Abstract
Postnatal early overnutrition (EO) is a risk factor for obesity in adult life. Rats raised in a small litter can develop hyperinsulinaemia, hyperphagia, hyperleptinaemia and hypertension as adults. Since leptin regulates the hypothalamic–pituitary–thyroid axis and the metabolism of thyroid hormones, we studied the leptin signalling pathway in pituitary and thyroid glands of the postnatal EO model. To induce EO, at the third day of lactation the litter size was reduced to three pups per litter (SL group). In control litters (NL group), the litter size was adjusted to 10 pups per litter. Body weight and food intake were monitored. Rat offspring were killed at 21 (weaning) and 180 days old (adulthood). Plasma thyroid hormones, thyroid-stimulating hormone (TSH) and leptin were measured by radioimmunoassay. Proteins of the leptin signalling pathway were analysed by Western blotting. Body weight of offspring in the SL group was higher from the seventh day of lactation (+33%, P < 0.05) until 180 days old (+18%, P < 0.05). Offspring in the SL group showed higher visceral fat mass at 21 and 180 days old (+176 and +52%, respectively, P < 0.05), but plasma leptin was higher only at 21 days (+88%, P < 0.05). The SL offspring showed higher plasma TSH, 3,5,3′-triiodothronine (T3) and thyroxine (T4) at 21 days (+60, +91 and +68%, respectively, P < 0.05), while the opposite was observed at 180 days regarding thyroid hormones (T3, −10%; and T4, −30%, P < 0.05), with no difference in TSH levels. In hypothalamus, no change was observed in the leptin signalling pathway at 21 days. However, lower janus thyrosine kinase 2 (JAK2) and phosphorilated-signal transducer and activator of transcription-3 (p-STAT3) content were detected in adulthood. In pituitary, the SL group presented higher leptin receptors (Ob-R), JAK2 and p-STAT3 content at 21 days and lower JAK2 and STAT3 content at 180 days old. In contrast, in thyroid, the Ob-R expression was lower in young SL rats, while the adult SL group presented higher Ob-R and JAK2 content. We showed that postnatal EO induces short- and long-term effects upon the hypothalamic–pituitary–thyroid axis. These changes may help to explain future development of metabolic and endocrine dysfunctions, such as metabolic syndrome and hypothyroidism.
It has been shown that adverse situations during lactation, such as malnutrition and hormonal changes, could permanently affect the nutritional and hormonal status of the progeny (Passos et al. 2004; Bonomo et al. 2007; Dutra et al. 2007; Fagundes et al. 2007; Trevenzoli, 2007). The prevalence of childhood obesity is increasing worldwide at alarming rates (Hedley et al. 2004). Besides genetic factors, epigenetic environmental factors, such as dietary intake, can contribute to development of obesity. This association has been termed metabolic imprinting or programming, which is defined as a biological phenomenon that determines the association between physical and chemical stimuli in early life and future functional status (Lucas, 1994; Barker, 2004; de Moura et al. 2008).
Overnutrition during early postnatal life represents a risk factor for persistent obesity and associated metabolic and cardiovascular disturbances. It has been shown that animals raised in small litters (SL) have an accelerated body weight gain before weaning, which is associated with permanent modulation of adiposity and hypothalamic circuits that control food intake and energy balance in adulthood (Faust et al. 1980; Plagemann et al. 1999a,b). Some experimental studies have reported that neonatal overfeeding causes persistent hyperphagia, obesity, elevated serum triacylglycerols, increased systolic blood pressure and hyperinsulinaemia in later life (You et al. 1990; Plagemann et al. 1992, 1999c; Paramore et al. 1999; Boullu-Ciocca et al. 2005; Davidowa & Plagemann, 2007; López et al. 2007; Rodrigues et al. 2007).
Leptin is a hormone secreted by adipose tissue, which reduces food intake and increases energy expenditure via specific hypothalamic signals, maintaining the body weight homeostasis (Pelleymounter et al. 1995; Schwartz et al. 1996; Friedman & Halaas, 1998). Leptin acts through the leptin receptors (Ob-R), which belong to the cytokine receptor class I superfamily. Five alternatively spliced isoforms of Ob-R (a, b, c, d and e) with different lengths of C-termini have been identified in mice (Lee et al. 1996). The long form (Ob-Rb) and the short form (Ob-Ra) are the most studied isoforms, and Ob-Rb is fully capable of activing intracellular signalling (Sahu, 2004). Leptin action mediated by leptin receptors involves multiple pathways, such as the janus thyrosine kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) pathway, which is the best characterized leptin signalling pathway. Leptin binding to the Ob-Rb initiates tyrosine phosphorylation by JAK2. Phosphorylated Ob-Rb recruits STAT3 proteins, which are activated through phosphorylation by JAK2 (Vaisse et al. 1996). The activated STAT3 proteins dimerize and translocate to the nucleus, stimulating gene transcription (Hekerman et al. 2005). The JAK2–STAT3 pathway stimulates transcription of suppressor of cytokine signalling 3 (SOCS3), a negative regulator of leptin signalling following Ob-Rb activation (Zhang et al. 1994; Vaisse et al. 1996).
It has been revealed that various peripheral organs have detectable levels of mRNA encoding the Ob-Rb, supporting the view that leptin has many peripheral actions, including suppression of insulin secretion, stimulation of cytokine production and control of development of reproductive organs (Muoio & Lynis Dohm, 2002). Leptin also has a well-known stimulatory role in thyroid function, mainly increasing thyrotrophin-releasing hormone (TRH) expression in the hypothalamus (Legradi et al. 1997). The detection of Ob-R in human (Jin et al. 1999, Knerr et al. 2001) and rodent pituitary (Jin et al. 2000; Sone et al. 2001; Vicente et al. 2004) reinforces its direct action on this gland, suggesting that leptin may act as a regulator of thyroid-stimulating hormone (TSH) release. In addition, Nowak et al. (2002) showed that rat thyroids express the Ob-Rb and the gland function is stimulated by leptin in euthyroid non-fasted female rats. Other studies have shown that leptin regulates thyroid function, but the investigations of the possible effects of leptin in this tissue are rather limited and controversial (Orban et al. 1998; Pinkney et al. 1998; Isozaki et al. 2004; de Oliveira et al. 2007). Initially, leptin was viewed as a hormone designed to prevent obesity, but several studies now suggest that leptin signals the switch from fed to starved state (Baskin et al. 2000; Flier et al. 2000), adapting the organism to a more economic energy expenditure. Disturbance of thyroid function is associated with marked changes in both energy expenditure and body weight, and it seems that leptin and thyroid hormones play mutual roles.
In obesity hyperleptinaemia and leptin resistance have been reported; therefore, it is interesting also to evaluate the peripheral action of leptin, since it was already demonstrated a dissociate effect for the sympathetic nervous system (Mark et al. 2004). Here, we investigated the short- and long-term effects of postnatal early overnutrition (EO) on thyroid gland function, through the evaluation of plasma thyroid hormones and TSH. We also evaluated the plasma leptin levels and the proteins of the leptin signalling pathway in the hypothalamic–pituitary–thyroid (HPT) axis of young and adult animals raised in small litters.
Methods
Experimental model
Wistar rats were housed in a room maintained at 23–25°C with a 12 h–12 h light–dark cycle. Three-month-old, virgin female rats were caged with male rats at a ratio of 3:1. During pregnancy and lactation, mothers were housed in individual cages and were provided with water ad libitum and standard pellet diet (commercial control diets for rats). To induce postnatal early overnutrition (EO), 3 days after birth, the litter size was adjusted to three male rats per litter (small litter; SL group; Plagemann et al. 1992; Rodrigues et al. 2007). In control litters, the litter size was adjusted to 10 pups per litter (normal litter; NL group). After weaning (day 21), the SL and NL pups received a commercial diet. The number of animals analysed in our study was 12 per group per period studied from 16 different litters (8 SL litters and 8 NL litters). Body weight gain was measured throughout life. Food intake was measured by determination of 24 h consumption of standard pellets from 30 to 180 days old. Both groups of offspring were killed when they were 21 or 180 days old, and we collected blood, hypothalamus, pituitary gland, thyroid gland and visceral fat mass. Animals were exsanguinated after anesthesia with pentobarbital. The use of the animals according our experimental design was approved by the Animal Care and Use Committee of the Biology Institute of the State University of Rio de Janeiro (CEA/184/2007), which based their analysis on the principles described in the Guide for the Care and Use of Laboratory Animals (Bayne, 1996).
