Abstract
Increased systemic myeloperoxidase (MPO) has been associated with both the presence and severity of heart failure (HF). This study tested the hypothesis that increased systemic MPO in apparently healthy elderly subjects may predict increased risk of developing HF. Systemic MPO was measured in all available samples from the 1992 to 1993 visit of the Cardiovascular Health Study (CHS). After excluding subjects without available blood samples or with a history of prevalent HF, myocardial infarction (MI), or stroke, 3,733 subjects were included. A total of 569 subjects developed incident HF during 7.2 ± 2.3 years of follow-up. Patients in the highest MPO quartile (>432 pmol/L) showed higher risk of developing incident HF after adjusting for MI, age, gender, systolic blood pressure, smoking, low-density lipoprotein cholesterol, diabetes mellitus, and any subclinical cardiovascular disease (hazard ratio 1.34, 95% confidence interval 1.06 to 1.72, p = 0.013). However, the relation was more apparent after censoring subjects with incident MI before incident HF, even when adjusted for C-reactive protein and cystatin C (hazard ratio 1.46, 95% confidence interval 1.08 to 1.97, p = 0.02). Interestingly, stratified analyses showed that the relation between increased MPO and HF risk was stronger in subjects without traditional cardiovascular risk factors (≤75 years old, systolic blood pressure ≤136 mm Hg, no subclinical cardiovascular disease, and no diabetes mellitus). In conclusion, an independent association between increased MPO and the development of HF in apparently healthy elderly subjects was observed, particularly beyond MI and traditional cardiac risk factors.
Myeloperoxidase (MPO) is a leukocyte-derived enzyme that catalyzes the formation of a number of reactive oxidant species and impacts on nitric oxide through complex mechanisms, including direct catalytic consumption resulting in endothelial dysfunction.1,2 MPO has been shown to provide prognostic value in the setting of chest pain and acute coronary syndromes.3–5 Recent studies of the community-based European Prospective Investigation of Cancer (EPIC)/Nolfork population also reported that systemic MPO independently predicted risk of the development of incident cardiovascular disease and death in apparently healthy middle-aged subjects.6 A potential pathogenic role of MPO in the development of left ventricular dysfunction and heart failure (HF) was also emerging.7–9 In animal models, MPO knockout mice showed an important role of MPO in facilitating HF disease progression.10 In humans, systemic MPO was increased in patients with established chronic systolic HF and correlated with diastolic dysfunction independent of age and plasma B-type natriuretic peptide.8 Recent studies also showed that systemic MPO in subjects with myocardial infarction (MI)7 or chronic systolic HF9 may predict long-term adverse clinical events. Here, we hypothesized that MPO may be associated with the long-term risk of developing HF in apparently healthy elderly subjects.
Methods
The Cardiovascular Heath Study (CHS) was a community-based longitudinal study of clinical and subclinical cardiovascular disease in 5,888 adults ≥65 years at baseline.11 The CHS recruited 5,201 subjects from 1989 to 1990 from the 4 communities of Sacramento County, California; Forsyth County, North Carolina; Washington County, Maryland; and Allegheny County, Pennsylvania. An additional 687 African-American subjects were recruited in 1992 to 1993. Potential study subjects were randomly sampled from Medicare eligibility lists in each area, and 57% of those invited chose to participate in the study. Subjects were excluded if they were institutionalized, required a proxy to give consent, were planning to move out of the area within 3 years after recruitment, required a wheelchair in the home, were receiving hospice care, or were undergoing radiation or chemotherapy for cancer. The investigational review boards of the 4 clinical sites and the Data Coordinating Center (University of Washington, Seattle, Washington) approved the CHS data collection procedures.
