Abstract
Inflammation has profound effects on the innervation of affected tissues, including altered neuronal excitability and neurotransmitter release. As Ca2+ influx through voltage-gated Ca2+ channels (VGCCs) is a critical determinant of excitation–secretion coupling in nerve terminals, the aim of this study was to characterize the effect of overnight incubation in the inflammatory mediator tumour necrosis factor α (TNFα; 1 nm) on VGCCs in dissociated neurons from mouse superior mesenteric ganglia (SMG). Voltage-gated Ca2+ currents (ICa) were measured using the perforated patch clamp technique and the VGCC subtypes present in SMG neurons were estimated based on inhibition by selective VGCC blockers: ω-conotoxin GVIA (300 nm; N-type), nifedipine (10 μm; L-type), and ω-conotoxin MVIIC (300 nm; N-, P/Q-type). We used intracellular Ca2+ imaging with Fura-2 AM to compare Ca2+ influx during depolarizations in control and TNFα-treated neurons. TNF receptor and VGCC mRNA expression were measured using PCR, and channel α subunit (CaV2.2) was localized with immunohistochemistry. Incubation in TNFα significantly decreased ICa amplitude and depolarization-induced Ca2+ influx. The reduction in ICa was limited to ω-conotoxin GVIA-sensitive N-type Ca2+ channels. Depletion of glial cells by incubation in cytosine arabinoside (5 μm) did not affect ICa inhibition by TNFα. Preincubation of neurons with SC-514 (20 μm) or BAY 11-7082 (1 μm), which both inhibit nuclear factor κB signalling, prevented the reduction in ICa by TNFα. Inhibition of N-type VGCCs following TNFα incubation was associated with a decrease in CaV2.2 mRNA and reduced membrane localization of CaV2.2 immunoreactivity. These data suggest that TNFα inhibits ICa in SMG neurons and identify a novel role for NF-κB in the regulation of neurotransmitter release during inflammatory conditions with elevated circulating TNFα, such as Crohn's disease and Guillain-Barré syndrome.
Voltage-gated Ca2+ channels (VGCCs) modulate a variety of neuronal functions including the coupling of membrane depolarization to neurotransmitter release, modification of gene transcription, neurite outgrowth and receptor clustering (Jarvis & Zamponi, 2007; Catterall & Few, 2008). VGCCs have been classified according to their voltage dependence of activation, the molecular identity of their pore-forming subunits, and their pharmacological sensitivity (Catterall et al. 2005). Currents through high voltage-activated VGCC subtypes (L, N, P/Q and R subtypes) are usually activated at depolarized membrane potentials whereas the T subtype of channel opens at more hyperpolarized potentials (Ertel et al. 2000). VGCCs are transiently regulated by G-protein-coupled receptor signalling (Tedford & Zamponi, 2006), and can be modified on a longer time scale by changes in channel trafficking or α subunit mRNA expression and stability (Schorge et al. 1999).
Although inflammation has profound effects on the innervation of affected tissues (Lomax et al. 2006; Dantzer et al. 2008), little is known about the cellular mechanisms underlying these neurophysiological changes. Circulating levels of a number of cytokines, including tumour necrosis factor α (TNFα) are increased during peripheral inflammation, which raises the possibility that direct effects of cytokines on neurons are responsible for neurological alterations during inflammation. Sympathetic prevertebral ganglia possess fenestrated capillaries (Szurszewski & Miller, 1994) which allow circulating macromolecules, such as cytokines, to access neurons within these ganglia. Therefore, these neurons may be particularly susceptible to increases in circulating cytokines, including TNFα, during inflammation.
Prevertebral ganglia, including the coeliac, superior mesenteric and inferior mesenteric ganglia, contain the cell bodies of postganglionic sympathetic neurons that regulate the function of the gastrointestinal tract, spleen, liver, pancreas and urogenital organs (Szurszewski & Miller, 1994; Miolan & Niel, 1996). The nerve terminals of these neurons release noradrenaline (norepinephrine), ATP and neuropeptide Y which, in the gastrointestinal tract, inhibit the activity of the enteric nervous system and modulate blood flow and secretion (Furness et al. 2003; Burnstock, 2004). The release of these neurotransmitters from sympathetic varicosities is dependent on activation of N-type VGCCs (Brock & Cunnane, 1999; Morris et al. 2004). Previous studies of the release of tritiated noradrenaline from sympathetic varicosities described a reduction in release in the colon during colitis (Swain et al. 1991; Jacobson et al. 1997; Blandizzi et al. 2003). We have recently reported that the reduction of noradrenaline release in the mouse model of dextran sulphate sodium (DSS)-induced colitis was due to selective inhibition of N-type VGCCs in sympathetic neurons (Motagally et al. 2009). We hypothesized that this defect is due to cytokine-induced inhibition of voltage-gated Ca2+ current (ICa) in sympathetic neurons. Therefore, in the present study we determined the effects of overnight exposure to TNFα on VGCCs in SMG neurons, as elevations of cytokines during disease typically last for an extended period of time, unlike transient events such as exposure to synaptically released neurotransmitter.
Methods
Isolation of SMG neurons
All reagents used in this study were obtained from Sigma Aldrich (St Louis, MO, USA), except when noted otherwise. The methods used for dissociating neurons were adapted from Lamas et al. (1997). Male CD1 mice (25–35 g) were deeply anaesthetized by isoflurane inhalation and killed by cervical dislocation in accordance with the principles and guidelines of the Canadian Council on Animal Care and Queen's University Animal Care Committee. Following a laparotomy, the SMG was quickly removed and placed in Hank's buffered salt solution (HBSS; Invitrogen, Carlsbad, CA, USA) maintained at 37°C. Ganglia were then washed with HBSS three times before being enzymatically dissociated by incubation for 25 min in HBSS containing collagenase (Type 1A) and bovine serum albumin (BSA; 6 mg ml−1), and 15 min in HBSS containing trypsin (Type XII-S) and BSA (6 mg ml−1). In order to neutralize the enzymatic activity of trypsin, 10% fetal calf serum (Invitrogen) was added. Single neurons were isolated by trituration through a series of fire-polished Pasteur pipettes of decreasing diameter. Following trituration, the cell suspension was centrifuged at 100g for 5 min and the supernatant was discarded. Dissociated neurons were plated on laminin-coated (10 μg ml−1) glass coverslips and maintained overnight at 37°C and 5% CO2 in Leibowitz medium supplemented with: 24 mm NaHCO3, 10% fetal calf serum, 38 mm d-glucose, 2 mm l-glutamine, 5000 IU penicillin–streptomycin, and 50 ng ml−1 nerve growth factor. To study the effects of TNFα, neurons were incubated overnight in media containing 1 nm recombinant mouse TNFα (PeproTech, Rocky Hill, NJ, USA).