Body fat and protein mass
At the day of killing, visceral fat mass was excised (mesenteric, epididymal and retroperitoneal white adipose tissue) and immediately weighed for evaluation of central adiposity (Toste et al. 2006b).
Fat and protein mass were determined by carcass analysis as reported previously (Fagundes et al. 2007). After killing, the offspring were eviscerated, carcasses were weighed, autoclaved for 1 h and homogenized in distilled water (1:1 w/v). Samples of the homogenate were stored at 4°C for analysis.
Three grams of homogenate were used to determine fat mass gravimetrically. Samples were hydrolysed in a shaking water bath at 70°C for 2 h with 30% KOH and ethanol. Total fatty acids and free cholesterol were removed by three successive washings with petroleum ether. After drying overnight in a vacuum, all tubes were weighed and data expressed as grams of fat per 100 g carcass.
Protein mass was determined in 1 g homogenate. Tubes were centrifuged at 2000g for 10 min. Total protein concentrations were determined by the method of Lowry et al. (1951). Results were expressed as grams of protein per 100 g carcass.
Western blotting analysis
To obtain the cell extracts, the hypothalamus, pituitary and thyroid were homogenized in ice-cold lysis buffer (50 mm Hepes, pH 6.4, 1 mm MgCl2, 10 mm EDTA and 1% Triton X-100) containing the following protease inhibitors: 10 μg/μl aprotinin, 10 μg/μl leupeptin, 2 μg/μl pepstatin and 1 mm phenylmethylsuphonic fluoride (Sigma-Aldrich, St Louis, MO, USA). The Ob-R, JAK2, STAT3 and p-STAT3 content was analysed by Western blotting, using actin as internal control.
Total protein content in the homogenate was determined (Bradford, 1976), and the cell lysates were denatured in sample buffer (50 mm Tris–HCl, pH 6.8, 1% SDS, 5% 2-mercaptoethanol, 10% glycerol and 0.001% bromophenol blue) and heated at 95°C for 5 min. Samples (50 μg total protein) were carried to 8, 10 or 12% SDS-PAGE, according to the molecular weight of each protein, and transferred to polyvinylidene filters (PVDF Hybond-P, Amersham Pharmacia Biotech, New Jersey, NJ, USA). Rainbow markers (Amersham Biosciences, Uppsala, Sweden) were run in parallel to estimate molecular weights. Membranes were blocked with 5% non-fat milk in the Tween–Tris-buffered saline (Tween–TBS; 20 mm Tris–HCl, pH 7.5, 500 mm NaCl and 0.1% Tween-20). Primary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) used were anti-Ob-R (1:5000); anti-JAK2 (1:500); anti-STAT3 (1:500); anti-p-STAT3 (1:500); and anti-actin (1:500). The PVDF filters were washed three times with Tween–TBS, followed by 1 h incubation with appropriate secondary antibody conjugated to biotin (Santa Cruz Biotechnology, Inc.). Then, filters were incubated with streptavidin-conjugated horseradish peroxidase (Caltag Laboratories, Burlingame, CA, USA). All Western blots were allowed to react with horseradish peroxidase substrate (ECL-plus; Amersham Pharmacia Biotech) and then exposed to X-ray film for between 10 s and 30 min. Images were scanned, and the bands were quantified by densitometry, using ImageJ 1.34s software (Wayne Rasband National Institute of Health, Boston, MA, USA).
Measurement of plasma hormones
Blood was obtained, just before sacrifice, by cardiac puncture and then quickly centrifuged (1000g, 4°C, 30 min) and plasma stored at −20°C until assayed. All measurements were performed in a unique assay. Plasma leptin concentration was determined by radioimmunoassay (RIA) kit (Linco Research, St Charles, MO, USA). Sensitivity limit and intra-assay variation were 0.5 ng ml−1 and 6.9%, respectively. Plasma total 3,5,3′-triiodothronine (TT3) and free thyroxine (FT4) were measured by RIA (ICN Pharmaceuticals, Inc., Los Angeles, CA, USA). Sensitivity limits were 25 ng dl−1 for T3 and 0.3 ng dl−1 for T4, and intra-assay variation was 3.6 and 7.5%, respectively. Plasma TSH was measured by specific RIA, using reagents supplied by the National Institute of Diabetes and Digestive and Kidney Diseases (NIH, Bethesda, MD, USA). Sensitivity limit was 0.18 ng ml−1, and intra-assay variation was 6.1%.
Statistical analysis
Data are reported as means ±s.e.m. GraphPad Prism 4 program was used for statistical analyses and graphics. Two-way ANOVA and Bonferroni's test were used to analyse body weight and food intake evolutions. A non-parametric test (Mann–Whitney U test) was used for plasma TSH. The other experimental observations were analysed by Student's unpaired t test, with significance level set at P < 0.05.
Results
Postnatal EO induces early-onset obesity, persistent hyperphagia and overweight in adult rats
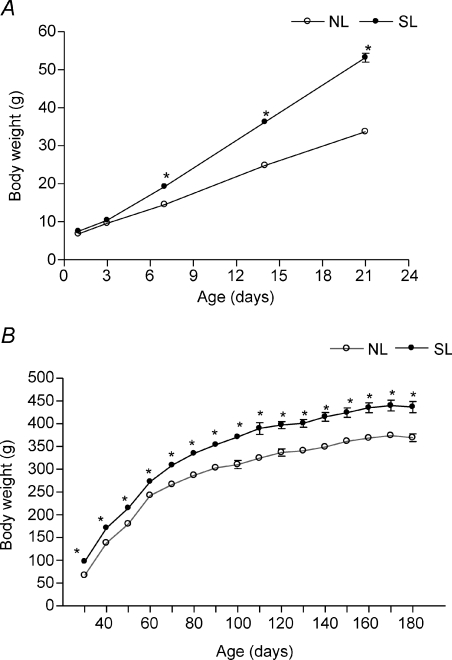
Body weight evolution from birth until adulthood is shown in Fig. 1. Rat offspring raised in small litters had a significantly higher body weight gain when they were 7 days old compared with control animals (+33%), 56% higher at 21 days old and 18% higher at 180 days old.
Figure 1. Postnatal EO induces early-onset obesity and overweight in adulthood.
Body weight was evaluated in NL (•; n= 12) and SL groups (○; n= 12) during lactaion (A) and after weaning (B). Results are expressed as means ±s.e.m.*P < 0.05.
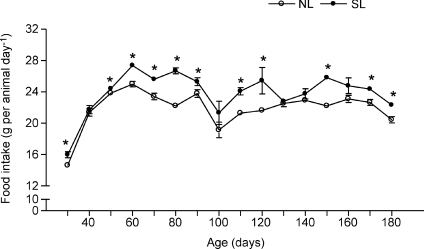
Figure 2 shows that SL rats presented higher daily relative food intake from weaning until adulthood (approximately 10%).
Figure 2. Postnatal EO induces persistent hyperphagia.
Mean food intake was evaluated in NL (•; n= 12) and SL groups (○; n= 12) during development. Results are expressed as means ±s.e.m.*P < 0.05.
Postnatal EO alters the fat and lean mass in both young and adult rats
Total and visceral fat mass were higher in both young (+237 and +176%, respectively, P < 0.05; Table 1) and old animals (+38 and + 52%, respectively, P < 0.05; Table 1). In contrast, postnatal EO induced an increase in protein mass in young rats, while a decrease was observed at adulthood (+41 and −14%, respectively, P < 0.05; Table 1).
Table 1.
Body composition and plasma hormone levels of young and adult rats
| 21 days |
180 days |
|||
|---|---|---|---|---|
| NL | SL | NL | SL | |
| Total fat mass (%) | 3.65 ± 0.27 | 12.32 ± 0.62* | 7.57 ± 0.50 | 10.50 ± 1.09* |
| Visceral fat mass (%) | 0.39 ± 0.05 | 1.08 ± 0.12* | 2.01 ± 0.17 | 3.06 ± 0.41* |
| Protein mass (%) | 5.82 ± 0.35 | 8.22 ± 0.45* | 6.88 ± 0.28 | 5.93 ± 0.13* |
| Leptin (ng ml−1) | 0.99 ± 0.23 | 1.88 ± 0.09* | 0.99 ± 0.10 | 1.06 ± 0.08 |
| TT3 (ng dl−1) | 48.21 ± 2.93 | 92.36 ± 3.48* | 53.07 ± 1.07 | 48.17 ± 2.07* |
| FT4 (ng dl−1) | 0.94 ± 0.05 | 1.58 ± 0.14* | 2.73 ± 0.15 | 1.92 ± 0.19* |
| TSH (ng ml−1) | 0.78 ± 0.09 | 1.26 ± 0.09* | 2.84 ± 0.37 | 2.76 ± 0.35 |
Results are expressed as means ±s.e.m. of 12 rats per group.
