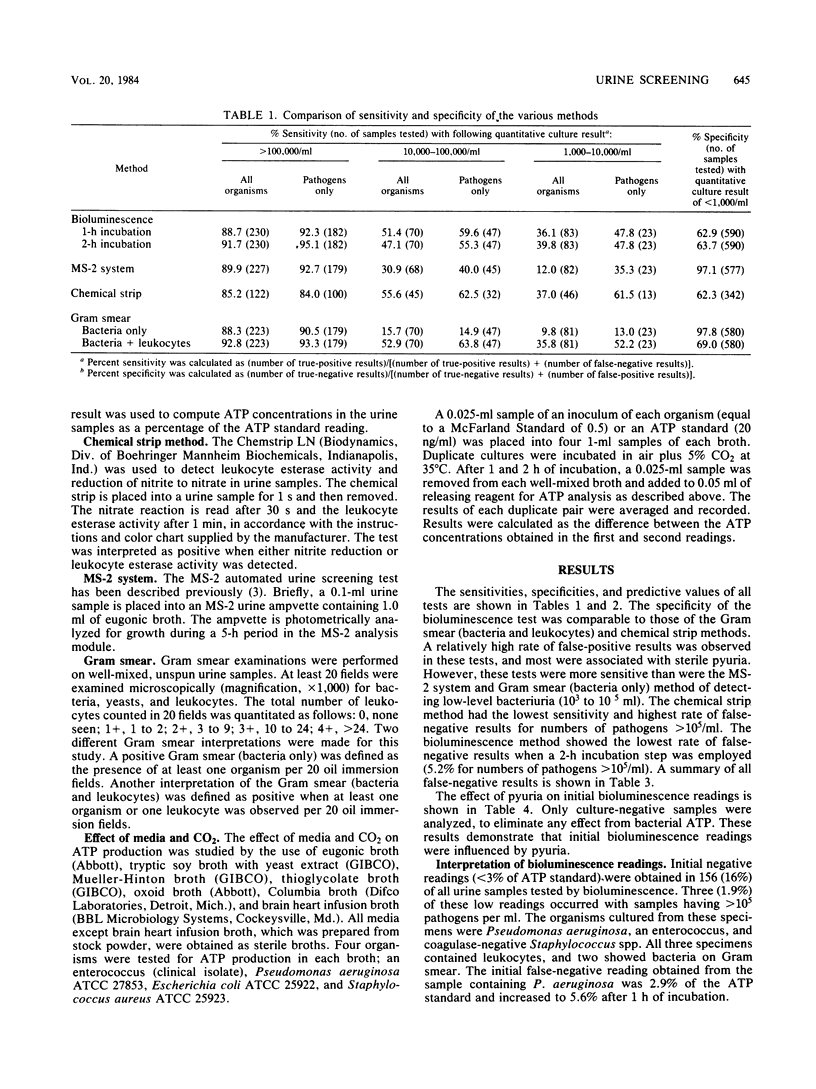
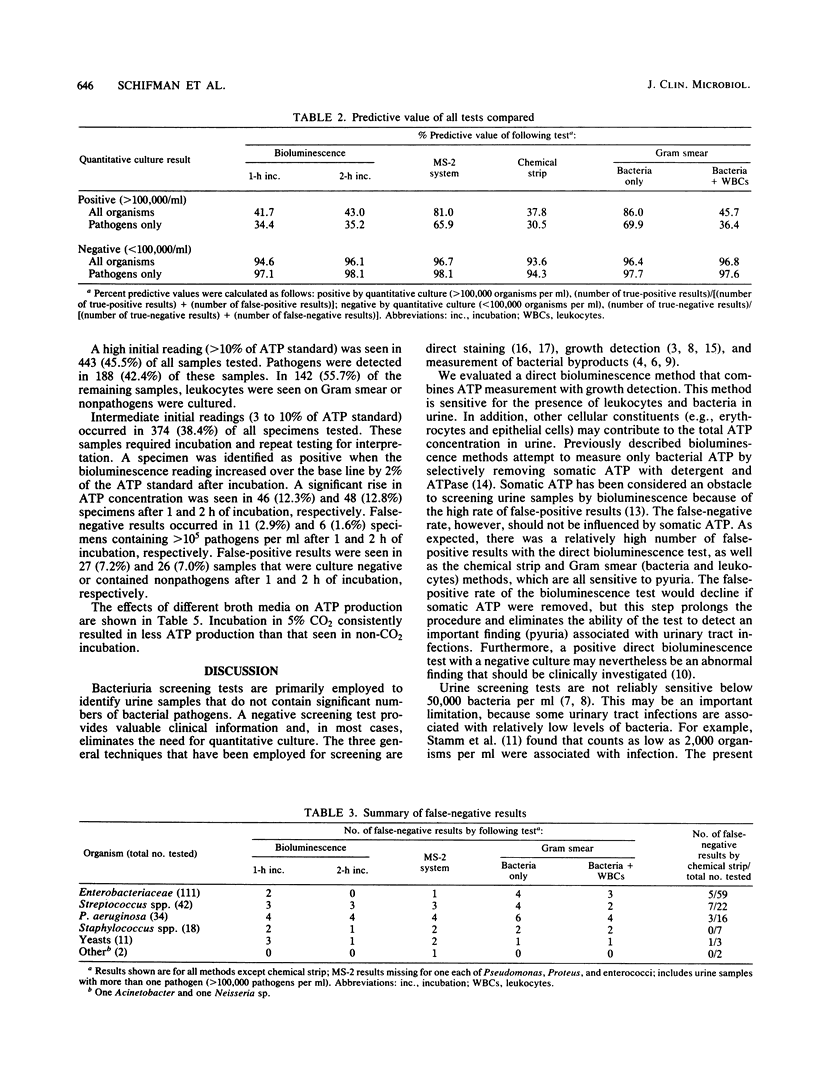
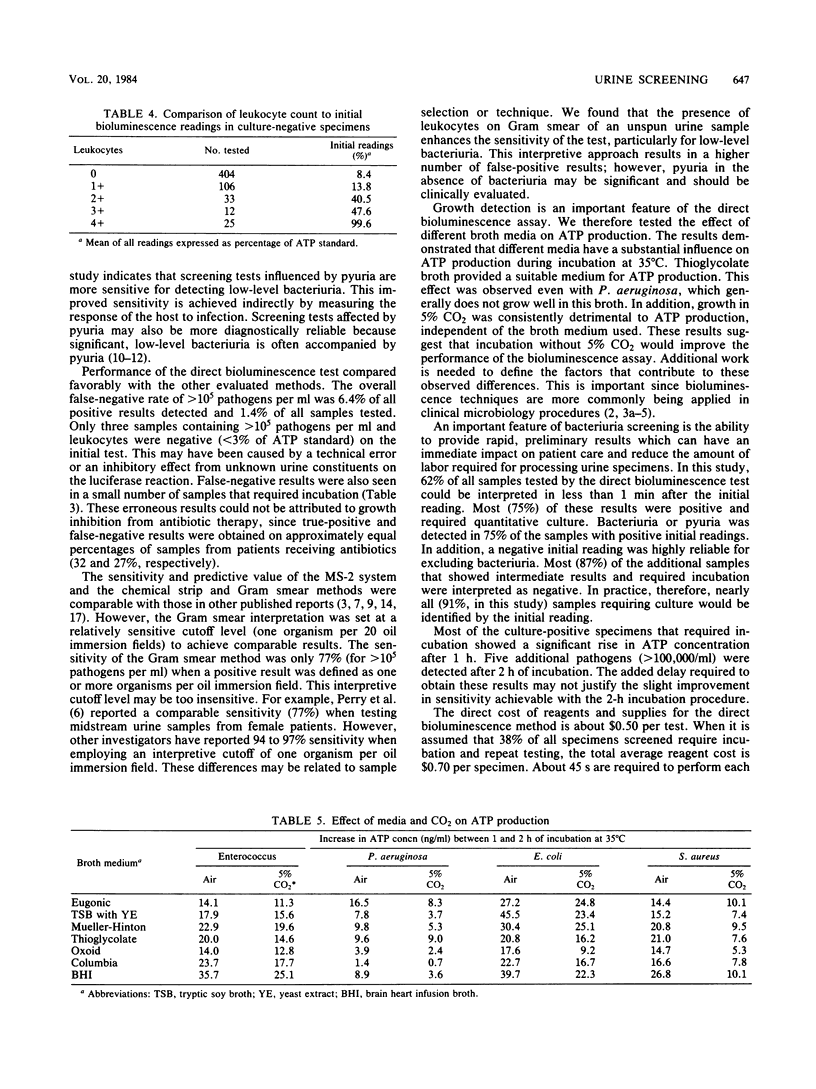
A direct bioluminescence assay for bacteriuria screening is described and compared with the MS-2 system (Abbott Laboratories, Irvine, Tex.) and the chemical strip, Gram smear, and calibrated-loop methods. A total of 973 specimens were tested. Unlike previously described bioluminescence methods, this test measures total ATP in urine without pretreatment of samples to remove somatic ATP. The result was compared with an ATP standard (20 ng/ml). A low result (less than 3% of standard) was interpreted as negative and a high result (greater than 10% of standard) as positive. Samples with intermediate results (38% of total) were incubated at 35 degrees C in thioglycolate broth (1:10). A 2% increase in ATP concentration was interpreted as positive. The sensitivity of this method for detecting greater than 10(5) pathogens per ml was 92.3% and was comparable to those of the MS-2 system (92.7%) and the Gram smear method (90.5%). The chemical strip method was less sensitive (84.0%). The direct bioluminescence method was more sensitive than were the MS-2 system and the Gram smear method for detecting low-level bacteriuria (less than 10(3) to 10(5) organisms per ml), primarily because of associated pyuria. Thioglycolate broth provided a suitable medium for ATP production, and 5% CO2 decreased bacterial ATP synthesis during log-phase growth. The direct bioluminescence assay is rapid, simple, cost-effective, and reliable for bacteriuria screening.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Höjer H., Nilsson L. Rapid determination of doxycycline based on luciferase assay of bacterial adenosine triphosphate. J Antimicrob Chemother. 1978 Nov;4(6):503–508. doi: 10.1093/jac/4.6.503. [DOI] [PubMed] [Google Scholar]