Patch clamping of dissociated neurons
All experiments were performed at room temperature (20–22°C). Patch pipettes were fabricated from borosilicate glass capillaries (Warner Instruments, Hamden, CT, USA) and had resistances between 2 and 4 MΩ when filled with internal solution of the following composition (mm): 120 CsCl, 1 MgCl2, 4 MgATP, 0.3 NaGTP, 10 EGTA and 10 Hepes (pH adjusted to 7.2 with CsOH). A calculated liquid junction potential (Clampex Junction Potential Assistant, MDS analytical technologies) of 10 mV was digitally subtracted. To achieve perforated patch configuration, pipettes were backfilled with internal solution containing amphotericin-B (50 μg ml−1, Sigma). After an acceptable and stable access resistance (8–15 MΩ) was acquired, the cell membrane capacitance and series resistance were electronically compensated. Voltage-clamp recording was performed using a Multiclamp 700B amplifier (MDS analytical technologies, Mississauga, Ontario, Canada). Data were acquired at 10 kHz for analysis using a Digidata 1440A analog to digital converter, and pCLAMP 10.1 software (all from MDS analytical technologies).
Currents through VGCCs were recorded using extracellular solution consisting of (in mm): 140 tetraethylammonium (TEA)-Cl, 2 MgCl2, 5 BaCl2, 10 glucose and 10 Hepes (pH adjusted to 7.4 with TEA-OH). ICa, carried by Ba2+, was elicited by a series of command potentials from −60 to 35 mV for 100 ms in 5 mV steps (10 s intervals) from a holding potential of −100 mV. In preliminary experiments, we explored whether SMG neurons possessed low voltage-activated T-type Ca2+ currents. Voltage steps between −100 mV and −60 mV did not evoke any inward current (n= 7 neurons). Therefore, T-type channels were not considered further in this study. Currents were allowed to stabilize for at least 5 min before recordings commenced. Series resistances were compensated 80–85%. The amplitude of inward currents was normalized to cell capacitance. To study the effects of specific Ca2+ channel blockers, the membrane potential was stepped to 0 mV from a holding potential of −100 mV for 100 ms to elicit ICa every 10 s before and during superfusion of antagonists. The steady-state inactivation of VGCCs was obtained by depolarizing cells to a series of prepulse potentials from −100 to 20 mV for 500 ms followed by a command potential to 0 mV for 150 ms. Activation and inactivation curves were fitted to a single Boltzmann function of the form: G/Gmax= 1/(1 + exp[V0.5−Vm/k]), where G is conductance, Vm is membrane voltage, k is the slope factor, and Gmax is maximal conductance.
Current clamp recordings were performed with the following solutions: extracellular (in mm): 140 NaCl, 10 Hepes, 10 glucose, 5 KCl, 2 CaCl2 and 1 MgCl2 (pH 7.4 with NaOH); intracellular: 110 potassium gluconate, 20 KCl, 10 EGTA, 10 Hepes, 4 Na2ATP, 1 CaCl2, 1 MgCl2, 0.2 GTP and 50 μg ml−1 amphotericin-B (pH 7.2 with KOH). Input resistance was calculated from the voltage change in response to a 10 pA hyperpolarizing current injection.
Ca2+ imaging
Intracellular calcium concentration ([Ca2+]i) measurements were recorded from isolated SMG neurons that were incubated in 2 μm Fura-2 acetoxymethylester (AM; dissolved in DMSO; Invitrogen) for 30 min at 37°C. Neurons were subsequently washed for 20 min with Hepes-buffered saline solution to remove the excess extracellular Fura-2 AM and to allow for intracellular de-esterification of the fluorophore. The saline solution was composed of (in mm): 140 NaCl, 10 Hepes, 10 glucose, 5 KCl, 2 CaCl2 and 1 MgCl2 (pH 7.4 with NaOH). Coverslips were mounted in a recording chamber on an inverted microscope (Olympus IX71, Markham, Ontario, Canada) and superfused with this solution at room temperature. Fura-2 AM-loaded cells were illuminated at 340 and 380 nm using a DeltaRamV high speed random access monochromator and images of fluorescence images at 510 nm were acquired every 1 s for 10 min using a Photometrics Cascade 512B CCD camera (Photon Technology International, London, Ontario, Canada). Regions of interest (ROIs) were defined within the cytoplasm of neurons, from which the averaged pixel intensity within the ROI was calculated. Paired 340 : 380 fluorescence ratios of each ROI were calculated every 1 s. [Ca2+]i was measured as the ratio of the fluorescence intensity (F) obtained at 405 nm following excitation at 340 and 380 nm. Changes in 340 : 380 ratios were measured using ImageMaster 5.0 software. To depolarize neurons and activate VGCCs, neurons were superfused with saline solution containing 40 mm K+ for 1 min, followed by washout. The high K+ saline solution was composed of (in mm): 105 NaCl, 10 Hepes, 10 glucose, 40 KCl, 2 CaCl2 and 1 MgCl2 (pH 7.4 with NaOH). To compare changes in [Ca2+]i during depolarizations between cell populations, the peak change in F340 : F380 was expressed as a percentage change relative to the baseline fluorescence prior to depolarization.
Drug application
To distinguish the relative contributions of different VGCC subtypes, neurons were superfused with the N-type channel blocker ω-conotoxin GVIA (300 nm; Tocris, Ellisville, MO, USA), the L-type channel blocker nifedipine (10 μm; Sigma) or the N- and P/Q-type channel blocker ω-conotoxin MVIIC (300 nm; Tocris), using concentrations that maximally inhibit channel current. In order to determine the contribution of P/Q-type channels to the total current, N-type channels were first blocked with ω-conotoxin GVIA, followed by superfusion of ω-conotoxin MVIIC. The difference in inhibition caused by these two blockers was taken as an estimate of P/Q-type current. In all cases, current inhibition was allowed to reach a steady state before measuring the extent of inhibition. These channel inhibitors were used in the same manner with TNFα-treated neurons to investigate the change in contribution of channel subtypes to current following incubation in TNFα.