P < 0.05.
Thyroid gland mass in young and adult NL and SL rats
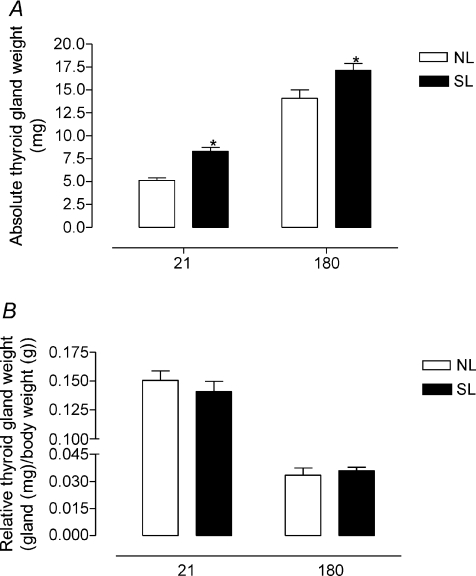
The SL rats presented had greater absolute thyroid gland mass at both time points (+62% at 21 days and +21% at 180 days, P < 0.05, Fig. 3A); however, no change was detected between groups in the relative thyroid mass (Fig. 3B).
Figure 3. Effect of postnatal EO on thyroid gland weight of young and adult NL and SL rats.
Thyroid weight was evaluated in NL (filled bars; n= 12) and SL groups (open bars; n= 12) at 21 and 180 days old. The figure shows the absolute (A) and relative thyroid weight (B). Results are expressed as means +s.e.m.*P < 0.05.
Plasma hormone concentrations
Animals raised in small litters had higher plasma leptin values when they were 21 days old (+88%, P < 0.05); however, no difference was observed when they were 180 days old. The SL offspring showed higher plasma T3 and T4 at weaning (+91 and +68%, respectively, P < 0.05), while the opposite profile was observed in adulthood (−10 and −30%, respectively, P < 0.05). Plasma TSH was higher (+62%, P < 0.05) in 21-day-old SL rats, and no difference was observed in adult rats (Table 1).
Leptin signalling pathway in the HPT axis
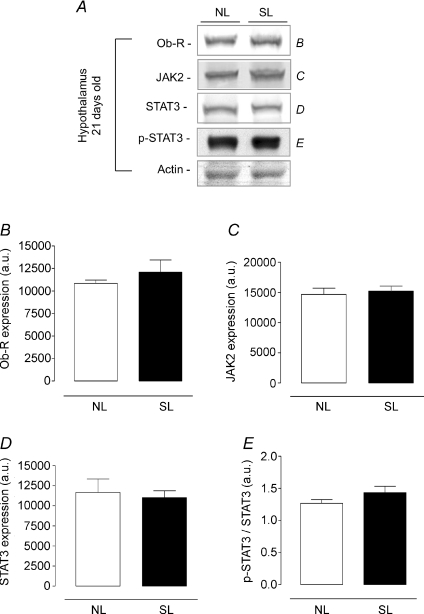
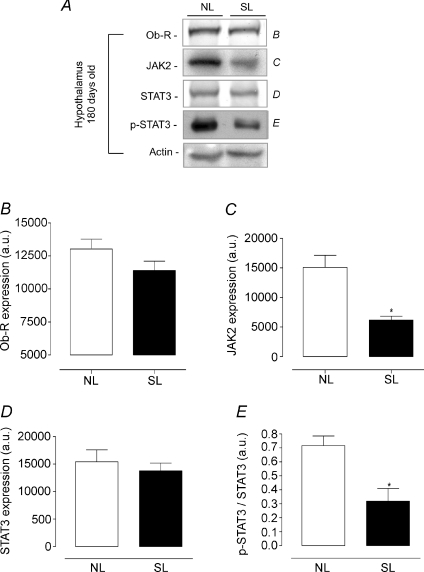
Figures 4 and 5 show the content of leptin signalling proteins (Ob-R, JAK2, STAT3 and p-STAT3) in the hypothalamus from NL and SL groups as assessed by Western blotting technique. At 21 days old, no change was detected in leptin signalling proteins (Fig. 4). However, 180-day-old SL animals had a lower hypothalamic JAK2 content (−59%, P < 0.05, Fig. 5A and C) and a decrease in STAT3 phosphorylation (−53%, P < 0.05, Fig. 5A and E) with no alteration in Ob-R and STAT3 expression (Fig. 5A, B and D).
Figure 4. Effect of postnatal EO on expression of proteins of the leptin signalling pathway in the hypothalamus of young rats (21 days).
Homogenates of hypothalamus from SL and NL groups were obtained, detection of Ob-R (B; n= 7), JAK2 (C; n= 7), STAT3 (D; n= 7) and p-STAT3 (E; n= 7) was done by Western blotting. The bands in A represent the expression of proteins in hypothalamus homogenate from individual NL and SL animals. The Ob-R, JAK2, STAT3 and p-STAT3 contents were quantified by scanning densitometry of the bands (expressed in arbitrary units, a.u.). Actin was loaded as a control. Results are expressed as means +s.e.m.*P < 0.05. A representative experiment is shown from three independent experiments.
Figure 5. Effect of postnatal EO on expression of proteins of the leptin signalling pathway in in hypothalamus of adult rats (180 days).
Homogenates of hypothalamus from SL and NL groups were obtained, and detection of Ob-R (B; n= 7), JAK2 (C; n= 7), STAT3 (D; n= 7) and p-STAT3 (E; n= 7) was done by Western blotting. The bands in A represent the expression of proteins in hypothalamus homogenate from individual NL and SL animals. The Ob-R, JAK2, STAT3 and p-STAT3 contents were quantified by scanning densitometry of the bands. Actin was loaded as a control. Results are expressed as means +s.e.m.*P < 0.05. A representative experiment is shown from three independent experiments.
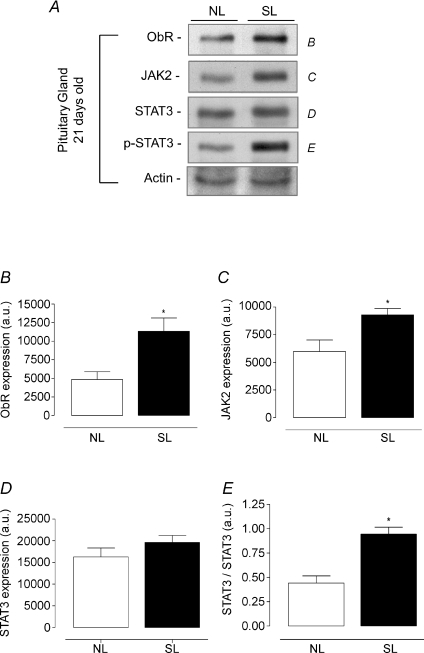
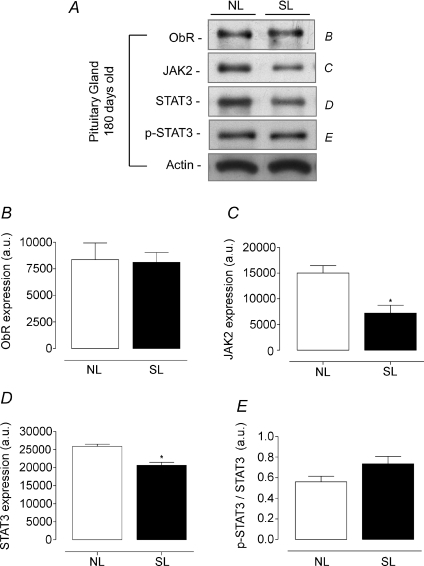
In the pituitary gland, young SL animals had a higher Ob-R and JAK2 content (+134 and +55%, respectively, P < 0.05, Fig. 6A, B and C) and consequently higher STAT3 phosphorylation (+110%, P < 0.05, Fig. 6A and E). In adulthood, we observed an opposite profile, with lower JAK2 and STAT3 content (−52 and −20%, respectively, P < 0.05, Fig. 7A, C and D). However, no change was detected in STAT3 phosphorylation in pituitary from SL adult animals (Fig. 7A and E).
Figure 6. Effect of postnatal EO on expression of proteins of the leptin signalling pathway in pituitary of young rats (21 days).
