Abstract
Sympathetic activation in chronic heart failure (CHF) is greatly augmented at rest but the response to exercise remains controversial. We previously demonstrated that single-unit muscle sympathetic nerve activity (MSNA) provides a more detailed description of the sympathetic response to physiological stress than multi-unit nerve recordings. The purpose of this study was to determine whether the reflex response and discharge properties of single-unit MSNA are altered during handgrip exercise (HG, 30% of maximum voluntary contraction for 3 min) in CHF patients (New York Heart Association functional class II or III, n= 16) compared with age-matched healthy control subjects (n= 13). At rest, both single-unit and multi-unit indices of sympathetic outflow were augmented in CHF compared with controls (P < 0.05). However, the percentage of cardiac intervals that contained one, two, three or four single-unit spikes were not different between the groups. Compared to the control group, HG elicited a larger increase in multi-unit total MSNA (Δ1002 ± 50 compared with Δ636 ± 76 units min−1, P < 0.05) and single-unit MSNA spike incidence (Δ27 ± 5 compared with Δ8 ± 2 spikes (100 heart beats)−1), P < 0.01) in the CHF patients. More importantly, the percentage of cardiac intervals that contained two or three single-unit spikes was increased (P < 0.05) during exercise in the CHF group only (Δ8 ± 2% and Δ5 ± 1% for two and three spikes, respectively). These results suggest that the larger multi-unit total MSNA response observed during HG in CHF is brought about in part by an increase in the probability of multiple firing of single-unit sympathetic neurones.
In chronic heart failure (CHF), excessive sympathetic activation under resting conditions has been shown to increase from the early stages of the disease and is related to prognosis (Cohn et al. 1984; Francis et al. 1990; Grassi et al. 1995; Barretto et al. 2008). As such, improvement of sympathetic overactivity has been recognized as an important therapeutic target in CHF. Exercise-induced sympathetic activity is thought to play a key role in the redistribution of cardiac output towards active skeletal muscle (Mitchell 1990). However, intense sympathoexcitation during exercise contributes to a reduced exercise capacity in CHF (Piepoli et al. 1996; Notarius et al. 1999, 2001; Crisafulli et al. 2007) via an increase in peripheral vascular resistance. Further investigation into the central sympathetic mechanisms associated with these clinical observations in CHF is warranted.
Direct recordings of multi-unit efferent muscle sympathetic nerve activity (MSNA) by microneurography has been used to evaluate the response of the sympathetic nervous system during exercise in CHF. This technique provides an estimation of overall sympathetic activity relating to the number of active firing units and/or the recruitment of new fibres from central or reflex effects. Increased multi-unit MSNA during exercise has been previously observed in CHF patients (Sterns et al. 1991; Notarius et al. 2001; Negrao et al. 2001). However, even including several measurements (Chidsey et al. 1962; Middlekauff et al. 2000; Piepoli et al. 1996; Rundqvist et al. 1997), the response to exercise in CHF remains a controversial issue.
Microneurographic recordings of single-unit muscle sympathetic nerve activity have been obtained from conscious humans, mainly under resting conditions (Macefield et al. 1994; Greenwood et al. 1999; Elam et al. 2002; Lambert et al. 2007). In CHF, despite an elevated level of multi-unit MSNA at rest, the firing pattern of single-unit sympathetic spikes was not different compared to healthy subjects (Macefield et al. 1999). Specifically, the percentage of cardiac intervals that contained one, two, three or four single-unit spikes was similar between the two groups. However, the incidence of single-unit sympathetic spikes that fired following a premature beat in CHF patients has been reported to shift towards a greater percentage of cardiac intervals that contained two spikes versus only one (Elam & Macefield, 2001). We have demonstrated that single-unit MSNA can also be recorded during periods of physiological stress (i.e. handgrip exercise and Valsalva's manoeuvre) and that reflex sympathoexcitation could be attributed to changes in the frequency of single-unit spike firing within each multi-unit sympathetic burst (Murai et al. 2006). In spite of these single-unit sympathetic investigations little is known about how the firing characteristics of single-unit vasoconstrictor neurones are altered during exercise in CHF.
Therefore, recording the firing properties of single muscle vasoconstrictor neurones might provide additional mechanisms for sympathetic augmentation in CHF. The purpose of the present investigation was to examine whether the response of single-unit MSNA during handgrip exercise (HG) was augmented in CHF patients, compared with age-matched healthy humans. We also determined whether the pattern of sympathoexcitation of single-unit MSNA was altered in CHF patients with high resting levels of sympathetic activity.