Non-membrane-bound TNFα signals via the TNFα receptor 1 (TNFR1) subtype (Grell et al. 1995). TNFR1 activation initiates a number of intracellular effector cascades, such as the nuclear factor κB (NF-κB) pathway and the p38 mitogen-activated protein kinase (MAPK) pathway (Baud & Karin, 2001). To determine whether activation of NF-κB or p38 MAPK contributed to inhibition of VGCCs by TNFα, neurons were pretreated with NF-κB inhibitors (20 μm SC-514 or 1 μm BAY 11 - 7082) or a p38 MAPK inhibitor (30 μm PD 169316) for 2 h prior to and throughout the application of the cytokine. These concentrations were chosen to maximally inhibit enzyme activity. Control neurons were pretreated with the vehicle (0.1% DMSO) for 2 h prior to and during incubation in normal culture medium or TNFα-containing medium. Following overnight incubation, ICa or intracellular Ca2+ transients were measured.
PCR
Total RNA was extracted using the Trizol method (Invitrogen, Canada) from dissociated SMG neurons that had been cultured overnight in control L-15 medium, or medium containing 1 nm TNFα. cDNA was then reverse transcribed from 1 μg of total RNA using Expand Reverse Transcriptase (Roche, Mississauga, Canada) and oligo(dT)12–18 primers (Invitrogen). Subsequently, real-time PCR was performed using a Roche Lightcycler and a QuantiTech SYBR Green PCR Kit (Qiagen, Mississauga, Canada). The target to reference ratio was then calculated using the Relquant software (Roche). The following intron-spanning primers were used. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (NM_008084, 130 bp product): forward, TAGACAAAATGGTGAAGGTCGG; reverse, AGTTGAGGTCAATGAAGGGGT; and CaV2.2 (NM_001042528, 124 bp product): forward, AGCCCTCAGATCCCAGCA; reverse, GCCTCCTTCTTGCCCTCT.
TNFR1 was detected in mRNA extracted from non-dissociated SMG. cDNA was reverse transcribed from 1 μg of total RNA using Expand Reverse Transcriptase (Roche) using oligo(dT)12–18 primer (Invitrogen). The primers for TNFR1 (NM_011609; 228 bp) were as follows: forward, TCACCCACAGGGAGTAGGGCA; reverse, GCCTGGCGGCGCCGCACGCCG. TNFR1 was amplified by PCR at an annealing temperature of 60°C for 30 cycles followed by a final elongation at 72°C for 10 min.
Immunohistochemistry
Dissociated SMG neurons grown overnight in the presence or absence of TNF-α were fixed for 10 min in ice-cold 4% paraformaldehyde. Following three washes in PBS, cells were blocked for 1 h using 10% normal goat serum in PBS. Rabbit anti-CaV2.2 antibody (Calbiochem, 1 : 1000) was added and allowed to incubate overnight at 4°C. The cells were washed 3 times in PBS and goat anti-rabbit Alexafluor 555 (Invitrogen, 1 : 2000) was added for 1 h. Following three further washes, coverslips were mounted in glycerol on glass microscope slides. Pre-adsorption of primary antiserum with antigenic peptide abolished immunoreactivity. Optical sections (0.5 μm) of cells were visualized using an Olympus IX70 confocal microscope and photographed using Fluoview software. The resultant images, from neurons dissociated from three animals, were analysed using ImageJ (http://rsbweb.nih.gov/ij/) following the method of Poole et al. (2008). Briefly, the nuclear staining was set as the background fluorescence, creating a thresholded image to identify the number of positively stained pixels and below-threshold pixels (valued at 255 or 0, respectively). The original grey scale images were used as a reference to define and trace the sub-membrane portion of the cell, since there was often little membrane staining in the TNF group. The total positive staining in the cell was compared with the staining in the cytoplasm alone, giving a measure of the membrane staining by subtraction. The ratio of membrane to cytoplasmic staining was calculated.
Trypan blue experiments
In order to assess the effects of the TNFα treatments on the viability of dissociated SMG neurons, trypan blue exclusion experiments were performed on populations of treated and control neurons. The trypan blue exclusion assay is based on the ability of viable cells to exclude the dye from their cytoplasm. A total of ten coverslips from four mice, each containing at least 100 neurons, were incubated overnight in either control media (n= 5 coverslips) or media containing TNFα (n= 5 coverslips). Trypan blue (0.2%) was added to individual coverslips following the overnight incubation and, after 5 min, the coverslips were examined and cell counts were performed. The number of viable cells was calculated as a percentage of the total cell population.
Statistical analysis
Electrophysiological data were analysed using Clampfit 10 (MDS analytical technologies). Statistical analysis was performed and graphs were formatted using GraphPad Prism 5. n values refer to the number of neurons used in assays unless otherwise stated. Population data were expressed as the mean ± standard error of the mean (s.e.m.). Statistical significance was reached when P≤ 0.05.
Results
Incubation in TNFα-reduced ICa
Reverse transcription PCR of mRNA from intact mouse SMG with primers for the TNFR1 gene revealed TNFR1 mRNA expression within these ganglia (Fig. 1A). Therefore, we investigated the effect of incubating neurons in TNFα on ICa. Neurons incubated in TNFα (1 nm) had markedly smaller ICa compared to untreated control neurons (Fig. 1B and C). Incubation in TNFα caused a significant decrease in ICa (P < 0.05; 2-way ANOVA with Bonferroni's post hoc test) in the treated neurons between −20 and +10 mV (TNFαn= 21; untreated n= 19; Fig. 1D).
Figure 1. Tumour necrosis factor α(TNFα) inhibited ICa in superior mesenteric ganglia (SMG) neurons.
A, expression of TNFα receptor 1 (TNFR1) in the SMG of mice, as detected by RT-PCR. B and C, comparison of ICa from an untreated control neuron (A) and a neuron incubated overnight in 1 nm TNFα (B). Voltage clamp protocol is illustrated in inset. D, mean ±s.e.m.I–V relations from a group of control neurons and TNFα-treated neurons. TNFα significantly reduced ICa. *P < 0.05, two-way ANOVA with Bonferroni's post hoc test.