Homogenates of pituitary from SL and NL groups were obtained, and detection of Ob-R (B; n= 6), JAK2 (C; n= 6), STAT3 (D; n= 6) and p-STAT3 (E; n= 6) was done by Western blotting. The bands in A represent the expression of proteins in pituitary homogenate from individual NL and SL animals. The Ob-R, JAK2, STAT3 and p-STAT3 contents were quantified by scanning densitometry of the bands. Actin was loaded as a control. Results are expressed as means +s.e.m.*P < 0.05. A representative experiment is shown from three independent experiments.
Figure 7. Effect of postnatal EO on expression of proteins of the leptin signalling pathway in pituitary of adult rats (180 days).
Homogenates of pituitary from SL and NL groups were obtained, and detection of Ob-R (B; n= 7), JAK2 (C; n= 7), STAT3 (D; n= 7) and p-STAT3 (E; n= 7) was done by Western blotting. The bands in A represent the expression of proteins in pituitary homogenate from individual NL and SL animals. The Ob-R, JAK2, STAT3 and p-STAT3 content were quantified by scanning densitometry of the bands. Actin was loaded as a control. Results are expressed as means +s.e.m.*P < 0.05. A representative experiment is shown from three independent experiments.
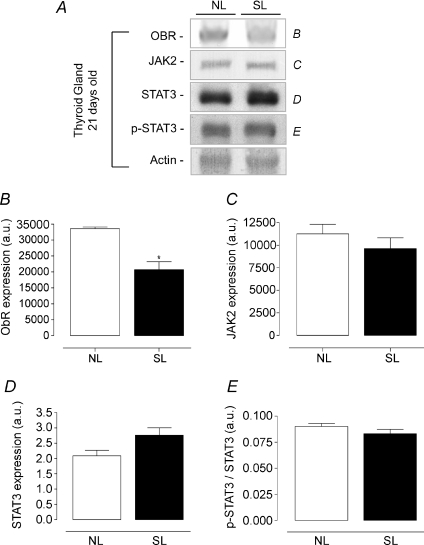
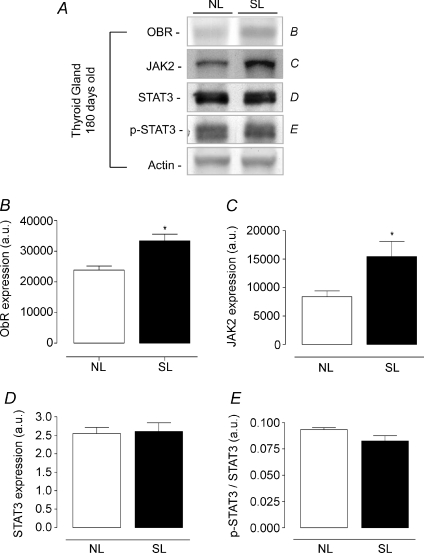
Regarding the leptin signalling proteins in the thyroid gland (Figs 8 and 9), Ob-R expression was lower in SL rats at 21 days old (−38%, P < 0.05, Fig. 8A and B), while at 180 days old, SL rats showed higher Ob-R and JAK2 contents (+40 and + 60%, respectively P < 0.05, Fig. 9A, B and C) when compared with NL animals. No difference was observed in thyroid STAT3 and p-STAT3 content at either studied time point.
Figure 8. Effect of postnatal EO on expression of proteins of the leptin signalling pathway in thyroid of young rats (21 days).
Homogenates of thyroid from SL and NL groups were obtained, and detection of Ob-R (B; n= 6), JAK2 (C; n= 6), STAT3 (D; n= 6) and p-STAT3 (E; n= 6) was done by Western blotting. The bands in A represent the expression of proteins in thyroid homogenate from individual NL and SL animals. The Ob-R, JAK2, STAT3 and p-STAT3 contents were quantified by scanning densitometry of the bands. Actin was loaded as a control. Results are expressed as means +s.e.m.*P < 0.05. A representative experiment is shown from three independent experiments.
Figure 9. Effect of postnatal EO on expression of proteins of the leptin signalling pathway in thyroid of adult rats (180 days).
Homogenates of thyroid from SL and NL groups were obtained, and detection of Ob-R (B; n= 7), JAK2 (C; n= 7), STAT3 (D; n= 7) and p-STAT3 (E; n= 7) was done by Western blotting. The bands in A represent the expression of proteins in thyroid homogenate from individual NL and SL animals. The Ob-R, JAK2, STAT3 and p-STAT3 contents were quantified by scanning densitometry of the bands. Actin was loaded as a control. Results are expressed as means +s.e.m.*P < 0.05. A representative experiment is shown from three independent experiments.
Discussion
In the present study, we observed that postnatal EO induced by small litter size causes a dramatic increase in body weight gain during lactation and programmes for overweight and persistent hyperphagia in later life. These findings are in accordance with earlier studies (Faust et al. 1980; Plagemann et al. 1992, 1997; Velkoska et al. 2005; Davidowa & Plagemann, 2007; Rodrigues et al. 2007). Interestingly, adult SL animals presented a downregulation of the leptin signalling pathway in the hypothalamus, characterizing a central leptin resistance. Leptin resistance has been well established in obesity (Sahu, 2002, 2003; Sandoval & Davis, 2003) and could explain the persistent hyperphagia observed in animals raised in small litters. In this study, we also showed that rats overfed in early life presented higher central adiposity at 21 and 180 days. Moreover, lower protein mass observed in adulthood indicates that the increased body weight is mainly due to a higher body fat mass. The adipose tissue distribution in obese subjects is important because the regional fat deposition confers different levels of cardiovascular risk (Grassi et al. 2004; Velkoska et al. 2005; Hamdy et al. 2006; Mathieu et al. 2008). Therefore, our findings suggest that neonatal obesity could be a risk factor for cardiovascular disease in later life, considering the higher visceral adiposity.
There are scarce data concerning the programming effect in females. Some programming models showed a sex-specific effect (Moura et al. 2002; Lu et al. 2007; Darnaudéry & Maccari, 2008). In the EO model, we found one only paper, by Basset & Craig (1998), which discusses this gender effect. However, generally in programming experiments the utilization of both males and females in the same experiment could be a confounding factor; for example, males can ingest more milk than females.
Recently, Xiao et al. (2007) showed lower uncoupling protein 1 (UCP1) expression in brown adipose tissue (BAT) from adult rats raised in small litters. Impaired BAT thermogenesis has been reported in genetic models of obesity (Commins et al. 1999; Masaki et al. 2000); conversely, mice overexpressing UCP1 are obesity resistant (Kopecky et al. 1995). Therefore, lower UCP1 in BAT reported previously in adult early overfed animals suggests reduced energy expenditure and consequently increased body weight in this experimental model (Xiao et al. 2007). However, those authors did not evaluate the thyroid function.
Corroborating previous studies, animals raised in small litters presented hyperleptinaemia at weaning (Plagemann et al. 1999c; Sanchez et al. 2003). However, no change was detected in plasma leptin of SL offspring at 180 days old. Two studies (Velkoska et al. 2005; López et al. 2007) showed that postnatal EO causes hyperleptinaemia until 60 days, and one of them (Velkoska et al. 2005) reported that this change was not observed in SL animals when they were 120 days old. To the best of our knowledge, there are no reports of leptin levels at 180 days old in animals raised in small litters. However, leptin is secreted mainly by the subcutaneous adipose tissue and here we showed an increase in only the visceral fat mass. Since most of the fat in adult rats is in the subcutaneous tissue, our data suggest that SL adult rats do not present increased subcutaneous fat mass when compared with control animals.
Thyroid gland can be regulated by nutritional status (Moura et al. 1987; Flier et al. 2000). Here we showed that nutritional changes in early life alter thyroid function during development. Animals raised in small litters presented higher plasma T3, T4 and TSH at weaning, while the opposite profile was detected at 180 days. Interestingly, some studies reported the same profile of TH and TSH levels in obese children (Zheng et al. 1996; Kopelman, 2000; Stichel et al. 2000). In obese children from China, higher T3 was positively correlated with body fat mass (Zheng et al. 1996). Similarly, other two studies showed a positive association between increased T3 and TSH levels and the degree of overweight in childhood (Kiortsis et al. 1999; Stichel et al. 2000). It has been reported that altered TSH and T3 in obese children can be normalized after weight reduction (Kiortsis et al. 1999), reinforcing the view that obesity alters thyroid function, possibly through the action of leptin. Since leptin is well known to stimulate thyroid function, mainly increasing TRH expression (Legradi et al. 1997), we hypothesized that the higher TSH levels in young SL rats may be due to an increase in leptin action at the hypothalamus. This increase in thyroid function could be physiologically important to counterbalance the higher adiposity induced by early overfeeding.