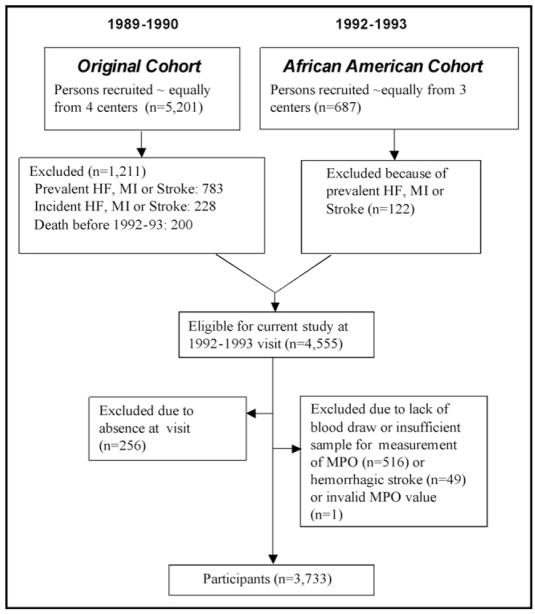
The present study used data from the 1992 to 1993 CHS examination inclusive of the African-American cohort. Our goal was to assess the risk of incident HF. Therefore, subjects were excluded if they had prevalent HF, prevalent MI, or prevalent stroke or died before the 1992 to 1993 visit. On this basis, 1,333 of 5,888 subjects were excluded. Of this eligible cohort, an additional 822 subjects were excluded for a variety of reasons (see Figure 1 for details), leaving 3,733 participants. Follow-up for events continued until June 30, 2001, with a median follow-up of 8.3 years (maximum 9.1). Factors assessed during the 1992 to 1993 CHS examination included age, race (self-report), gender, diabetes, hypertension, smoking, subclinical cardiovascular disease, alcohol use, current medications, height, weight, blood pressure, and laboratory measurements (MPO and lipids) and were included as covariates in multivariable analysis. For the diagnosis of prevalent HF, self reports were confirmed by components of the physical examination or, if necessary, a validation protocol that included surveys of treating physicians or review of medical records.12 For the diagnosis of incident HF, a physician’s diagnosis of HF was followed by a review of the subject’s medical records. The incidence of HF was then determined by the CHS Cardiovascular Events Committee based on diagnosis from a physician, as well as consideration of symptoms, signs, chest X-ray findings, and treatment of HF (current prescription for a diuretic agent and either digitalis or a vasodilator).13,14 Diabetes was defined as a history of diabetes mellitus, use of a diabetic medication, or fasting blood glucose ≥126 mg/dl. Systolic and diastolic blood pressures were calculated from the mean of 2 consecutive readings in the seated position. Smoking was defined as current versus not. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula.15 Subclinical cardiovascular diseases documented in the CHS included abnormalities in carotid intima-media thickness monitored using ultrasound, ankle-arm index, increased left ventricular mass using electrocardiography, and major electrocardiographic abnormalities.16 Follow-up interviews for events (including MI and stroke) occurred every 6 months and in person annually.
Figure 1.
Selection of subjects. Schematic diagram for blood sampling from 2 subject cohorts in the CHS.
MPO was measured from frozen samples collected at the 1992 to 1993 CHS examination. Plasma MPO was determined using the CardioMPO test (PrognostiX Inc., Cleveland, Ohio). This was a Food and Drug Administration–approved sandwich enzyme-linked immunosorbent assay for the measurement of MPO. Normal control values from a middle-aged healthy population have been reported to be <640 pmol/L. The minimum detection limit (calculated using interpolation of the mean plus 2 SDs) was 30 pmol/L, with within-run precision of 4.8%.
A prespecified statistical analysis plan was performed at the Data Coordinating Center for CHS. Quartile-based analyses were performed, and comparisons were made of the distribution of demographic characteristics and traditional cardiovascular risk factors across quartile groups. Differences in baseline characteristics were compared using Cochrane-Armitage trend tests for continuous variables and chi-square tests for categorical variables. The association between MPO and HF was determined using multivariate Cox proportional hazards regression models. To evaluate the contribution of MPO quartiles as a marker of risk, models were generated in stages as unadjusted, then adjusted for demographics and cardiovascular risk factors. Analyses were performed with a time-dependent variable for incident MI added to the model to evaluate the effect of controlling for this intervening event on the association of baseline characteristics with risk of incident HF. We also ran a model adjusted for clinical risk characteristics censoring for subjects with incident MI. Based on the skewed distribution and risk profiles across MPO quartiles, Kaplan-Meier survival analysis was performed dichotomizing MPO at the top quartile (>432 pmol/L) versus lower 3 quartiles (≤432 pmol/L). S-Plus (release 6.1; Insightful Inc., Seattle, Washington) and SPSS statistical software (version 15.0.1; SPSS Inc., Chicago, Illinois) were used for analyses. A p value <0.05 was considered statistically significant.
Results
In our study cohort, median MPO was 258 pmol/L (interquartile range 168 to 424). Table 1 lists subject characteristics according to quartiles of MPO. Subjects were followed up for a mean of 9.2 ± 3.1 years. Of 3,733 subjects included, 569 (15.2%) developed incident HF during follow-up. Patients with incident HF were more likely to be older and men, have higher systolic blood pressure, and have a history of diabetes mellitus and subclinical cardiovascular disease (all p <0.01). Overall, systemic MPO was similar in subjects with and without incident HF. Median MPO levels were 257 (25th, 75th percentiles 173, 451) and 258 pmol/L (25th, 75th percentiles 168, 419), respectively (p = 0.259).