We next investigated whether TNFα selectively inhibited specific Ca2+ channel subtypes. In order to do this we first identified which VGCC subtypes contributed to ICa in SMG neurons. ICa amplitude was measured during 100 ms depolarizations to 0 mV every 10 s before and following the superfusion of subtype-specific channel blockers (Fig. 2A). Figure 2B summarizes the percentage of current inhibition caused by the inhibitors in control neurons. The greatest inhibition was caused by the N-type channel blocker ω-conotoxin (CTX) GVIA (300 nm; 60.6 ± 5.8%; n= 11). Application of nifedipine (10 μm), an L-type channel blocker, resulted in a 21 ± 6% (n= 17) reduction in the current. The reduction caused by ω-conotoxin MVIIC, a blocker of N- and P/Q-type channels did not significantly differ from the inhibition caused by the specific N-type channel blocker (56.4 ± 5.9%, n= 12; Kruskal–Wallis test followed by Dunn's multiple comparison test), suggesting that the contribution of P/Q channel subtypes was small or absent. Consistent with this, the combination of ω-conotoxin GVIA and nifedipine completely abolished ICa (Fig. 2A), which also rules out a role for R-type currents in SMG somata. The amplitude of ω-conotoxin GVIA-sensitive N-type current was significantly reduced after TNFα incubation (P < 0.001, unpaired 2-tailed t test), whereas the amplitude of nifedipine-sensitive L-type current was not altered (P= 0.517; Fig. 2C and D). These data suggest that TNFα treatment caused a selective inhibition of N-type Ca2+ channels, while L-type channels were unaffected.
Figure 2. TNFα selectively inhibited N-type current.
Examples of ICa elicited by a step to 0 mV from a holding potential of −100 mV from an untreated (A) and a TNFα-treated (C) neuron before and during superfusion of 300 nmω-conotoxin GVIA (CTX) and 10 μm nifedipine. B, percentage inhibition of ICa in control neurons by ω-conotoxin GVIA (300 nm), the N- and P/Q-type blocker ω-conotoxin MVIIC (300 nm) and nifedipine (10 μm), indicating that ICa in control SMG neurons is primarily carried by N-type channels. *P < 0.05, Kruskal–Wallis test with Dunn's multiple comparison test. D, current density (pA pF−1) inhibition by 300 nmω-conotoxin GVIA (estimate of N-type current) and 10 μm nifedipine (estimate of L-type current) in control and TNFα-treated neurons (ω-conotoxin GVIA). *P < 0.05 versus untreated controls, unpaired 2-tailed t test. Number of neurons is indicated within bars.
Mechanisms of N-type inhibition by TNFα
Paired-pulse voltage clamp protocols were utilized to determine whether TNFα changed the voltage dependence of activation or inactivation of ICa. The voltage dependence of inactivation and activation were unchanged following incubation in TNFα (Fig. 3). These data suggest that the reduction of ICa caused by TNFα is not associated with altered voltage-dependent channel gating. We also examined whether direct interaction of βγ subunits of G-protein-coupled receptors might account for the reduction of ICa following incubation in TNFα. It has previously been shown that inhibition of N-type Ca2+ channels by βγ subunits of G-protein-coupled receptors can be transiently reversed following large membrane depolarizations (Zamponi & Snutch, 1998). A step to +150 mV for 150 ms prior to a test pulse to 0 mV caused a 17.2 ± 0.05% (n= 10) increase in ICa in control neurons and an 18.6 ± 0.06% (n= 8) increase in TNFα-treated neurons (P= 0.76, Mann–Whitney test), suggesting that while βγ subunits tonically modulate ICa, they have no role in the TNFα suppression of N-type currents.
Figure 3. Voltage dependence of activation and inactivation of ICa was not altered by TNFα.
A, Boltzmann fits of mean normalized conductances showing the voltage dependence of steady-state inactivation of ICa in control neurons (n= 10) and TNFα-treated neurons (n= 12). The V0.5 was −72.6 ± 0.8 and −70 ± 2.1 mV in control and TNFα-treated neurons, respectively. The slope factor was −17.1 ± 0.7 and −18.4 ± 1.4 in control and TNFα-treated neurons, respectively. B, voltage-dependent activation curves for control neurons (n= 14) and 1 nm TNFα-treated neurons (n= 9). The V0.5 was −20.9 ± 0.6 and −20.8 ± 1.3 mV in control and TNFα-treated neurons, respectively. The slope factor was −3.9 ± 0.6 and −5.1 ± 1.3 in control and TNFα-treated neurons, respectively.
The reduction in ICa caused by TNFα could be a non-specific effect of a change in the viability of the neurons. Current clamp recordings of both control and treated neurons revealed no effects of overnight TNFα incubation on neuronal resting membrane potential (Control: −54.9 ± 1.4 mV, n= 53 neurons; TNFα: −55.9 ± 1.8 mV, n= 21; P= 0.66, unpaired t test) or input resistance (Control: 596 ± 71 MΩ, n= 53; TNFα: 709 ± 118 MΩ, n= 21; P= 0.42, unpaired t test). In addition, we assessed cell viability directly by determining the proportion of cells that excluded trypan blue following TNFα incubation. Of control neurons 77.7 ± 0.8% were viable and of TNFα-treated neurons 79.3 ± 2.1% were viable (n= 5 coverslips each; P= 0.69, Mann–Whitney test), suggesting that TNFα did not affect neuronal viability.
SC-514 inhibited reduction of ICa by TNFα
TNFR1 activation mediates the effects of soluble TNFα on a variety of cell types via downstream intracellular signalling pathways, including effects on gene transcription due to activation of the NF-κB signalling complex (Baud & Karin, 2001). We initially utilized SC-514, which inhibits Iκ kinase 2 (IKK−2), an enzyme that facilitates NF-κB nuclear signalling, to investigate whether the decrease in ICa caused by TNFα was dependent on NF-κB signalling. Incubation of neurons in 20 μm SC-514 2 h prior to and during incubation in TNFα blocked the reduction in ICa caused by TNFα (Fig. 4A). At 0 mV, ICa amplitude was significantly reduced in neurons treated with TNFα alone (n= 17), compared with either untreated controls (n= 20, P < 0.05), SC-514-treated controls (n= 11, P < 0.05) and, importantly, neurons co-incubated with SC-514 and TNFα (n= 16, P < 0.05; one-way ANOVA with Bonferroni's post hoc test for each; Fig. 4B). These data suggest that TNFα inhibits ICa via activation of IKK-2 and thereby identify a central role for NF-κB signalling in the reduction of ICa by TNFα.
Figure 4. SC-514 blocked the inhibition of ICa by TNFα.