An association between increased T3 and TSH levels and excess body weight has also been reported in obese human adults (Bray et al. 1973, 1976). In the present study, adult animals raised in small litters were programmed for lower thyroid hormone levels, with normal TSH. In accordance with our data, some studies have also shown no association between TSH and body weight (Rosenbaum et al. 2000; Roti et al. 2000; Tagliaferri et al. 2001; Ritz et al. 2002). Probably, weight- or nutrition-related factors affect the long-term regulation of the HPT axis, but the mechanisms involved are still unclear.
According to some authors (Dussault et al. 1982; Walker and Courtin, 1985; Pracyk et al. 1992; Wilcoxon & Redei, 2004; Moura et al. 2008), neonatal transient hyperthyroidism programmes for future hypothyroidism. In the adult animals, despite the lower thyroid hormone serum levels of SL rats, TSH was inappropriately normal. Thus, it is possible that, after weaning, an increase in thyroid hormone feedback could permanently inhibit the TRH–TSH axis, maybe through the increase in central deiodinase expression and/or activity of SL rats. Also the TSH level of these animals might be only immunologically normal but its bioactivity could be lower, in the case of lower TRH (Gesundheit et al. 1986).
Despite a lack of change in the expression of proteins of the leptin signalling pathway in hypothalamus at 21 days old, the hyperleptinaemia per se can increase TRH production, which consequently stimulates TSH secretion. In contrast, López et al. (2005) showed lower hypothalamic Ob-Rb mRNA expression in EO animals when they were 24 days old. However, in our study we showed the Ob-R protein content that included the Ob-Rb and other isoforms of the leptin receptor, while López et al. (2005) reported the expression of Ob-Rb mRNA. It is possible that the metabolism of this protein can also be altered and, despite the lower synthesis, a reduction in catabolism might have kept the content unchanged. However, although the antibody used in this study was not specific to the long form, it is unlikely that isoforms other than Ob-Rb are present in hypothalamus in significant amounts. Thus, although the results of López et al. (2005) could explain the apparent leptin resistance at this age, probably it is not related to the leptin signalling pathway and could be related to other mechanisms, such as leptin transport through the blood–brain barrier or influences over the peptides regulated by leptin. Moreover, until now, there have been no reports about leptin signalling proteins in hypothalamus from young animals submitted to postnatal EO.
In other models of neonatal programming, our group showed that leptin injections on the first 10 days of life programmed for hyperleptinaemia and hypothyroidism at 30 days old, as well as for hyperleptinaemia and hyperthyroidism at 150 days old (Toste et al. 2006a,b). In addition, these offspring when adult presented lower expression of Ob-Rb in the hypothalamus (Toste et al. 2006b). In our study, we did not show lower hypothalamic Ob-R expression; however, the JAK2 content and STAT3 phosphorylation were reduced, also indicating downregulation of the central leptin action. In earlier studies, we also showed an association between maternal malnutrition during lactation and higher serum thyroid hormone levels in adulthood (Passos et al. 2002; Lisboa et al. 2008). In addition, the malnutrition of the offspring induced by blockage of the maternal serum prolactin at the end of lactation is related to hypothyroidism in adult life (Bonomo et al. 2008). Taken together, these data suggest an association between neonatal nutritional and hormonal status and thyroid function in adult life. In the two models mentioned above, leptin was increased in the neonatal period (Teixeira et al. 2002; Bonomo et al. 2005).
Initially, leptin was viewed as a hormone designed to prevent obesity, but nowadays this concept has been extended because leptin regulates many other systems, including the immune, reproductive and thyroid systems (Karlsson et al. 1997; Jin et al. 1999; Liu et al. 2007). It has been shown that leptin, in vivo, regulates the pituitary gland function, directly stimulating TSH production (Ahima et al. 1996; Seoane et al. 2000; Ortiga-Carvalho et al. 2002). Ortiga-Carvalho et al. (2002) showed that acute leptin injection in rats increased serum TSH levels. In their study, leptin-incubated pituitary glands showed a dose-dependent decrease in TSH release, and the presence of antiserum against leptin resulted in an approximately 40% increase in TSH release. Several studies have demonstrated the presence of Ob-R in pituitary glands from humans (Jin et al. 1999; Knerr et al. 2001) and rats (Jin et al. 2000; Sone et al. 2001; Vicente et al. 2004), suggesting that leptin has direct a effect in pituitary gland to regulate TSH secretion. Therefore, we investigated the content of leptin signalling proteins in pituitary gland from neonatal overfed animals.
At 21 days old, we observed an overexpression of Ob-R and JAK2 in pituitary gland, followed by a higher basal phosphorylation of STAT3 protein. Since young SL animals also showed higher serum leptin and TSH levels, we suggest that leptin could play a direct stimulatory effect upon TSH secretion, higher than in the control group. We observed an opposite profile in adult SL animals, which showed lower JAK2 and STAT3 expression. However, the lower JAK2 and STAT3 content did not alter the basal p-STAT3. Interesting, adult SL rats presented the same profile of leptin signalling pathway in the hypothalamus. We observed a decrease in JAK2 and p-STAT3 content in hypothalamus from adult rats submitted to postnatal EO. Curiously, when adult, the SL animals showed no change in TSH and leptin levels, suggesting that other regulatory factors explain the leptin signalling changes in adulthood. We hypothesized that the lower expression of leptin signalling proteins in hypothalamus and pituitary gland could interfere in the negative feedback of thyroid hormones, which could explain the decrease in thyroid hormone levels without a change in plasma TSH levels in the adult SL group. In addition, it is possible that adult animals submitted to postnatal EO showed either an increase in pituitary sensitivity to thyroid hormones or a decrease in TSH bioactivity.
Several cytokines are produce by adipocytes (Lumeng et al. 2007; Zeyda & Stulnig, 2007; Heilbronn & Campbell, 2008). Interesting, animals raised in small litters have been shown to have a high content of tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in mesenteric adipose tissue at 150 days old (Boullu-Ciocca et al. 2008). Tumour necrosis factor-α has previously been shown to induce formation of reactive oxygen species in a variety of tissues (Goossens et al. 1995; Javesghani et al. 2003). Reactive oxygen species are known to activate nuclear factor-κB (Mercurio & Manning, 1999), which could regulate the expression of leptin signalling proteins in the hypothalamus and pituitary. Moreover, IL-6 and TNF-α have been reported to decrease HPT axis activity and TSH secretion (Torpy et al. 1998; Mönig et al. 1999). Based on these findings, we suggest that TNF-α and IL-6 could be other factors involved in the regulation of the leptin signalling pathway in hypothalamus and pituitary from SL animals.
Thyroid dysfunctions are associated with marked changes in body weight and energy expenditure, and it seems that both thyroid hormones and leptin could play a synergistic role. Several animal and human studies have investigated the association between leptin, thyroid hormones and thyroid dysfunction (Legradi et al. 1997; Seoane et al. 2000; Nowak et al. 2002; Ortiga-Carvalho et al. 2002; Zimmermann-Belsing et al. 2003; Isozaki et al. 2004; de Oliveira et al. 2007; Fagundes et al. 2007). In rodents, but not in humans, leptin receptors have been identified on thyroid cells (Nowak et al. 2002). The function of leptin in peripheral tissues has yet to be determined, but it has been suggested that systemic leptin administration in rats stimulates thyroid gland growth and secretion thyroid hormones by mechanisms involving Ob-R (Nowak et al. 2002). Accordingly, EO animals showed higher absolute thyroid weight and changes in thyroid function when both young and adult. However, when we correlated the thyroid weight with the body weight (relative thyroid weight), no difference was observed between the groups. Thus, the thyroid gland mass is higher in SL offspring, but this increase is proportional to the higher body mass observed in these animals.
In this study, we demonstrated for the first time the content of proteins of the leptin signalling pathway in the thyroid gland. At 21 days old, SL offspring showed lower Ob-R expression in thyroid. Curiously, once again we observed an opposite profile in these animals when they were 180 days old, when there was an overexpression of thyroid Ob-R and JAK2 protein content. This inverse association between thyroid hormone levels and the content of some proteins in the leptin signalling pathway at both time points studied is remarkable. Interestingly, this profile is opposite to that observed in pituitary and thyroid glands. Few studies have shown the expression of thyroid Ob-R (Nowak et al. 2002; Isozaki et al. 2004), and little is known about the regulation of leptin receptors in thyroid gland. Nevertheless, in animals submitted to postnatal EO there are some putative factors, known to be altered in obesity, which may regulate the leptin signalling pathway at the pituitary and/or thyroid level. For example, TNF-α has been reported to inhibit thyroid specific gene expression, such as the Na+–I– symporter (Pekary & Hershman, 1998) and TSH-induced hydrogen peroxide production (Kimura et al. 1997). Thus, it is possible that some factors, such as TNF-α and IL-6, could also interfere with leptin signalling in thyroid cells. Also, TSH, thyroid hormones or leptin could play a role in this process.