Table 1.
Baseline characteristics according to quartiles of systemic myeloperoxidase (MPO)
| Characteristics | MPC Quartiles (pmol/L) |
||||
|---|---|---|---|---|---|
| <170 (n = 950) | 170–261 (n = 938) | 262–432 (n = 949) | >432 (n = 896) | p Value (trend) | |
| Age (yrs) | 75 ± 5 | 75 ± 5 | 75 ± 5 | 74 ± 5 | 0.068 |
| Men | 364 (38%) | 342 (37%) | 350 (37%) | 387 (43%) | 0.041 |
| African Americans | 142 (15%) | 149 (16%) | 167 (17%) | 166 (19%) | 0.023 |
| Systolic blood pressure (mm Hg) | 138 (21) | 136 (22) | 137 (21) | 134 (20) | 0.001 |
| Diastolic blood pressure (mm Hg) | 71 (12) | 72 (11) | 72 (10) | 71 (11) | 0.742 |
| Antihypertensive therapy | 421 (44%) | 410 (44%) | 437 (46%) | 416 (46%) | 0.252 |
| Smoker (current) | 79 (9%) | 84 (9%) | 93 (10%) | 112 (13%) | 0.003 |
| LDL cholesterol (mg/dl) | 128 ± 33 | 129 ± 33 | 127 ± 34 | 126 ± 33 | 0.124 |
| High-density lipoprotein cholesterol (mg/dl) | 53 ± 14 | 55 ± 15 | 55 ± 15 | 54 ± 14 | 0.077 |
| Diabetes mellitus | 148 (16%) | 135 (14%) | 111 (12%) | 117 (13%) | 0.039 |
| Subclinical cardiovascular diseases | 609 (66%) | 615 (67%) | 625 (68%) | 574 (65%) | 0.993 |
| C-Reactive protein (mg/L) | 2.03 (0.99–4.03) | 2.18 (1.12–5.03) | 2.54 (1.19–6.09) | 3.93 (1.65–8.56) | <0.001 |
| Cystatin C (mg/L) | 1.06 ± 0.26 | 1.07 ± 0.26 | 1.10 ± 0.28 | 1.10 ± 0.32 | <0.001 |
Values expressed as mean ± SD, number (percent), or median (interquartile range).
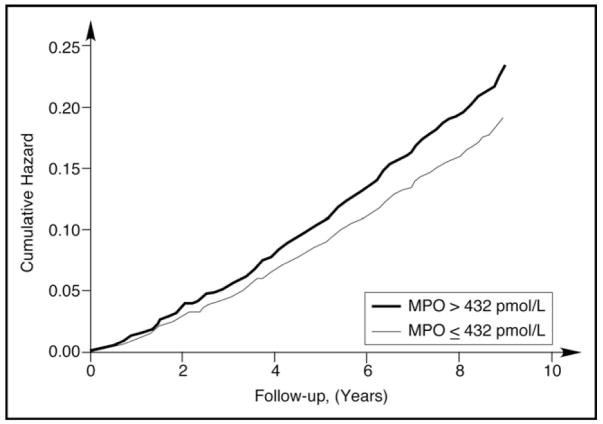
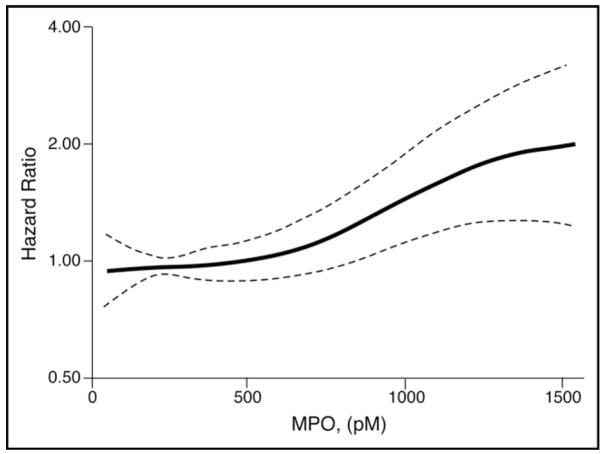
We first explored whether MPO was associated with incident HF in unadjusted survival analysis of events. Both curves began to diverge between 1 and 2 years and continued to diverge for the rest of follow-up (p = 0.014; Figure 2). We observed that higher MPO was associated with higher incident HF (hazard ratio [HR] 1.14, 95% confidence interval [CI] 1.01 to 1.28, p = 0.04, spline curve for HR shown in Figure 3).