Methods
Subjects
CHF patients
Data were obtained from 16 CHF patients (11 males and 5 females) aged 62 ± 3 years with left ventricular systolic dysfunction (ejection fraction by 2D echocardiography of 38 ± 2%). Patients were classified as being in the New York Heart Association functional class II (12 patients) or III (4 patients). Nine patients had ischaemic heart disease, five patients had dilated cardiomyopathy and two patients had undergone valvular replacement surgery. One patient had aortic regurgitation and another mitral regurgitation. The drug regimen for this cohort included: angiotensin-converting enzyme inhibitors (n= 7), angiotensin receptor blockers (n= 9), diuretics (n= 16), statins (n= 6) and β-adrenergic receptor blockers (n= 12). No patients underwent regimented exercise training. To minimize the confounding effects of a distended bladder on sympathetic nerve recordings (Fagius & Karhuvaara, 1989), diuretics were withheld on the morning of the study but other medications were continued as prescribed. Patients were free of clinical symptoms for at least 6 months.
Healthy subjects
We recruited and screened 13 healthy volunteers (9 male and 4 female, aged 61 ± 3 years). Subjects were screened by physical examination, baseline electrocardiogram evaluation, echocardiography and a complete blood work examination to exclude diseases that may affect cardiovascular status, such as cardiac dysfunction, metabolic syndrome, diabetes mellitus, chronic kidney disease and hypertension. All subjects were non-smokers with average physical fitness and none took any medications known to influence cardiovascular function.
All subjects gave written informed consent to participate in the study. The study protocol was approved by the ethics review panel of the Graduate School of Medical Science at Kanazawa University.
Measurements
All experiments were conducted in a quiet, electrically shielded room with the subjects in the supine position. Heart rate was determined from the time between successive R-wave intervals recorded from an electrocardiogram sampled at 1000 Hz. Arterial pressure was recorded continuously from the radial artery and digitized at 800 Hz using a non-invasive tonometry monitoring system (JENTOW-7700; Nihon Colin, Komaki, Japan). Postganglionic muscle sympathetic nerve activity was recorded from the left peroneal nerve, as described previously (Nakata et al. 1998). Briefly, the common peroneal nerve was located posterior to the head of the fibular bone by palpation and surface electrical stimulation. A high-impedance tungsten microelectrode (type 25-5-1; Frederick Haer, Brunswick, ME, USA) was inserted percutaneously into a motor fascicle and then adjusted until spontaneous pulse-synchronous multi-fibre bursts of sympathetic activity were observed. To determine the shape of a single-unit sympathetic action potential further adjustment of the microelectrode was required until a large unitary spike discharge could be easily separated from the background noise and the multi-unit bursts in the raw nerve recording. In this study, the single-unit and multi-unit MSNA were simultaneously recorded from the same microelectrodes.
Data collection
Sympathetic neural activity was amplified (×70 000), band-pass filtered (0.5–3.0 kHz), sampled at 12 kHz and stored on a digital audio tape recorder (DT120RT; Sony, Tokyo). To produce a mean voltage neurogram for the analysis of multi-unit MSNA, the amplified and filtered nerve activity was full-wave rectified and passed through a resistance-capacitance integrating circuit with a time constant of 0.1 s and connected to an audio speaker. Multi-unit integrated nerve activity was digitized at a sampling rate of 1000 Hz. Both the raw nerve signals and the mean voltage neurogram were visually displayed on an oscilloscope (Neuropack 2; Nihon Kohden, Tokyo).
Data analysis
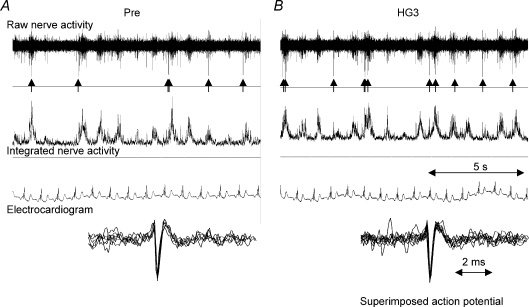
During off-line analysis, the morphology of single-unit MSNA spikes were carefully inspected using Sony PC Scan II software. All candidate action potentials were then superimposed in an attempt to determine that the single-unit spikes of MSNA were indeed from a single fibre as previously described (Murai et al. 2006). We used three criteria to identify single-unit spikes of MSNA: (1) synchronization with multi-unit MSNA bursts, (2) triphasic spike morphology with the main phase being negative, and (3) superimposabilty. When the single-unit MSNA was superimposed, minimal variation was found in single-unit spike amplitude or shape (Fig. 1).
Figure 1. Typical recording of single-unit and multi-unit MSNA in a CHF patient during baseline (A) and the third minute of handgrip exercise (HG3) (B).
Single-unit MSNA was identified on the raw nerve recording and highlighted by the arrows (↑). Superimposed spikes (bottom) demonstrated a consistent spike morphology suggesting that the action potentials originated from the same single sympathetic axon. The shape of the single-unit MSNA remained unchanged during HG.