Hyperleptinaemia at 21 days could downregulates thyroid Ob-R expression, and higher TSH could have the same effect at this time. However, at 180 days, changes in the leptin signalling pathway could not be explained by TSH or leptin, because their levels were unchanged. Thus, it is possible that thyroid hormones could directly or indirectly regulate the leptin pathway in thyroid, suppressing this pathway at 21 days and failing to suppress it at 180 days. Studies with different models of hyper- and hypothyroidism, using antithyroid drugs, TSH or TRH injection, are necessary to elucidate the role of thyroid hormones in the leptin signalling pathway in thyroid gland.
In general terms, epigenetic mechanisms, such as DNA methylation or histone acetylation–deacetylation, induced by neonatal nutritional, hormonal or environmental factors, may lead to an increased risk of metabolic disorders in the adult offspring (de Moura et al. 2008). Thus, this explanation may help in understanding the mechanism involved in the permanent changes of thyroid function induced by overnutrition during lactation. Since this alteration might make overfed children more susceptible to thyroid disorders in adult life, it deserves epidemiological and prospective studies.
In summary, postnatal EO induces short- and long-term effects upon thyroid function that can directly or indirectly modulate the leptin signalling pathway in the rat HPT axis. Since changes in the leptin signalling pathway seem to be similar in hypothalamus and pituitary gland, and in the opposite direction in thyroid gland, we suggest that different regulatory factors are responsible for these differences in our programming model.
Acknowledgments
The research was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico; CNPq), Co-ordination for the Enhancement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; CAPES) and State of Rio de Janeiro Carlos Chagas Filho Research Foundation (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro; FAPERJ). A. L. Rodrigues is recipient of a CNPq fellowship. All authors are grateful to Mr Carlos Roberto, Miss Monica Moura and Mr Luciano Martins da Silva Santos for technical assistance.
Glossary
Abbreviations
- EO
early overnutrition
- HPT axis
hypothalamic–pituitary–thyroid axis
- IL-6
interleukin-6
- JAK2
janus thyrosine kinase 2
- NL
normal litter
- Ob-R
leptin receptor
- p-STAT3
phosphorilated-signal transducer and activator of transcription-3
- SL
small litter
- STAT3
signal transducer and activator of transcription 3
- T3
3,5,3′-triiodothronine
- T4
thyroxine
- TNF-α
tumour necrosis factor-α
- TRH
thyrtotrophin-releasing factor
- TSH
thyroid-stimulating hormone
References
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Breininger JF, Schwartz MW. SOCS-3 expression in leptin-sensitive neurons of the hypothalamus of fed and fasted rats. Regul Pept. 2000;92:9–15. doi: 10.1016/s0167-0115(00)00143-9. [DOI] [PubMed] [Google Scholar]
- Bassett DR, Craig BW. Influence of early nutrition on growth and adipose tissue characteristics in male and female rats. J Appl Physiol. 1998;64:1249–1256. doi: 10.1152/jappl.1988.64.3.1249. [DOI] [PubMed] [Google Scholar]
- Bayne K. Revised Guide for the Care and Use of Laboratory Animals available. American Physiological Society. Physiologist. 1996;39:208–111. 199. [PubMed] [Google Scholar]
- Bonomo IT, Lisboa PC, Passos MC, Alves SB, Reis AM, de Moura EG. Prolactin inhibition at the end of lactation programs for a central hypothyroidism in adult rat. J Endocrinol. 2008;198:331–337. doi: 10.1677/JOE-07-0505. [DOI] [PubMed] [Google Scholar]
- Bonomo IT, Lisboa PC, Passos MCF, Pazos-Moura CC, Reis AM, Moura EG. Prolactin inhibition in lactating rats changes leptin transfer through the milk. Horm Metab Res. 2005;37:220–225. doi: 10.1055/s-2005-861381. [DOI] [PubMed] [Google Scholar]
- Bonomo IT, Lisboa PC, Pereira AR, Passos MC, de Moura EG. Prolactin inhibition in dams during lactation programs for overweight and leptin resistance in adult offspring. J Endocrinol. 2007;192:339–344. doi: 10.1677/joe.1.06952. [DOI] [PubMed] [Google Scholar]
- Boullu-Ciocca S, Achard V, Tassistro V, Dutour A, Grino M. Postnatal programming of glucocorticoid metabolism in rats modulates high-fat diet-induced regulation of visceral adipose tissue glucocorticoid exposure and sensitivity and adiponectin and proinflammatory adipokines gene expression in adulthood. Diabetes. 2008;57:669–677. doi: 10.2337/db07-1316. [DOI] [PubMed] [Google Scholar]
- Boullu-Ciocca S, Dutour A, Guillaume V, Achard V, Oliver C, Grino M. Postnatal diet-induced obesity in rats upregulates systemic and adipose tissue glucocorticoid metabolism during development and in adulthood: its relationship with the metabolic syndrome. Diabetes. 2005;54:197–203. doi: 10.2337/diabetes.54.1.197. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray GA, Fisher DA, Chopra IJ. Relation of thyroid hormones to body-weight. Lancet. 1976;1:1206–1208. doi: 10.1016/s0140-6736(76)92158-9. (7971) [DOI] [PubMed] [Google Scholar]
- Bray GA, Melvin KE, Chopra IJ. Effect of triiodothyronine on some metabolic responses of obese patients. A J Clin Nutr. 1973;26:715–721. doi: 10.1093/ajcn/26.6.715. [DOI] [PubMed] [Google Scholar]
- Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140:292–300. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- Darnaudéry M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Davidowa H, Plagemann A. Insulin resistance of hypothalamic arcuate neurons in neonatally overfed rats. Neuroreport. 2007;18:521–524. doi: 10.1097/WNR.0b013e32805dfb93. [DOI] [PubMed] [Google Scholar]
- de Moura EG, Lisboa PC, Passos MC. Neonatal programming of neuroimmunomodulation – role of adipocytokines and neuropeptides. Neuroimmunomodulation. 2008;15:176–188. doi: 10.1159/000153422. [DOI] [PubMed] [Google Scholar]
- de Oliveira E, Fagundes ATS, Bonomo IT, Curty FH, Passos MCF, de Moura EG, Lisboa PC. Acute and chronic leptin effect upon in vivo and in vitro rat thyroid iodide uptake. Life Sciences. 2007;81:1241–1246. doi: 10.1016/j.lfs.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Dussault JH, Coulombe P, Walker P. Effects of neonatal hyperthyroidism on the development of the hypothalamic-pituitary-thyroid axis in the rat. Endocrinology. 1982;110:1037–1042. doi: 10.1210/endo-110-3-1037. [DOI] [PubMed] [Google Scholar]
- Dutra S, Moura E, Rodrigues A, Lisboa P, Bonomo I, Toste F, Passos M. Cold exposure restores the decrease in leptin receptors (OB-Rb) caused by neonatal leptin treatment in 30-day-old rats. J Endocrinol. 2007;195:351–358. doi: 10.1677/JOE-07-0366. [DOI] [PubMed] [Google Scholar]
- Fagundes AT, Moura EG, Passos MC, Oliveira E, Toste FP, Bonomo IT, Trevenzoli IH, Garcia RM, Lisboa PC. Maternal low-protein diet during lactation programmes body composition and glucose homeostasis in the adult rat offspring. Br J Nutr. 2007;98:922–928. doi: 10.1017/S0007114507750924. [DOI] [PubMed] [Google Scholar]
- Faust IM, Johnson PR, Hirsch J. Long-term effects of early nutritional experience on the development of obesity in the rat. J Nutr. 1980;110:2027–2034. doi: 10.1093/jn/110.10.2027. [DOI] [PubMed] [Google Scholar]
- Flier JS, Harris M, Hollenberg AN. Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring. J Clin Invest. 2000;105:859–861. doi: 10.1172/JCI9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gesundheit N, Magner JA, Chen T, Weintraub BD. Differential sulfation and sialylation of secreted mouse thyrotropin (TSH) subunits: regulation by TSH-releasing hormone. Endocrinology. 1986;119:455–463. doi: 10.1210/endo-119-2-455. [DOI] [PubMed] [Google Scholar]
- Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci U S A. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Dell’Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens. 2004;22:2363–2369. doi: 10.1097/00004872-200412000-00019. [DOI] [PubMed] [Google Scholar]
- Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, Joost HG, Becker W. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J. 2005;272:109–119. doi: 10.1111/j.1742-4658.2004.04391.x. [DOI] [PubMed] [Google Scholar]
- Isozaki O, Tsushima T, Nozoe Y, Miyakawa M, Takano K. Leptin regulation of the thyroids: negative regulation on thyroid hormone levels in euthyroid subjects and inhibitory effects on iodide uptake and Na+/I– symporter mRNA expression in rat FRTL-5 cells. Endocr J. 2004;51:415–423. doi: 10.1507/endocrj.51.415. [DOI] [PubMed] [Google Scholar]
- Javesghani D, Hussain SN, Scheidel J, Quinn MT, Magder SA. Superoxide production in the vasculature of lipopolysaccharide-treated rats and pigs. Shock. 2003;19:486–493. doi: 10.1097/01.shk.0000054374.88889.37. [DOI] [PubMed] [Google Scholar]
- Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, Kulig E, Lloyd RV. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;84:2903–2911. doi: 10.1210/jcem.84.8.5908. [DOI] [PubMed] [Google Scholar]
- Jin L, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141:333–339. doi: 10.1210/endo.141.1.7260. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Lindell K, Svensson E, Bergh C, Lind P, Billig H, Carlsson LM, Carlsson B. Expression of functional leptin receptors in the human ovary. J Clin Endocrinol Metab. 1997;82:4144–4148. doi: 10.1210/jcem.82.12.4446. [DOI] [PubMed] [Google Scholar]
- Kimura T, Okajima F, Kikuchi T, Kuwabara A, Tomura H, Sho K, Kobayashi I, Kondo Y. Inhibition of TSH-induced hydrogen peroxide production by TNF-α through a sphingomyelinase signaling pathway. Am J Physiol Endocrinol Metab. 1997;273:E639–E643. doi: 10.1152/ajpendo.1997.273.3.E638. [DOI] [PubMed] [Google Scholar]
- Kiortsis DN, Durack I, Turpin G. Effects of a low-calorie diet on resting metabolic rate and serum tri-iodothyronine levels in obese children. Eur J Pediatr. 1999;158:446–450. doi: 10.1007/s004310051117. [DOI] [PubMed] [Google Scholar]
- Knerr I, Schuster S, Nomikos P, Buchfelder M, Dotsch J, Schoof E, Fahlbusch R, Rascher W. Gene expression of adrenomedullin, leptin, their receptors and neuropeptide Y in hormone-secreting and non-functioning pituitary adenomas, meningiomas and malignant intracranial tumours in humans. Neuropathol Appl Neurobiol. 2001;27:215–222. doi: 10.1046/j.0305-1846.2001.00324.x. [DOI] [PubMed] [Google Scholar]
- Kopecky J, Clarke G, Enerbäck S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- Lisboa PCFA, Denolato AT, Oliveira E, Bonomo IT, Alves SB, Curty FH, Passos MC, Moura EG. Neonatal low-protein diet changes deiodinase activities and pituitary TSH response to TRH in adult rats. Exp Biol Med. 2008;233:57–63. doi: 10.3181/0705-RM-146. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Bian J, Liu J, Endoh A. Obesity reduced the gene expressions of leptin receptors in hypothalamus and liver. Horm Metab Res. 2007;39:489–494. doi: 10.1055/s-2007-981680. [DOI] [PubMed] [Google Scholar]
- López M, Seoane LM, Tovar S, García MC, Nogueiras R, Diéguez C, Señarís RM. A possible role of neuropeptide Y, agouti-related protein and leptin receptor isoforms in hypothalamic programming by perinatal feeding in the rat. Diabetologia. 2005;48:140–148. doi: 10.1007/s00125-004-1596-z. [DOI] [PubMed] [Google Scholar]
- López M, Tovar S, Vázquez MJ, Nogueiras R, Seoane LM, García M, Señarís RM, Diéguez C. Perinatal overfeeding in rats results in increased levels of plasma leptin but unchanged cerebrospinal leptin in adulthood. Int J Obes (Lond) 2007;31:371–377. doi: 10.1038/sj.ijo.0803425. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lu F, Bytautiene E, Tamayo E, Gamble P, Anderson GD, Hankins GD, Longo M, Saade GR. Gender-specific effect of overexpression of sFlt-1 in pregnant mice on fetal programming of blood pressure in the offspring later in life. Am J Obstet Gynecol. 2007;197:418. doi: 10.1016/j.ajog.2007.06.064. [DOI] [PubMed] [Google Scholar]
- Lucas A. Role of nutritional programming in determining adult morbidity. Arch Dis Child. 1994;71:288–290. doi: 10.1136/adc.71.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- Mark AL, Correia ML, Rahmouni K, Haynes WG. Loss of leptin actions in obesity: two concepts with cardiovascular implications. Clin Exp Hypertens. 2004;26:629–636. doi: 10.1081/ceh-200031948. [DOI] [PubMed] [Google Scholar]
- Masaki T, Yoshimatsu H, Chiba S, Sakata T. Impaired response of UCP family to cold exposure in diabetic (db/db) mice. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1305–R1309. doi: 10.1152/ajpregu.2000.279.4.R1305. [DOI] [PubMed] [Google Scholar]
- Mathieu P, Pibarot P, Larose E, Poirier P, Marette A, Despres JP. Visceral obesity and the heart. Int J Biochem Cell Biol. 2008;40:821–836. doi: 10.1016/j.biocel.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Manning AM. Multiple signals converging on NF-κB. Curr Opin Cell Biol. 1999;11:226–232. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- Mönig H, Arendt T, Meyer M, Kloehn S, Bewig B. Activation of the hypothalamo-pituitary-adrenal axis in response to septic or non-septic diseases – implications for the euthyroid sick syndrome. Intensive Care Med. 1999;25:1402–1406. doi: 10.1007/s001340051088. [DOI] [PubMed] [Google Scholar]
- Moura AS, Franco de Sá CC, Lopez da Costa C, Vicente LL, Guerreiro SM, Pinto AM. Association between nutrition and gender during lactation influencing glucose homeostasis and blood pressure of the adult offspring. Biol Neonate. 2002;82:263–270. doi: 10.1159/000065889. [DOI] [PubMed] [Google Scholar]
- Moura EG, Ramos CF, Nascimento CC, Rosenthal D, Breitenbach MM. Thyroid function in fasting rats: variations in 131I uptake and transient decrease in peroxidase activity. Braz J Med Biol Res. 1987;20:407–410. [PubMed] [Google Scholar]
- Moura EG, Santos RS, Lisboa PC, Alves SB, Bonomo IT, Fagundes AT, Oliveira E, Passos MC. Thyroid function and body weight programming by neonatal hyperthyroidism in rats – the role of leptin and deiodinase activities. Horm Metab Res. 2008;40:1–7. doi: 10.1055/s-2007-1004554. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002;16:653–666. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- Nowak KW, Kaczmarek P, Mackowiak P, Ziolkowska A, Albertin G, Ginda WJ, Trejter M, Nussdorfer GG, Malendowicz LK. Rat thyroid gland expresses the long form of leptin receptors, and leptin stimulates the function of the gland in euthyroid non-fasted animals. Int J Mol Med. 2002;9:31–34. [PubMed] [Google Scholar]
- Orban Z, Bornstein SR, Chrousos GP. The interaction between leptin and the hypothalamic-pituitary-thyroid axis. Horm Metab Res. 1998;30:231–235. doi: 10.1055/s-2007-978872. [DOI] [PubMed] [Google Scholar]
- Ortiga-Carvalho TM, Oliveira KJ, Soares BA, Pazos-Moura CC. The role of leptin in the regulation of TSH secretion in the fed state: in vivo and in vitro studies. J Endocrinol. 2002;174:121–125. doi: 10.1677/joe.0.1740121. [DOI] [PubMed] [Google Scholar]
- Paramore DS, Fanelli CG, Shah SD, Cryer PE. Hypoglycemia per se stimulates sympathetic neural as well as adrenomedullary activity, but, unlike the adrenomedullary response, the forearm sympathetic neural response is not reduced after recent hypoglycemia. Diabetes. 1999;48:1429–1436. doi: 10.2337/diabetes.48.7.1429. [DOI] [PubMed] [Google Scholar]
- Passos MC, da Fonte Ramos C, Dutra SC, Mouco T, de Moura EG. Long-term effects of malnutrition during lactation on the thyroid function of offspring. Horm Metab Res. 2002;34:40–43. doi: 10.1055/s-2002-19966. [DOI] [PubMed] [Google Scholar]