A, sample recordings of ICa from a control neuron, a TNFα-treated neuron and a neuron that was pretreated with 20 μm SC-514 and subsequently exposed to SC-514 and TNFα. B, mean ±s.e.m. peak ICa amplitude at 0 mV in untreated controls, SC-514-treated controls, TNFα-treated neurons, and SC-514-pretreated neurons that subsequently were exposed to SC-514 and TNFα overnight. *P < 0.05, one-way ANOVA with Bonferroni's post hoc test. Number of neurons is indicated within bars.
Intracellular Ca2+ imaging
Intracellular Ca2+ imaging with Fura-2 AM was used to further study the effect of TNFα on voltage-dependent Ca2+ entry into SMG neurons. Baseline 340 : 380 ratios in unstimulated cells, a correlate of resting intracellular [Ca2+], did not differ between control and TNFα-treated neurons (0.5686 ± 0.019 and 0.5318 ± 0.015; n= 64 each; P= 0.2, Mann–Whitney test). Superfusion of SMG neurons with a high [K+] extracellular solution transiently increased neuronal 340 : 380 ratio (Fig. 5A). TNFα-treated neurons had significantly reduced responses to raised extracellular [K+] (P < 0.001, Kruskal–Wallis test and Dunn's multiple comparison test; Fig. 5B). These data are consistent with voltage-clamp evidence of reduced voltage-gated ICa. Pretreatment and co-incubation with 20 μm SC-514 prevented this effect of TNFα. Furthermore, the amplitude of Ca2+ transients in SC-514 plus TNFα did not differ significantly from untreated controls. Similar findings were obtained using another NF-κB antagonist, BAY 11-7082 (1 μm). These data support the notion that incubation in TNFα leads to NF-κB-mediated reduction in voltage-gated Ca2+ entry into SMG neurons. We examined the role of p38 MAPK signalling in channel inhibition by using the p38 MAPK inhibitor PD 169316. In contrast to inhibition of NF-κB signalling, blocking the p38 MAPK pathway did not affect the reduction of Ca2+ influx by TNFα (Fig. 5B).
Figure 5. Inhibition of NF-κB blocked the reduction in depolarization-induced Ca2+ transients by TNFα.
A, representative Ca2+ imaging recording of an isolated SMG neuron displaying a peak response following superfusion with a 40 mm K+-containing saline solution. B, percentage changes in F340 : F380 during high K+ depolarization in control, 1 nm TNFα-treated neurons, neurons treated with 20 μm SC-514 and TNFα, neurons treated with 1 μm BAY 11 - 7082 and TNFα, and neurons treated with the p38 mitogen-activated protein kinase (MAPK) inhibitor PD 169316 (30 μm) and TNFα. *P < 0.001 versus TNFα-treated neurons, Kruskal–Wallis test and Dunn's multiple comparisons test. Number of neurons is indicated within bars.
Penicillin and streptomycin (Pen–Strep) are routinely used in cell culture media to prevent microbial contamination. However, it was possible that the presence of Pen–Strep in the culture medium affected voltage-dependent Ca2+ influx or the inhibition of Ca2+ influx by TNFα. The percentage increase in 340 : 380 ratio relative to baseline, in response to superfusion of high K+ saline solution in neurons that were cultured overnight in the presence of Pen–Strep was 226.8 ± 20 (n= 48 neurons), compared to neurons cultured without Pen–Strep (236.8 ± 35%, n= 21 neurons, P= 0.96 versus Pen–Strep treatment, Mann–Whitney test). Ca2+ influx in neurons that were incubated overnight in Pen–Strep plus TNFα was reduced to 168.3 ± 7% (n= 77 neurons), compared to neurons cultured in TNFα (152.6 ± 7%, n= 34 neurons, P= 0.4 versus Pen–Strep treatment, Mann–Whitney test). These data indicate that Pen–Strep had no effect on VGCCs and did not influence the inhibition of ICa by TNFα.
Role of sympathetic glial cells in Ca2+ channel inhibition
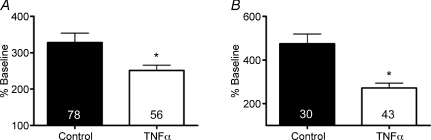
It is possible that the inhibitory effect of TNFα on N-type VGCCs in SMG neurons occurs indirectly, via activation of glial cells. We examined this possibility by selectively depleting glial cells from SMG cultures using cytosine arabinoside (ara-C) at a concentration (5 μm) which prevents glial proliferation without causing neuronal damage (Besirli et al. 2003). Dissociated ganglia were cultured for 4 days, with ara-C added to the culture medium after 1 day and present for the remainder of the experiment. On the third day, TNFα was added and left overnight. Ca2+ imaging was then performed on day 4. Control populations were cultured without ara-C and with or without TNFα.
SMG neurons that were incubated for 4 days had larger percentage increases in F340 : F380 ratio compared to neurons that were cultured overnight (overnight: 160.4 ± 4.1%, n= 65 neurons, versus 4 days: 328.1 ± 26%, n= 78; P < 0.01, Mann–Whitney test). Neurons that were cultured in the presence of cytosine arabinoside had larger increases than neurons cultured in the absence of the gliotoxin (control: 328.1 ± 26%, n= 78 versus ara-C: 474.4 ± 45%, n= 30; P < 0.01). Incubation in TNFα significantly reduced Ca2+ influx in cells that were treated in ara-C (Fig. 6A), as it did in cells that were not cultured in ara-C (Fig. 6B). Taken together, these data suggest that glial cells do not participate in the inhibition of VGCCs by TNFα.
Figure 6. Depletion of glial cells did not affect the reduction of Ca2+ influx by TNFα.
A, mean ±s.e.m. percentage increase in Ca2+ influx in response to 40 mm K+ in cells cultured for 4 days, with TNFα added to some cultures for the final day. B, neurons cultured in the presence of cytosine arabinoside (ara-C; 5 μm) for 3 days exhibited the same reduction in Ca2+ influx following overnight incubation in TNFα.
Effects of TNFα on channel expression and trafficking
Our pharmacological data suggested that TNFα selectively inhibited N-type VGCCs, whose pore-forming α subunit is α1B, and is encoded by the CaV2.2 gene (Catterall et al. 2005). This channel can be modulated by short term effects on channel gating and by longer term effects on channel transcription and insertion into the membrane (Catterall & Few, 2008). NF-κB signalling complexes regulate gene expression in a variety of cell types during inflammation (Baud & Karin, 2001). Therefore, we examined whether the inhibition of N-type channels was due to a reduction in CaV2.2 gene expression. Real-time PCR analysis of CaV2.2 expression relative to GAPDH, a loading control, demonstrated that CaV2.2 mRNA was significantly reduced in neurons exposed to TNFα (Fig. 7, P= 0.05, n= 3 wells for each condition, Mann–Whitney test) suggesting that a decrease in channel mRNA may contribute to reduced N-type current.