From the mean voltage neurogram multi-unit MSNA burst frequency (bursts per minute) and burst incidence (bursts per 100 heart beats) were determined. Normalized mean burst amplitude was calculated by assigning the largest absolute burst amplitude recorded at rest an arbitrary value of ‘100’ and expressing all other burst amplitudes as a percentage of this maximum burst height. Total MSNA activity was then calculated as the product of mean burst frequency by the mean normalized burst amplitude. Single-unit MSNA was expressed as both the total number of spikes per minute (spike frequency) and per 100 heart beats (spike incidence). Firing probability was defined as the percentage of heart beats during which one or more spikes occurred and the probability of multiple spikes was defined as the percentage of heart beats that contained two or more spikes among heart beats with any spike. Additionally, the percentage of cardiac intervals in which one, two, three or four single-unit spikes were separately calculated for the number of cardiac intervals in which one, two, three or four single-unit spikes fired as a percentage of all heart beats with at least one spike (Macefield et al. 1999).
Study protocol
Each subject performed a brief (5 s) maximum voluntary contraction (MVC) using their dominant hand prior to the baseline recording period. Following 15 min of quiet rest, baseline values for heart rate, arterial pressure, multi-unit and single-unit MSNA were recorded for 5 min. After baseline measurements were collected, subjects performed a sustained isometric handgrip at 30% of their MVC for 3 min. All parameters were continuously measured during the handgrip exercise period and later divided into 1 min epochs (HG1, HG2 and HG3).
Statistical analysis
Results are expressed as the mean ±s.e.m. Group physical characteristics, resting discharge of MSNA and handgrip-mediated responses were compared between CHF patients and healthy subjects with a Student's unpaired t test. A two-way analysis of variance (ANOVA) with repeated measures was performed to compare between- and within-group differences during handgrip exercise. A Fisher's post hoc test was applied to assess individual point differences. All calculations were performed on a personal computer using the statistical package Stat View (1995; Abacas Concepts, Inc., Berkeley, CA, USA). A value of P < 0.05 was considered statistically significant.
Results
Baseline comparisons
Baseline characteristics of healthy subjects and CHF patients are shown in Table 1. There were no significant differences between healthy subjects and CHF patients with respect to heart rate, arterial pressure, body height and weight. CHF patients exhibited a significantly lower left ventricular ejection fraction (LVEF) with an augmented resting level of multi-unit MSNA burst frequency and burst incidence compared with the healthy subjects. Furthermore, single-unit MSNA spike frequency, spike incidence and firing probability were all higher in CHF patients. However, the firing probability of multiple spikes per cardiac interval was not different between the two groups.
Table 1.
Baseline characteristics in controls and CHF patients
| Controls | CHF patients | |
|---|---|---|
| Number (women) | 13 (4) | 16 (5) |
| Age (years) | 61 ± 3 | 62 ± 3 |
| Height (cm) | 164 ± 2 | 161 ± 2 |
| Body weight (kg) | 62 ± 3 | 61 ± 2 |
| Heart rate (beats min−1) | 64 ± 2 | 62 ± 2 |
| SAP (mmHg) | 116 ± 2 | 111 ± 3 |
| DAP (mmHg) | 62 ± 2 | 59 ± 2 |
| MAP (mmHg) | 80 ± 2 | 76 ± 2 |
| LVEF (%) | 65 ± 2 | 38 ± 2* |
| Multi-unit MSNA | ||
| Burst frequency (bursts min−1) | 26 ± 2 | 44 ± 3* |
| Burst incident (bursts (100 heart beats)−1) | 40 ± 4 | 70 ± 4* |
| Single-unit MSNA | ||
| Spike frequency (spikes min−1) | 22 ± 2 | 42 ± 4* |
| Spike incidence (spikes (100 heart beats)−1) | 35 ± 2 | 67 ± 5* |
| Firing probability (%) | 26 ± 2 | 50 ± 4* |
| Probability of multiple spikes (%) | 25 ± 2 | 26 ± 2 |
Results are expressed as means ±s.e.m.
P < 0.05 compared to healthy controls.
SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; LVEF, left ventricular ejection fraction; MSNA, muscle sympathetic nerve activity.
Typical recordings of single-unit and multi-unit MSNA at rest (A) and during the third minute of handgrip exercise (B) in a CHF patient are shown in Fig. 1. The characteristic triphasic action potential shape of an unmyelinated axon that included a large single main negative phase was observed in the raw nerve recording that occurred in synchrony with the multi-unit MSNA discharges. Superimposed single-unit spike action potentials displayed on an expanded time scale highlight the constancy of the spike shapes between HG and baseline conditions (Fig. 1, bottom). In this study, the maximum number of single-unit MSNA spikes identified within any one cardiac interval was four in both groups.