- Passos MC, Vicente LL, Lisboa PC, de Moura EG. Absence of anorectic effect to acute peripheral leptin treatment in adult rats whose mothers were malnourished during lactation. Horm Metab Res. 2004;36:625–629. doi: 10.1055/s-2004-825927. [DOI] [PubMed] [Google Scholar]
- Pekary AE, Hershman JM. Tumor necrosis factor, ceramide, transforming growth factor-β1, and aging reduce Na+/I– symporter messenger ribonucleic acid levels in FRTL-5 cells. Endocrinology. 1998;139:703–712. doi: 10.1210/endo.139.2.5760. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Pinkney JH, Goodrick SJ, Katz J, Johnson AB, Lightman SL, Coppack SW, Mohamed-Ali V. Leptin and the pituitary-thyroid axis: a comparative study in lean, obese, hypothyroid and hyperthyroid subjects. Clin Endocrinol. 1998;49:583–588. doi: 10.1046/j.1365-2265.1998.00573.x. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Kohlhoff R, Rohde W, Dorner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord. 1997;21:451–456. doi: 10.1038/sj.ijo.0800429. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Melchior K, Rohde W, Dorner G. Increased number of galanin-neurons in the paraventricular hypothalamic nucleus of neonatally overfed weanling rats. Brain Res. 1999a;818:160–163. doi: 10.1016/s0006-8993(98)01264-5. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dorner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999b;836:146–155. doi: 10.1016/s0006-8993(99)01662-5. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dorner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999c;11:541–546. doi: 10.1046/j.1365-2826.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Heidrich I, Götz F, Rohde W, Dörner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154–158. doi: 10.1055/s-0029-1211159. [DOI] [PubMed] [Google Scholar]
- Pracyk JB, Seidler FJ, McCook EC, Slotkin TA. Pituitary-thyroid axis reactivity to hyper- and hypothyroidism in the perinatal period: ontogeny of regulation of regulation and long-term programming of responses. J Dev Physiol. 1992;18:105–109. [PubMed] [Google Scholar]
- Ritz P, Dumas JF, Salle A, Simard G, Malthiery Y, Rohmer V. Thyroid hormones and obesity. Ann Endocrinol (Paris) 2002;63:135–139. [PubMed] [Google Scholar]
- Rodrigues AL, De Souza EP, Da Silva SV, Rodrigues DS, Nascimento AB, Barja-Fidalgo C, De Freitas MS. Low expression of insulin signaling molecules impairs glucose uptake in adipocytes after early overnutrition. J Endocrinol. 2007;195:485–494. doi: 10.1677/JOE-07-0046. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71:1421–1432. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]
- Roti E, Minelli R, Salvi M. Thyroid hormone metabolism in obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):113–115. doi: 10.1038/sj.ijo.0801293. [DOI] [PubMed] [Google Scholar]
- Sahu A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol. 2002;14:796–804. doi: 10.1046/j.1365-2826.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Sahu A. Minireview: a hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 2004;145:2613–2620. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Toledo-Pinto EA, Menezes ML, Pereira OC. Changes in norepinephrine and epinephrine concentrations in adrenal gland of the rats submitted to acute immobilization stress. Pharmacol Res. 2003;48:607–613. doi: 10.1016/s1043-6618(03)00241-x. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Davis SN. Leptin: metabolic control and regulation. J Diabetes Complications. 2003;17:108–113. doi: 10.1016/s1056-8727(02)00167-8. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane LM, Carro E, Tovar S, Casanueva FF, Dieguez C. Regulation of in vivo TSH secretion by leptin. Regul Pept. 2000;92:25–29. doi: 10.1016/s0167-0115(00)00145-2. [DOI] [PubMed] [Google Scholar]
- Sone M, Nagata H, Takekoshi S, Osamura RY. Expression and localization of leptin receptor in the normal rat pituitary gland. Cell Tissue Res. 2001;305:351–356. doi: 10.1007/s004410100407. [DOI] [PubMed] [Google Scholar]
- Stichel H, l’Allemand D, Grüters A. Thyroid function and obesity in children and adolescents. Horm Res. 2000;54:14–19. doi: 10.1159/000063431. [DOI] [PubMed] [Google Scholar]
- Tagliaferri M, Berselli ME, Calo G, Minocci A, Savia G, Petroni ML, Viberti GC, Liuzzi A. Subclinical hypothyroidism in obese patients: relation to resting energy expenditure, serum leptin, body composition, and lipid profile. Obes Res. 2001;9:196–201. doi: 10.1038/oby.2001.21. [DOI] [PubMed] [Google Scholar]
- Teixeira C, Passos M, Ramos C, Dutra S, Moura E. Leptin serum concentration, food intake and body weight in rats whose mothers were exposed to malnutrition during lactation. J Nutr Biochem. 2002;13:493. doi: 10.1016/s0955-2863(02)00197-3. [DOI] [PubMed] [Google Scholar]
- Toste FP, Alves SB, Dutra SC, Bonomo IT, Lisboa PC, Moura EG, Passos MC. Temporal evaluation of the thyroid function of rats programed by leptin treatment on the neonatal period. Horm Metab Res. 2006a;38:827–831. doi: 10.1055/s-2006-956502. [DOI] [PubMed] [Google Scholar]
- Toste FP, de Moura EG, Lisboa PC, Fagundes AT, de Oliveira E, Passos MC. Neonatal leptin treatment programmes leptin hypothalamic resistance and intermediary metabolic parameters in adult rats. Br J Nutr. 2006b;95:830–837. doi: 10.1079/bjn20061726. [DOI] [PubMed] [Google Scholar]
- Torpy DJ, Tsigos C, Lotsikas AJ, Defensor R, Chrousos GP, Papanicolaou DA. Acute and delayed effects of a single-dose injection of interleukin-6 on thyroid function in healthy humans. Metabolism. 1998;47:1289–1293. doi: 10.1016/s0026-0495(98)90338-9. [DOI] [PubMed] [Google Scholar]
- Trevenzoli IHV, Valle MMR, Machado FB, Garcia RMG, Lisboa PC, Passos MCF, Moura EG. Neonatal hyperleptinemia programmes adrenal medulary function in adult rats: effects on cardiovascular parameters. J Physiol. 2007;580:629–637. doi: 10.1113/jphysiol.2006.126151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- Velkoska E, Cole TJ, Morris MJ. Early dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, and adiposity markers. Am J Physiol Endocrinol Metab. 2005;288:E1236–E1243. doi: 10.1152/ajpendo.00505.2004. [DOI] [PubMed] [Google Scholar]
- Vicente LL, de Moura EG, Lisboa PC, Monte Alto Costa A, Amadeu T, Mandarim-de-Lacerda CA, Passos MC. Malnutrition during lactation in rats is associated with higher expression of leptin receptor in the pituitary of adult offspring. Nutrition. 2004;20:924–928. doi: 10.1016/j.nut.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Walker P, Courtin F. Transient neonatal hyperthyroidism results in hypothyroidism in the adult rat. Endocrinology. 1985;116:2246–2250. doi: 10.1210/endo-116-6-2246. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab. 2004;287:E318–E326. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]
- Xiao XQ, Williams SM, Grayson BE, Glavas MM, Cowley MA, Smith MS, Grove KL. Excess weight gain during the early postnatal period is associated with permanent reprogramming of brown adipose tissue adaptative thermogenesis. Endocrinology. 2007;148:4150–4159. doi: 10.1210/en.2007-0373. [DOI] [PubMed] [Google Scholar]
- You S, Gotz F, Rohde W, Dorner G. Early postnatal overfeeding and diabetes susceptibility. Exp Clin Endocrinol. 1990;96:301–306. doi: 10.1055/s-0029-1211023. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zheng J, Yao X, Chen Z. [Relationship between body composition and serum thyroid hormone in children aged 8 to 12 years] Zhonghua Yu Fang Yi Xue Za Zhi. 1996;30:279–281. [PubMed] [Google Scholar]
- Zimmermann-Belsing T, Brabant G, Holst JJ, Feldt-Rasmussen U. Circulating leptin and thyroid dysfunction. Eur J Endocrinol. 2003;149:257–271. doi: 10.1530/eje.0.1490257. [DOI] [PubMed] [Google Scholar]