Figure 7. Overnight incubation in TNFα reduced mRNA of the N-type channel α subunit.
Real-time PCR analysis of CaV2.2 mRNA expression relative to GAPDH in neurons incubated overnight in control medium containing TNFα. *P= 0.05, Mann–Whitney test. Number of wells is indicated within bars, with each well containing neurons dissociated from two SMG.
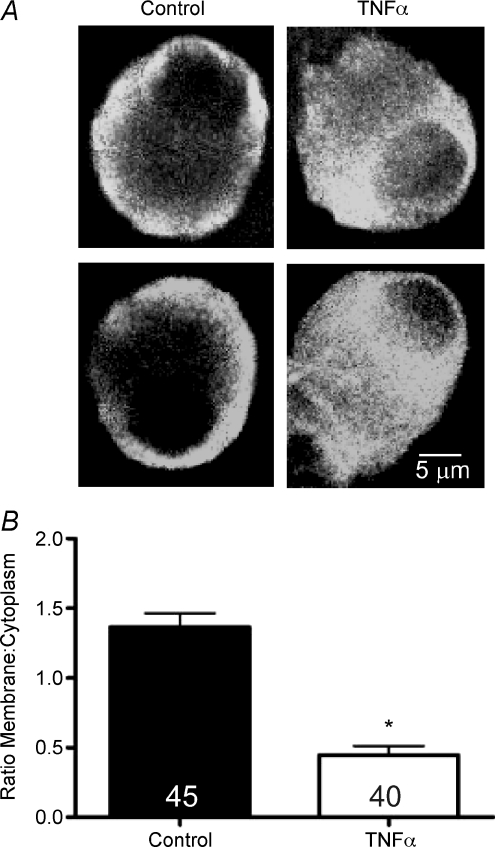
We examined the cellular distribution of CaV2.2 channel α subunits using immunohistochemistry and confocal microscopy (Fig. 8A). In untreated control cells, immunoreactivity for CaV2.2 was predominantly localized to the cell membrane and submembrane space. In contrast, the distribution of CaV2.2 was dramatically different in cells that were incubated overnight in TNFα: the α subunit was distributed throughout the cytoplasm with little to no membrane expression. Comparison of the ratios of membrane to cytoplasm CaV2.2 immunoreactivity in control and TNFα-treated neurons revealed that TNFα significantly decreased the relative membrane expression of CaV2.2 (Fig. 8B, P < 0.001, Mann–Whitney test, n= 45 untreated neurons and 40 TNFα-treated neurons).
Figure 8. TNFα reduced membrane localization of CaV2.2 protein.
A, sample confocal micrographs of CaV2.2 immunoreactivity in dissociated SMG neurons incubated overnight in the absence or presence of TNFα. In untreated controls, channel immunoreactivity was localized to the plasmalemma and sub-membrane space, whereas in TNFα-treated neurons, membrane localization was reduced relative to cytoplasmic immunoreactivity. B, quantification of the ratio of membrane to cytoplasmic CaV2.2 immunoreactivity in control and TNFα-treated neurons. *P < 0.001, Mann–Whitney test. Number of neurons examined is indicated within bars.
Discussion
The aim of the present study was to determine whether overnight incubation of sympathetic neurons in TNFα affects voltage-gated calcium channels. Our data suggest that TNFα selectively inhibits N-type Ca2+ channels via initiation of NF-κB-dependent signalling pathways. The decrease in N-type current may be due to reduction of CaV2.2 gene transcription and redistribution of channel α subunits away from the plasma membrane.
Ca2+ channels and sympathetic neurotransmitter release
N- and L-type channels are the predominant subtypes of VGCCs in the cell bodies of SMG neurons. This conclusion is based on the sensitivity of ICa to blockade by ω-conotoxin GVIA and nifedipine, respectively. P/Q, R and T channels do not appear to contribute to ICa, as conotoxin MVIIC (which blocks N and P/Q channels) did not inhibit ICa to a greater extent than the N-type channel-selective ω-conotoxin GVIA, and the combination of ω-conotoxin GVIA and nifedipine abolished ICa in SMG neurons. Previous studies have shown that release of neurotransmitter from sympathetic varicosities within the viscera is dependent on N-type VGCCs, and is inhibited by ω-conotoxin GVIA (Wright & Angus, 1996; Smith & Cunnane, 1997; Waterman, 1997; Serone & Angus, 1999; Tanaka et al. 1999; Brock & Cunnane, 1999; Motagally et al. 2009). Taken together, this suggests that although L-type channels are present in the cell body of SMG neurons, they may not be involved in synaptic vesicle release.
TNFα and inflammation
Tumour necrosis factor α is a multifunctional proinflammatory cytokine produced by many cell types, including macrophages, lymphocytes, monocytes and fibroblasts. It initiates cellular responses including apoptosis, cell proliferation, differentiation and angiogenesis depending on the target cell type and the receptors expressed. Infliximab, a monoclonal antibody against TNFα, has shown promising results in treating both Crohn's disease and rheumatoid arthritis, highlighting the importance of this cytokine in chronic inflammation (Sandborn & Hanauer, 1999; Lipsky et al. 2000). Prevertebral ganglia have fenestrated capillaries, similar to the circumventricular organs. Consequently, resident neurons are exposed to higher levels of circulating cytokines than many brain regions and other sympathetic ganglia, including the superior cervical ganglion (Baker et al. 1989; Szurszewski & Miller, 1994; Chau & Lu, 1995). Therefore, it seems likely that the TNFα-induced decrease in N-type Ca2+ channels in SMG neurons observed in the present study might occur during many inflammatory diseases. Indeed, a recent study from our laboratory reported a selective decrease in SMG neuron N-type current during DSS-induced colitis in mice, which led to a reduction in noradrenaline release from sympathetic varicosities (Motagally et al. 2009). Our current findings of ICa reduction are consistent with a recent study by Czeschik et al. (2008) of rat dorsal root ganglion (DRG) neurons which found that acute exposure to TNFα inhibited ICa. In contrast, other authors have reported that acute application of TNFα caused facilitation of transmitter release from vagal afferent terminals in the rat nucleus of the solitary tract (Rogers et al. 2006). Together, these data suggest that TNFα can have differing effects on neuronal Ca2+ signalling, depending on the neurons involved and the time course of exposure to the cytokine. Other inflammation-associated molecules have also been shown to modulate ICa: prostaglandin E2 inhibited ICa in dissociated trigeminal neurons (Borgland et al. 2002) and TRPV1 activation on DRG neurons downregulated ICa (Wu et al. 2005).