Haemodynamic and multi-unit MSNA responses to HG exercise
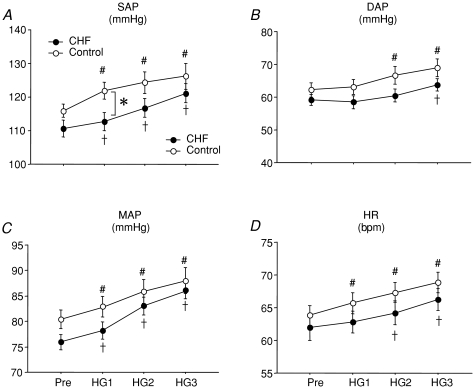
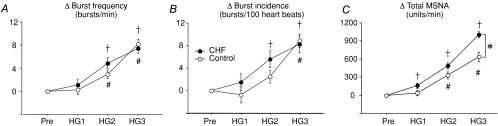
Figure 2 shows the effect of HG on systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP) and heart rate (HR). HG significantly increased SAP, MAP and HR in both groups over 3 min. No significant between-group differences were found in the time course of DAP, MAP and HR responses to HG. However, the initial minute of handgrip exercise resulted in a significantly greater increase in SAP in the healthy control group. Multi-unit MSNA responses to HG in CHF patients and healthy subjects are presented in Table 2. Burst frequency and burst incidence in CHF patients were higher at baseline and remained significantly greater during handgrip exercise compared to healthy subjects. There were no HG exercise-mediated changes of multi-unit MSNA in either the burst frequency or burst incidence (Fig. 3A and B) between the two groups. However, the change in normalized total MSNA was significantly greater in CHF patients compared with the healthy subjects during the third minute (HG3) of exercise (Δ1002 ± 50 compared with Δ636 ± 76 units min−1, respectively, P < 0.05, Fig. 3C).
Figure 2. Systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP) and heart rate (HR) during HG in control subjects (○) and CHF patients (•).
Pre, Pre-HG; HG1, HG2 and HG3, first, second and third minutes of HG exercise, respectively. Data are expressed as means ±s.e.m. †P < 0.05 compared to CHF at baseline. #P < 0.05 compared to control subjects at baseline. *P < 0.05 compared to control subjects at the same time point.
Table 2.
Effect of handgrip exercise on multi-unit MSNA
| Controls |
CHF patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Pre | HG1 | HG2 | HG3 | Pre | HG1 | HG2 | HG3 |
| Burst frequency (bursts min−1) | 26 ± 2 | 26 ± 2 | 28 ± 2# | 34 ± 3# | 44 ± 3* | 45 ± 3* | 48 ± 3*# | 52 ± 3*# |
| Burst incident (bursts (100 heart beats)−1) | 40 ± 4 | 40 ± 3 | 43 ± 3 | 49 ± 4# | 70 ± 4* | 71 ± 4* | 75 ± 4*# | 78 ± 4*# |
| Total MSNA (units min−1) | 1036 ± 109 | 1070 ± 97 | 1367 ± 98# | 1673 ± 156# | 2337 ± 166 | 2497 ± 141# | 2824 ± 153# | 3339 ± 171# |
The values are expressed as the mean ±s.e.m.
P < 0.05 compared to corresponding baseline (Pre).
P < 0.01 compared to healthy controls at the same time point.
Pre, Pre-HG; HG1, HG2 and HG3, first, second and third minutes of HG exercise, respectively.
Figure 3. Handgrip exercise-mediated changes of multi-unit MSNA parameters in control subjects (○) and CHF patients (•).
Changes in MSNA burst frequency (A) and burst incidence (B) were similar between the two groups. However, changes in normalized total MSNA activity (C) were significantly greater at HG3 in CHF patients versus the healthy subjects. The values are expressed as means ±s.e.m. †P < 0.05 compared to CHF at baseline. #P < 0.05 compared to control subjects at baseline. *P < 0.05 compared to control subjects at the same time point.
Single-unit MSNA responses to HG exercise
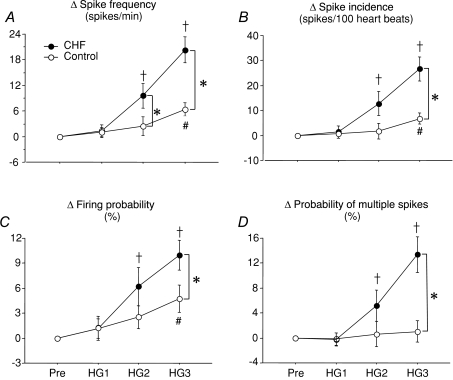
The effect of HG on single-unit MSNA is represented in Table 3. Spike frequency, spike incidence and firing probability at baseline in CHF patients were higher and remained significantly greater during handgrip exercise compared to healthy controls. On the other hand, probability of multiple spikes in CHF patients was similar at baseline, but significantly increased at HG3 compared to healthy controls at the same time point. The exercise-induced changes in single-unit MSNA spike frequency and incidence, firing probability and probability of multiple spike firing are shown in Fig. 4. During the third minute of static handgrip exercise at 30% MVC, the CHF patients demonstrated an augmented rise in spike frequency (Δ20 ± 3 spikes min−1 compared with Δ6 ± 2 spikes min−1, P < 0.01, Fig. 4A), spike incidence (Δ27 ± 5 spikes (100 heart beats)−1 compared with Δ8 ± 2 spikes (100 heart beats)−1, P < 0.01, Fig. 4B), overall firing probability (Δ10.0 ± 2% compared with Δ5 ± 2%, P < 0.05, Fig. 4C), and probability of multiple spikes (Δ13 ± 3% compared with Δ1 ± 2%, P < 0.05, Fig. 4D) compared to the healthy subjects.