Figure 2.
Unadjusted risk of developing incident HF comparing cumulative hazards according to MPO at 432-pmol/L cutoff.
Figure 3.
Spline curve analysis for HRs for MPO shows modest increase in unadjusted HRs with increasing systemic MPO.
Of 569 subjects with incident HF, 188 (33%) had an MI after their blood draw, but before developing HF. We developed 2 multivariable models to explore interactions between MPO with traditional cardiac risk factors and MI. In model 1, we treated MI as a time-dependent covariate (Table 2). After adjusting for age, gender, systolic blood pressure, smoking, LDL cholesterol, diabetes mellitus, any subclinical cardiovascular disease, and MI as a time-dependent covariate, increased MPO (>432 pmol/L) predicted subjects at increased risk of developing incident HF (HR 1.34, 95% CI 1.06 to 1.72, p = 0.013; Table 2). As expected, MI was the strongest predictor of incident HF in this model. However, adding C-reactive protein and cystatin C to the model, high systemic MPO no longer was independently predictive of incident HF (Table 2).
Table 2.
Multivariable model with myeloperoxidase (MPO) expressed in quartiles, including subjects with history of myocardial infarction (MI)
| HR (95% CI) | p Value | HR* (95% CI) | p Value | |
|---|---|---|---|---|
| MPO quartiles (pmol/L) | ||||
| <170 | 1.0 (reference) | 1.0 (reference) | ||
| 170–261 | 1.2 (0.94–1.52) | 0.138 | 1.18 (0.92–1.50) | 0.190 |
| 262–432 | 0.94 (0.73–1.21) | 0.623 | 0.92 (0.71–1.18) | 0.505 |
| >432 | 1.34 (1.06–1.72) | 0.013 | 1.22 (0.96–1.57) | 0.108 |
| MI | 4.91 (4.08–5.91) | <0.01 | 4.73 (3.92–5.71) | <0.01 |
| Age (yrs) | 1.08 (1.07–1.10) | <0.01 | 1.08 (1.06–1.10) | <0.01 |
| Men | 1.16 (0.98–1.39) | 0.09 | 1.21 (1.01–1.44) | 0.034 |
| Systolic blood pressure (mm Hg)† | 1.26 (1.16–1.36) | <0.01 | 1.21 (1.12–1.31) | <0.01 |
| Smoker (current) | 1.50 (1.15–1.97) | <0.01 | 1.44 (1.10–1.90) | <0.01 |
| LDL cholesterol (mg/dl)† | 0.93 (0.85–1.01) | 0.13 | 0.93 (0.85–1.02) | 0.103 |
| Diabetes mellitus | 1.92 (1.56–2.36) | <0.01 | 1.86 (1.50–2.30) | <0.01 |
| Subclinical cardiovascular diseases | 1.93 (1.53–2.43) | <0.01 | 1.87 (1.48–2.36) | <0.01 |
| C-Reactive protein† | 1.13 (1.06–1.20) | <0.01 | ||
| Cystatin C† | 1.22 (1.15–1.29) | <0.01 | ||
When CRP and cystatin C were added to the multivariate model.
Per standard deviation increments (systolic blood presure = 21 mmHg, LDL cholesterol = 33 mg/dL, C-reactive protein = 9.4 mg/L, cystatin C = 0.28 mg/L).
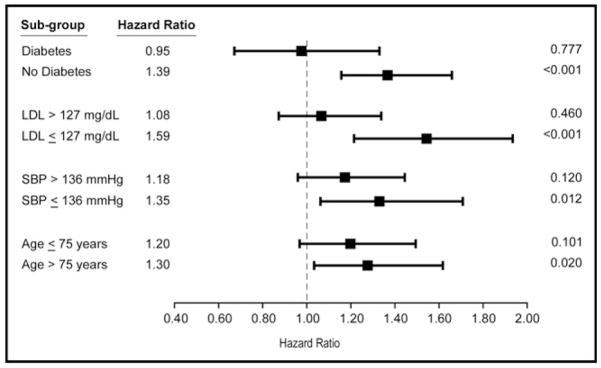
In model 2, we performed a tiered analysis by censoring patients who had an interim MI before the onset of HF (Table 3). After adjusting for age, gender, systolic blood pressure, smoking, LDL cholesterol, diabetes, and any subclinical cardiovascular disease, higher MPO remained an independent predictor of incident HF, even in the presence of C-reactive protein and cystatin C as covariates (HR 1.46, 95% CI 1.08 to 1.97, p = 0.02; Table 3). These results were similar when analyzed with MPO as a continuous variable under log transformation. Furthermore, subgroups that showed statistically significant relative risks of developing incident HF with increased MPO were more likely to not have diabetes, have systolic blood pressure <136 mm Hg, be >75 years, or have LDL cholesterol <127 mg/dl (Figure 4). Test for interaction showed no significant interactions between these variables with MPO (age, p = 0.726; systolic blood pressure, p = 0.085; subclinical disease, p = 0.062; and diabetes mellitus, p = 0.106).