- McCarthy L. R., Gavan T. L., Robson J., Corlett C. Evaluation of the MS-2 urine screening method for detection of bacteriuria. J Clin Microbiol. 1982 Aug;16(2):250–252. doi: 10.1128/jcm.16.2.250-252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWalter P. W. Determination of susceptibility of Staphylococcus aureus to methicillin by luciferin-luciferase assay of bacterial adenosine triphosphate. J Appl Bacteriol. 1984 Feb;56(1):145–150. doi: 10.1111/j.1365-2672.1984.tb04706.x. [DOI] [PubMed] [Google Scholar]
- Molin O., Nilsson L., Anséhn S. Rapid detection of bacterial growth in blood cultures by bioluminescent assay of bacterial ATP. J Clin Microbiol. 1983 Sep;18(3):521–525. doi: 10.1128/jcm.18.3.521-525.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Curtis G. D., Johnston H. H. Analysis of the disagreement between automated bioluminescence-based and culture methods for detecting significant bacteriuria, with proposals for standardizing evaluations of bacteriuria detection methods. J Clin Microbiol. 1982 May;15(5):802–809. doi: 10.1128/jcm.15.5.802-809.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L., Matthews J. S., Weesner D. E. Evaluation of leukocyte esterase activity as a rapid screening technique for bacteriuria. J Clin Microbiol. 1982 May;15(5):852–854. doi: 10.1128/jcm.15.5.852-854.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzlo M. T. Automated methods for detection of bacteriuria. Am J Med. 1983 Jul 28;75(1B):71–78. doi: 10.1016/0002-9343(83)90075-x. [DOI] [PubMed] [Google Scholar]