Table 3.
Effect of handgrip exercise on single-unit MSNA
| Controls |
CHF patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Pre | HG1 | HG2 | HG3 | Pre | HG1 | HG2 | HG3 |
| Spike frequency (spikes min−1) | 22 ± 2 | 23 ± 2 | 25 ± 2 | 29 ± 3# | 42 ± 4* | 43 ± 4* | 52 ± 6*# | 62 ± 6*# |
| Spike incidence (spikes (100 heart beats)−1) | 35 ± 2 | 35 ± 2 | 37 ± 3 | 42 ± 4# | 67 ± 5* | 68 ± 6* | 80 ± 8*# | 94 ± 9*# |
| Firing probability (%) | 26 ± 2 | 27 ± 2 | 29 ± 2 | 31 ± 3# | 50 ± 4* | 51 ± 4* | 56 ± 1*# | 60 ± 5*# |
| Probability of multiple spikes (%) | 25 ± 2 | 25 ± 2 | 26 ± 2 | 26 ± 1 | 26 ± 2 | 26 ± 2 | 31 ± 2# | 40 ± 3*# |
The values are expressed as the mean ±s.e.m.
P < 0.05 compared to corresponding baseline (Pre).
P < 0.01 compared to healthy controls at the same time point.
Pre, Pre-HG; HG1, HG2 and HG3, first, second and third minutes of HG exercise, respectively.
Figure 4. Handgrip exercise-mediated changes of single-unit MSNA parameters in control subjects (○) and CHF patients (•).
The changes of single-unit MSNA spike frequency (A), spike incidence (B), firing probability (C) and the probability of multiple firing (D) in CHF patients were significantly increased at HG3, compared to healthy subjects. The values are expressed as the mean ±s.e.m. †P < 0.05 compared to CHF at baseline. #P < 0.05 compared to control subjects at baseline. *P < 0.05 compared to control subjects at the same time point.
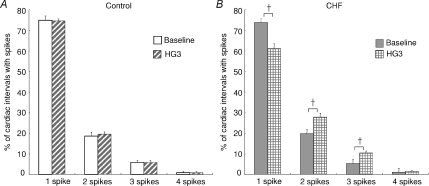
The percentage of cardiac intervals in which one, two, three or four single-unit spikes fired at baseline and during handgrip exercise (HG3) in the healthy controls and CHF patients are shown in Fig. 5. At rest, there were no differences in the probability of firing of one, two, three or four spikes per cardiac interval between the two groups. Furthermore, there were no changes in this distribution during the final minute of handgrip exercise in the healthy subjects (Fig. 5A). Conversely, there was a decrease in the percentage of cardiac intervals that contained 1 spike (from 74 ± 2 to 61 ± 2%, P < 0.05) and an increase in the proportion of cardiac intervals that had two and three spikes (from 20 ± 2 to 28 ± 2% and from 5 ± 1 to 10 ± 1%, respectively, P < 0.05) between baseline and handgrip exercise (HG3) in CHF patients (Fig. 5B).
Figure 5. Percentage of cardiac intervals in which 1, 2, 3 or 4 single-unit spikes fired during baseline and HG3 in control subjects (A) and CHF patients (B).
The percentage of multiple spike firing did not differ between baseline and HG exercise in healthy subjects. However, during HG in CHF there was a decrease in the firing probability of 1 spike per cardiac interval but an increase in the percentage of cardiac intervals that contained 2 or 3 spikes. The values are expressed as the mean ±s.e.m. †P < 0.05 compared with baseline.
Discussion
This is the first report to examine the effects of static exercise on the firing pattern of single-unit MSNA in CHF patients compared with age-matched healthy humans. The main findings of this investigation were: (1) single-unit MSNA recordings are feasible to evaluate sympathetic neural activity at rest and during static exercise in CHF patients, (2) total MSNA activity and single-unit MSNA spike frequency responses to exercise were significantly augmented in CHF, whereas multi-unit burst frequency and burst incidence responses were similar in both groups, and (3) the firing pattern of single-unit MSNA spikes was altered such that there was a shift from single to multiple firings per cardiac cycle during exercise in CHF but not in healthy subjects. The analysis of single-unit MSNA provides further understanding of sympathetic augmentation during static exercise in CHF. Our results suggest that the larger multi-unit total MSNA response of CHF patients during exercise is brought about in part by an increase in the probability of a single-unit spike firing more than once per cardiac cycle.