An important issue to consider is whether the inhibition of ICa in SMG neurons is due to direct effects of the cytokine on neurons or whether glial cells may play a role. Enteric glia have previously been found to play important roles in regulating gastrointestinal inflammation, and release neurotrophic factors in response to cytokines (Cabarrocas et al. 2003; Sharkey et al. 2004; von Boyen et al. 2006; Savidge et al. 2007). However, it is unknown whether glia in prevertebral ganglia play similar roles. Our finding that depleting sympathetic glia by ara-C treatment did not affect the inhibition of ICa by TNFα suggests that glial cells do not participate in this phenomenon.
Mechanism of VGCC inhibition by TNFα
One of the better characterized signalling pathways that is initiated following activation of TNFR1, the receptor for soluble TNFα (Grell et al. 1995), is the NF-κB pathway (Perkins, 2007). In the absence of stimulation, NF-κB is maintained in an inactive state by inhibitory IκB proteins. Following cytokine stimulation, IκB proteins are phosphorylated by IKK-2 which leads to ubiquitination and eventual degradation of IκB. This releases NF-κB, which translocates to the nucleus and influences gene transcription. The present study is, to our knowledge, the first to document changes in N-type Ca2+ channels in neurons due to NF-κB signalling. Interestingly, it has recently been reported that TNFα-mediated NF-κB signalling can induce N-type channel mRNA expression in CD4+ve T-cells (Lee et al. 2008). Several other cytokines signal through NF-κB pathways (Perkins, 2007), suggesting that the effects on VGCCs observed in the present study may be a redundant response to NF-κB activation in SMG neurons.
Current through N-type Ca2+ channels is regulated by a variety of means, including protein–protein interactions (Jarvis & Zamponi, 2007; Davies et al. 2007; Catterall & Few, 2008). Following G-protein-coupled receptor activation, βγ subunits of the receptor complex can bind to N-type channel subunits, resulting in decreased current (Zamponi & Snutch, 1998). We examined whether this mechanism accounted for TNFα-induced current inhibition by examining the effect of depolarizing neurons to +150 mV before measuring ICa and found no difference between control and TNFα-treated neurons. This strongly suggests that interaction of G-protein βγ subunits with N-type channels does not underlie the inhibition of ICa by TNFα.
It appears that activation of NF-κB signalling is essential for the reduction in ICa following TNFα exposure (Figs 4 and 5). As the p65 subunit of NF-κB is a potent transcription factor, we used real-time PCR to measure mRNA of the gene encoding the α subunit of N-type VGCCs (CaV2.2) and examined whether decreased CaV2.2 transcription and/or decreased CaV2.2 mRNA stability was responsible for the decrease in Ca2+ current. We found a significant reduction in mRNA encoding CaV2.2 following TNFα treatment. In addition, immunohistochemical analysis of channel α subunit localization revealed that TNFα caused a redistribution of channel from the membrane to the cytoplasm, which may account for the marked decrease of N-type current in cells treated with TNFα. Therefore, it seems plausible that NF-κB-mediated effects on the expression of genes encoding Ca2+ channel pore-forming subunits and those that regulate channel trafficking and function, such as syntaxin and CASK (Catterall et al. 2006; Jarvis & Zamponi, 2007; Catterall & Few, 2008), may underlie the reduction in current amplitude in neurons incubated in TNFα.
Potential significance
The present study reveals that TNFα selectively inhibits N-type channels and is the first demonstration of NF-κB regulation of these channels. If the inhibition of N-type channels at the cell bodies also occurs in sympathetic nerve terminals, this would drastically reduce the release of sympathetic neurotransmitters, including noradrenaline, ATP and neuropeptide Y. Evidence in support of this extrapolation comes from studies of noradrenaline release in models of inflammatory bowel disease that are associated with elevated levels of TNFα. We recently found that DSS colitis reduced noradrenaline release in the colon due to inhibition of N-type VGCCs in sympathetic neurons (Motagally et al. 2009). Given the similarity of the effects of colitis and overnight exposure to cytokines on N-type channels in SMG neurons, it seems likely that similar intracellular mechanisms underlie each. It will be important to determine whether inhibition of neuronal NF-κB signalling reverses the inhibition of ICa during inflammation and restores normal sympathetic regulation.
Acknowledgments
This work was supported by grants from the Crohn's and Colitis Foundation of Canada, the Canadian Institutes of Health Research (CIHR) and the Canadian Association of Gastroenterology to A.E.L., M.A.M. and S.P.C. were recipients of graduate scholarships from a Canadian Institutes of Health Research training grant in digestive sciences. We are grateful to Paul Bertrand, Lysa Boissé and Ian Spreadbury for their insightful comments on an earlier draft of this manuscript, and to Daniel Poole for advice on confocal image analysis.