- Pezzlo M. T., Tan G. L., Peterson E. M., de la Maza L. M. Screening of urine cultures by three automated systems. J Clin Microbiol. 1982 Mar;15(3):468–474. doi: 10.1128/jcm.15.3.468-474.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley D. L., Dittmann A. N. Use of leukocyte esterase-nitrate activity as predictive assays of significant bacteriuria. J Clin Microbiol. 1983 Nov;18(5):1256–1257. doi: 10.1128/jcm.18.5.1256-1257.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm W. E., Counts G. W., Running K. R., Fihn S., Turck M., Holmes K. K. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982 Aug 19;307(8):463–468. doi: 10.1056/NEJM198208193070802. [DOI] [PubMed] [Google Scholar]
- Stamm W. E. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983 Jul 28;75(1B):53–58. doi: 10.1016/0002-9343(83)90073-6. [DOI] [PubMed] [Google Scholar]
- Stamm W. E., Wagner K. F., Amsel R., Alexander E. R., Turck M., Counts G. W., Holmes K. K. Causes of the acute urethral syndrome in women. N Engl J Med. 1980 Aug 21;303(8):409–415. doi: 10.1056/NEJM198008213030801. [DOI] [PubMed] [Google Scholar]
- Thore A., Lundin A., Anséhn S. Firefly luciferase ATP assay as a screening method for bacteriuria. J Clin Microbiol. 1983 Feb;17(2):218–224. doi: 10.1128/jcm.17.2.218-224.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadke M., McDonnell C., Ashton J. K. Rapid processing of urine specimens by urine screening and the AutoMicrobic system. J Clin Microbiol. 1982 Oct;16(4):668–672. doi: 10.1128/jcm.16.4.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Longoria C. J. Colorimetric method for rapid determination of bacteriuria. J Clin Microbiol. 1981 Sep;14(3):342–346. doi: 10.1128/jcm.14.3.342-346.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