The mean firing frequency of individual sympathetic vasoconstrictor neurones in healthy people has previously been shown to range from 0.24 to 0.47 Hz (Macefield et al. 1994; Greenwood et al. 1999). In this study, single-unit MSNA mean frequency was 0.37 ± 0.04 Hz in the healthy control group, which is similar to these previous findings. Macefield et al. (1999) reported a single-unit MSNA mean firing frequency of 0.98 ± 0.22 Hz in CHF, which is higher than our CHF group (0.70 ± 0.60 Hz). An explanation for the higher mean firing frequency in this previous study may be attributed to differences in the cardiovascular status between the two CHF groups. Specifically, compared to Macefield et al. (1999), the disease severity of our CHF patients was less as characterized by a higher left ventricular ejection fraction (38 ± 2 versus 29 ± 5%) and lower magnitude of resting MSNA burst incidence (70 ± 4 versus 88 ± 5%). Furthermore, they also demonstrated that the probability of multiple single-unit spike firing per cardiac interval was less than 30% (2 spikes, 18.2 ± 2.4%; 3 spikes, 7.3 ± 2.6%; 4 spikes, 3.0 ± 1.6%), which was similar to our study (2 spikes, 19.8 ± 1.7%; 3 spikes, 5.3 ± 0.9%; 4 spikes, 1.2 ± 0.4%). These results suggest that the probability of multiple spikes might not be affected by the severity of heart failure in the stable state.
Baseline levels of multi-unit MSNA in CHF patients may be considered to be at near-maximal and therefore cannot be increased further. Due to its pulse-synchronous nature, multi-unit MSNA burst frequency cannot increase more than the heart rate (i.e. 100% burst incidence). Multi-unit burst amplitude and area can also be measured to evaluate relative changes in the strength of MSNA within a recording session (Sverrisdóttir et al. 2000). Lambert et al. (2007) demonstrated that different mechanisms are responsible for the increased levels of total MSNA activity in hypertensive individuals and suggested that total multi-unit MSNA measures could be used as an indicator to represent a greater recruitment of vasoconstrictor fibres. Total multi-unit MSNA activity cannot distinguish between changes in sympathetic nerve firing due to the recruitment of additional single-unit vasoconstrictor neurones and that due to an increase in the firing rate of already active single-unit fibres. In this study, the larger baseline total MSNA in CHF may indicate that multi-unit MSNA in CHF is composed of a higher number of total active fibres.
In this study, we also measured the total MSNA activity before and during HG. The underlying mechanism responsible for increasing total MSNA activity may be differentiated between healthy subjects and CHF patients by the simultaneous measurements of single-unit and multi-unit MSNA. Accordingly, in CHF patients, central sympathetic augmentation may depend on increasing the firing frequency of individual vasoconstrictor fibres as we observed a shift towards multiple spike firing of an active neurone within a cardiac interval and/or the recruitment of additional sympathetic fibres. Conversely, in healthy subjects the increase in total multi-unit MSNA could be attributed solely to the recruitment of previously silent sympathetic neurones without changing the multiple firing rate of currently active neurones.
Elam & Macefield (2001) analysed a total of 60 premature ventricular beats in patients with congestive heart failure and reported that the incidence of multiple within-burst firing increased markedly, with two spikes being more common than one. The analysis of single-unit MSNA allows a more detailed examination of sympathetic nerve activity by specifically recording spike firing within each cardiac interval. Consistent with this idea, we previously demonstrated the importance of single-unit MSNA compared with multi-unit MSNA during reflex-mediated sympathetic activation (Murai et al. 2006). Furthermore, Greenwood et al. (1998) demonstrated a significant increase of single-unit MSNA (86 compared with 47 impulses min−1) during HG in pregnant women. However, until now, there have been no studies that have characterized the effect of exercise on the firing pattern of single-unit MSNA in CHF patients.
In the present study, the firing pattern of single-unit MSNA was significantly altered during HG. Although the significance of increasing the firing rate of a single active neurone remains uncertain, these reactions would be consistent with the generation of an intense sympathoexcitatory state. Previous reports demonstrated that intense sympathoexcitation during exercise in CHF was correlated to a reduction of exercise capacity (Notarius et al. 2001). We suggest that the single-unit sympathetic firing pattern observed in the present investigation may also contribute to this exercise intolerance. Muscle metaboreceptors, mechanoreceptors and/or central command play an important role in sympathoexcitation during static exercise (Mitchell, 1990; Rowell & O’Leary, 1990). Piepoli et al. (1999) first described the concept of a muscle hypothesis in which CHF lowers the threshold for muscle ischaemia and muscle reflex sympathetic stimulation during exercise. The mechanisms underlying the sympathoexcitatory activity in CHF patients remain unknown. However, we found that the single-unit MSNA response to HG in CHF patients was augmented by an increase in the firing frequency of single-unit neurones per cardiac interval. Single-unit MSNA elucidates two possible scenarios to explain an increase in sympathetic outflow: (1) increasing the overall mean spike firing frequency without an increase the rate of multiple firing during per cardiac interval, and (2) increasing the firing frequency by shifting towards multiple spike firing within one cardiac interval. Previous reports in an animal model demonstrated that short-term irregular electrical stimulation to mesenteric arteries induced vasoconstriction more than regular stimulation (Nilsson et al. 1985). Mental stress and cycling exercise can elicit further increases in cardiac noradrenaline (norepinephrine) spillover in heart failure (Kaye et al. 1995), indicating that considerable adrenergic reserve remains. Augmentation of multiple firing within one cardiac interval might play a role in generating more intense sympathoexcitation through the release of vasoconstrictor neurotransmitters in CHF patients with high levels of resting sympathetic activity.