References
- Baker DM, Santer RM, Blaggan AS. Morphometric studies on the microvasculature of pre- and paravertebral sympathetic ganglia in the adult and aged rat by light and electron microscopy. J Neurocytol. 1989;18:647–660. doi: 10.1007/BF01187084. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Besirli CG, Deckwerth TL, Crowder RJ, Freeman RS, Johnson EM., Jr Cytosine arabinoside rapidly activates Bax-dependent apoptosis and a delayed Bax-independent death pathway in sympathetic neurons. Cell Death Differ. 2003;10:1045–1058. doi: 10.1038/sj.cdd.4401259. [DOI] [PubMed] [Google Scholar]
- Blandizzi C, Fornai M, Colucci R, Baschiera F, Barbara G, De Giorgio R, De Ponti F, Breschi MC, Del Tacca M. Altered prejunctional modulation of intestinal cholinergic and noradrenergic pathways by α2-adrenoceptors in the presence of experimental colitis. Br J Pharmacol. 2003;139:309–320. doi: 10.1038/sj.bjp.0705249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Ryan RM, Ball HJ, Christie MJ. Prostaglandin E2 inhibits calcium current in two sub-populations of acutely isolated mouse trigeminal sensory neurons. J Physiol. 2002;539:433–444. doi: 10.1113/jphysiol.2001.013322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Effects of Ca2+ concentration and Ca2+ channel blockers on noradrenaline release and purinergic neuroeffector transmission in rat tail artery. Br J Pharmacol. 1999;126:11–18. doi: 10.1038/sj.bjp.0702256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Cabarrocas J, Savidge TC, Liblau RS. Role of enteric glial cells in inflammatory bowel disease. Glia. 2003;41:81–93. doi: 10.1002/glia.10169. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Hulme JT, Jiang X, Few WP. Regulation of sodium and calcium channels by signalling complexes. J Recept Signal Transduct Res. 2006;26:577–598. doi: 10.1080/10799890600915100. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Chau YP, Lu KS. Investigation of the blood-ganglion barrier properties in rat sympathetic ganglia by using lanthanum ion and horseradish peroxidase as tracers. Acta Anat (Basel) 1995;153:135–144. doi: 10.1159/000313647. [DOI] [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-α differentially modulates ion channels of nociceptive neurons. Neurosci Lett. 2008;434:293–298. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Furness JB, Clerc N, Vogalis F, Stebbing MJ. The enteric nervous system and its extrinsic connections. In: Yamada T, Alpers DH, editors. Textbook of Gastroenterology. Lippincot Williams; 2003. pp. 12–34. [Google Scholar]
- Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Jacobson K, McHugh K, Collins SM. The mechanism of altered neural function in a rat model of acute colitis. Gastroenterology. 1997;112:156–162. doi: 10.1016/s0016-5085(97)70230-0. [DOI] [PubMed] [Google Scholar]
- Jarvis SE, Zamponi GW. Trafficking and regulation of neuronal voltage-gated calcium channels. Curr Opin Cell Biol. 2007;19:474–482. doi: 10.1016/j.ceb.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IKM) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. Eur J Neurosci. 1997;9:605–616. doi: 10.1111/j.1460-9568.1997.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Lee LF, Lih CJ, Huang CJ, Cao T, Cohen SN, McDevitt HO. Genomic expression profiling of TNF-α-treated BDC2.5 diabetogenic CD4+ T cells. Proc Natl Acad Sci U S A. 2008;105:10107–10112. doi: 10.1073/pnas.0803336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky PE, Van Der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Infliximab and methotrexate in the treatment of rheumatoid arthritisAnti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Linden DR, Mawe GM, Sharkey KA. Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci. 2006;126–127:250–257. doi: 10.1016/j.autneu.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Miolan JP, Niel JP. The mammalian sympathetic prevertebral ganglia: integrative properties and role in the nervous control of digestive tract motility. J Auton Nerv Syst. 1996;58:125–138. doi: 10.1016/0165-1838(95)00128-x. [DOI] [PubMed] [Google Scholar]
- Morris JL, Ozols DI, Lewis RJ, Gibbins IL, Jobling P. Differential involvement of N-type calcium channels in transmitter release from vasoconstrictor and vasodilator neurons. Br J Pharmacol. 2004;141:961–970. doi: 10.1038/sj.bjp.0705712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motagally MA, Neshat S, Lomax AE. Inhibition of sympathetic N-type voltage-gated Ca2+ current underlies the reduction in noradrenaline release during colitis. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00006.2009. DOI 10.1152/ajpgi.00006.2009. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Poole DP, Amadesi S, Rozengurt E, Thacker M, Bunnett NW, Furness JB. Stimulation of the neurokinin 3 receptor activates protein kinase Cɛ and protein kinase D in enteric neurons. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1245–G1256. doi: 10.1152/ajpgi.00521.2007. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Van Meter MJ, Hermann GE. Tumor necrosis factor potentiates central vagal afferent signalling by modulating ryanodine channels. J Neurosci. 2006;26:12642–12646. doi: 10.1523/JNEUROSCI.3530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Schorge S, Gupta S, Lin Z, McEnery MW, Lipscombe D. Calcium channel activation stabilizes a neuronal calcium channel mRNA. Nat Neurosci. 1999;2:785–790. doi: 10.1038/12153. [DOI] [PubMed] [Google Scholar]
- Serone AP, Angus JA. Role of N-type calcium channels in autonomic neurotransmission in guinea-pig isolated left atria. Br J Pharmacol. 1999;127:927–934. doi: 10.1038/sj.bjp.0702629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KA, Nasser Y, Ruhl A. Enteric glia. Gut. 2004;53:1390. [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Cunnane TC. Multiple calcium channels control neurotransmitter release from rat postganglionic sympathetic nerve terminals. J Physiol. 1997;499:341–349. doi: 10.1113/jphysiol.1997.sp021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain MG, Blennerhassett PA, Collins SM. Impaired sympathetic nerve function in the inflamed rat intestine. Gastroenterology. 1991;100:675–682. doi: 10.1016/0016-5085(91)80011-w. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH, Miller SM. Physiology of prevertebral ganglia. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 795–877. [Google Scholar]
- Tanaka Y, Mochizuki Y, Tanaka H, Shigenobu K. Significant role of neuronal non-N-type calcium channels in the sympathetic neurogenic contraction of rat mesenteric artery. Br J Pharmacol. 1999;128:1602–1608. doi: 10.1038/sj.bjp.0702954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- von Boyen GB, Steinkamp M, Geerling I, Reinshagen M, Schafer KH, Adler G, Kirsch J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: a key to the regulation of epithelial apoptosis in Crohn's disease. Inflamm Bowel Dis. 2006;12:346–354. doi: 10.1097/01.MIB.0000219350.72483.44. [DOI] [PubMed] [Google Scholar]
- Waterman SA. Role of N-, P- and Q-type voltage-gated calcium channels in transmitter release from sympathetic neurones in the mouse isolated vas deferens. Br J Pharmacol. 1997;120:393–398. doi: 10.1038/sj.bjp.0700948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CE, Angus JA. Effects of N-, P- and Q-type neuronal calcium channel antagonists on mammalian peripheral neurotransmission. Br J Pharmacol. 1996;119:49–56. doi: 10.1111/j.1476-5381.1996.tb15676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J Biol Chem. 2005;280:18142–18151. doi: 10.1074/jbc.M501229200. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gβ subunit. Proc Natl Acad Sci U S A. 1998;95:4035–4039. doi: 10.1073/pnas.95.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]