Exaggerated sympathetic outflow contributes to a worsening prognosis in CHF, such as beta receptor downregulation (Böhm et al. 1997), cardiac myocyte apoptosis (Communal et al. 1998) and calcium overload (Chaudhri et al. 2002). Several therapeutic approaches have demonstrated that the reduction of sympathetic activity improves mortality in CHF (Packer et al. 1996; Pfeffer et al. 2003). However, exercise capacity was restored with exercise training (Roveda et al. 2003) but not beta-blocker therapy (Gullestad et al. 2001), although these treatments have been reported to attenuate sympathetic activity at rest. The difference in exercise capacity between two recommended therapies might be attributed to the single-unit sympathetic activation response to exercise through an increase in afterload and a decrease in blood flow to exercising muscle. Although further study is required, exercise-induced multiple single-unit firing per cardiac interval in CHF could become the next therapeutic target for exercise intolerance and disease progression.
Several limitations may be associated with the present study. First, we measured multi-unit and single-unit MSNA but did not evaluate cardiac sympathetic activity. However, there is evidence to support a close relationship between resting MSNA in the peroneal nerve and cardiac noradrenaline spillover in healthy normotensive individuals (Wallin et al. 1992). In the present study, we found that handgrip exercise augmented heart rate in the presence of beta blocker therapy. Therefore, sympathetic drive directed to the heart was significantly enhanced during handgrip exercise as well as to muscle. Second, not all medications were discontinued in the CHF patients. Angiotensin-converting enzyme inhibitors attenuate sympathetic activity under resting conditions (Grassi et al. 1997). Chronic treatment with beta-blockers have been shown to decrease (De Matos et al. 2004) or to have no effect on MSNA (Azevedo et al. 2001), but their effect on single-unit MSNA during exercise remains unclear. By maintaining the medication schedule, we avoided possible rebound cardiovascular responses and the adverse effects of drug withdrawal. These drugs might affect our results in terms of the response of arterial pressure and heart rate during exercise. However, we have demonstrated that sympathetic hyperactivity exists during exercise, even when optimal management for CHF is maintained.
In conclusion, single-unit MSNA was markedly augmented during exercise and single-unit sympathetic firing pattern was shifted towards a greater probability of multiple spike firing per cardiac interval in CHF patients. We conclude that these responses contributed to the exaggerated sympathoexcitation measured during exercise in CHF patients, which may contribute to exercise intolerance and disease progression.
Acknowledgments
We thank Professor T. Mano (Gifu University of Medical Science) for his invaluable help with the development of the microneurographic technique in our department.
References
- Azevedo ER, Kubo T, Mak S, Floras JS. Nonselective versus selective β-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation. 2001;104:2194–2199. doi: 10.1161/hc4301.098282. [DOI] [PubMed] [Google Scholar]
- Barretto AC, Santos AC, Munhoz R, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.03.056. in press. [DOI] [PubMed] [Google Scholar]
- Böhm M, Flesch M, Schnabel P. Beta-adrenergic signal transduction in the failing and hypertrophied myocardium. J Mol Med. 1997;75:842–848. doi: 10.1007/s001090050175. [DOI] [PubMed] [Google Scholar]
- Chaudhri B, Del Monte F, Hajjar RJ, Harding SE. Interaction between increased SERCA2a activity and beta-adrenoceptor stimulation in adult rabbit myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H2450–H2457. doi: 10.1152/ajpheart.00391.2002. [DOI] [PubMed] [Google Scholar]
- Chidsey CA, Harrison DC, Braunwald E. Augmentation of the plasma nor-epinephrine response to exercise in patients with congestive heart failure. N Engl J Med. 1962;267:650–654. doi: 10.1056/NEJM196209272671305. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;27:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the ß-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Salis E, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol. 2007;292:H2988–H2996. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- De Matos LD, Gardenghi H, Negrao CE. Impact of 6 months of therapy with carvedilol on muscle sympathetic nerve activity in heart failure patients. J Card Fail. 2004;10:496–502. doi: 10.1016/j.cardfail.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Elam M, Macefield VG. Multiple firing of single muscle vasoconstrictor neurones during cardiac dysrhythmias in human heart failure. J Appl Physiol. 2001;91:717–724. doi: 10.1152/jappl.2001.91.2.717. [DOI] [PubMed] [Google Scholar]
- Elam M, McKenzie D, Macefield VG. Mechanisms of sympathoexcitation: single-unit analysis of muscle vasoconstrictor neurones in awake OSAS subjects. J Appl Physiol. 2002;93:297–303. doi: 10.1152/japplphysiol.00899.2001. [DOI] [PubMed] [Google Scholar]
- Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension. 1989;14:511–517. doi: 10.1161/01.hyp.14.5.511. [DOI] [PubMed] [Google Scholar]
- Francis GS, Benedict C, Johnstone DE. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure: A substudy of studies of left ventricular dysfunction (SOLVD) Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995;92:3206–3211. doi: 10.1161/01.cir.92.11.3206. [DOI] [PubMed] [Google Scholar]
- Grassi GM, Cattaneo BM, Servalle S. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation. 1997;96:1173–1179. doi: 10.1161/01.cir.96.4.1173. [DOI] [PubMed] [Google Scholar]
- Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge quantitative assessment in human hypertensive disease. Circulation. 1999;1100:1305–1310. doi: 10.1161/01.cir.100.12.1305. [DOI] [PubMed] [Google Scholar]
- Greenwood JP, Stoker JB, Walker JJ, Mary DA. Sympathetic nerve discharge in normal pregnancy and pregnancy-induced hypertension. J Hypertens. 1998;16:617–624. doi: 10.1097/00004872-199816050-00009. [DOI] [PubMed] [Google Scholar]
- Gullestad L, Manhenke C, Aarsland T. Effect of metoprolol CR/XL on exercise in chronic heart failure – a substudy to the MERIT-HF trial. Eur J Heart Fail. 2001;3:463–468. doi: 10.1016/s1388-9842(01)00146-5. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Lefkovits J, Cox H. Regional epinephrine kinetics in human heart failure: Evidence for extra-adrenal, nonneural release. Am J Physiol Heart Circ Physiol. 1995;269:H182–H188. doi: 10.1152/ajpheart.1995.269.1.H182. [DOI] [PubMed] [Google Scholar]
- Lambert E, Straznicky N, Esler M, Dawood Tye, Hotchkin E, Lambert G. Different pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50:862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo ÅB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J Physiol. 1994;481:799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Rundqvist B, Sverrisdottir YB, Wallin BG, Elam M. Firing properties of single muscle vasoconstrictor neurones in the sympathoexcitation associated with congestive heart failure. Circulation. 1999;100:1708–1713. doi: 10.1161/01.cir.100.16.1708. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Nitzsche EU, Hoh CK. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation. 2000;101:784–789. doi: 10.1161/01.cir.101.7.784. [DOI] [PubMed] [Google Scholar]
- Mitchell JH. Neural control of the circulation during exercise. Med Sci Sport Exer. 1990;22:141–154. [PubMed] [Google Scholar]
- Murai H, Takata S, Maruyama M. The activity of a single muscle sympathetic vasoconstrictor nerve unit is affected by physiological stress in humans. Am J Physiol Heart Circ Physiol. 2006;290:H853–H860. doi: 10.1152/ajpheart.00184.2005. [DOI] [PubMed] [Google Scholar]
- Nakata A, Takata S, Yuasa T, Kobayashi K. Spectral analysis of heart rate, arterial pressure, and muscle sympathetic nerve activity in normal humans. Am. J Physiol Heart Circ Physiol. 1998;274:H1211–H1217. doi: 10.1152/ajpheart.1998.274.4.H1211. [DOI] [PubMed] [Google Scholar]
- Negrao CE, Rondon MU, Tinucci T. Abnormal neurovascular control during exercise is linked to heart failure severity. Am J Physiol Heart Circ Physiol. 2001;280:H1286–H1292. doi: 10.1152/ajpheart.2001.280.3.H1286. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Ljung B, Sjoblem N, Wallin BG. The influence of sympathetic impulse pattern on contractile response of rat mesenteric arteries and vein. Acta Physiol Scand. 1985;123:303–309. doi: 10.1111/j.1748-1716.1985.tb07592.x. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Ando S, Floras JS. Resting muscle sympathetic nerve activity and peak oxygen uptake in heart failure and normal subjects. Eur Heart J. 1999;20:880–887. doi: 10.1053/euhj.1998.1447. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280:H969–H976. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Volterrani M. Contribution of muscle afferents to hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Ponikowski PP, Clark AL. A neural link to explain the muscle hypothesis of exercise intolerance in chronic heart failure. Am Heart J. 1999;137:1050–1056. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- Roveda F, Middlekauff HR, Negrão CE. The effects of exercise training on sympathetic neural activation in advanced heart failure; a randomized control trial. J Am Coll Cardiol. 2003;42:854–860. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Rundqvist B, Eisenhofer G, Elam M, Friberg P. Attenuated cardiac sympathetic responsiveness during dynamic exercise in patients with heart failure. Circulation. 1997;95:940–945. doi: 10.1161/01.cir.95.4.940. [DOI] [PubMed] [Google Scholar]
- Sterns DA, Ettinger SM, Gray KS. Skeletal muscle metaborecepter exercise responses are attenuated in heart failure. Circulation. 1991;84:2034–2039. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- Sverrisdóttir YB, Rundqvist B, Johannsson G, Elam M. Sympathetic neural burst amplitude distribution. A more specific indicator of sympathoexcitation in human heart failure. Circulation. 2000;102:2076–2081. doi: 10.1161/01.cir.102.17.2076. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Klangos I. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]