Table 3.
Multivariable Cox regression model with myeloperoxidase (MPO) expressed in quartiles in subjects without a history of myocardial infarction (MI)
| HR (95% CI) | p value | HR* (95% CI) | p Value | |
|---|---|---|---|---|
| MPO quartiles (pmol/L) | ||||
| <170 | 1.0 (reference) | 1.0 (reference) | ||
| 170–261 | 1.42 (1.06–1.91) | 0.02 | 1.42 (1.05–1.90) | 0.02 |
| 262–432 | 0.96 (0.70–1.33) | 0.81 | 0.91 (0.66–1.25) | 0.55 |
| >432 | 1.62 (1.20–2.17) | <0.01 | 1.46 (1.08–1.97) | 0.02 |
| Age (yrs) | 1.09 (1.07–1.11) | <0.01 | 1.08 (1.06–1.10) | <0.01 |
| Men | 1.31 (1.06–1.62) | 0.01 | 1.33 (1.07–1.64) | 0.01 |
| Systolic blood pressure (mm Hg)† | 1.29 (1.17–1.42) | <0.01 | 1.28 (1.16–1.41) | <0.01 |
| Smoker (current) | 1.42 (1.01–2.00) | 0.05 | 1.35 (0.96–1.90) | 0.09 |
| LDL cholesterol (mg/dl)† | 0.90 (0.81–1.00) | 0.06 | 0.92 (0.82–1.02) | 0.11 |
| Diabetes mellitus | 1.96 (1.51–2.53) | <0.01 | 1.82 (1.40–2.36) | <0.01 |
| Subclinical cardiovascular diseases | 1.89 (1.44–2.48) | <0.01 | 1.82 (1.39–2.39) | <0.01 |
| C-Reactive protein† | — | — | 1.12(1.04–1.22) | 0.04 |
| Cystatin C† | — | — | 1.26(1.19–1.34) | <0.01 |
When CRP and cystatin C were added to the multivariate model.
Per standard deviation increments (systolic blood presure = 21 mmHg, LDL cholesterol = 33 mg/dL, C-reactive protein = 9.4 mg/L, cystatin C = 0.28 mg/L).
Figure 4.
Adjusted risk of developing incident HF. HRs of subjects stratified by traditional risk factors, including history of diabetes mellitus, LDL cholesterol, systolic blood pressure (SBP), and age.
Discussion
MPO has often been associated with the development and progression of atherosclerotic coronary artery disease. Taken together, our data suggested that in the elderly population, increased systemic MPO was associated with increased risk of developing incident HF. This increased risk was apparent when censoring the incidence of interim MI and was particularly apparent in those without significant cardiovascular risk factors, the group of subjects for whom existing screening methods fail to identify risks. Our findings were concordant with previous animal data suggesting a role of MPO in modulating disease progression of cardiac remodeling that was independent of MI or extent of myocardial ischemia.17
The association between MPO and cardiac dysfunction was evident in a community screening population. In a recent cross-sectional study performed in a non-HF outpatient setting, systemic MPO was independently associated with the presence of left ventricular dysfunction (defined as ejection fraction <40% using echocardiography), with predictive values synergistic with plasma N-terminal pro-B natriuretic peptide and C-reactive protein.18 Unlike natriuretic peptides that are often produced in response to increased myocardial stress and dysfunction, higher systemic MPO may indicate the presence of an underlying inflammatory process leading to enhanced oxidative stress, including increased generation of reactive oxygen species. When generated in excess, prolonged exposure to reactive oxygen species may lead to ventricular injury, activation of specific pathways, and depletion of nitric oxide. This may ultimately lead to subsequent myocyte apoptosis and pathologic remodeling seen in the HF phenotype.17 Naturally, these pathogenic processes were highly prevalent in patients with hypertension, diabetes mellitus, and many other co-morbid diseases predicting the incident development of HF.13 However, it was apparent that most of our apparently healthy elderly population in the CHS had relatively normal systemic MPO. Moreover, the clinical signal observed between increased MPO and HF risk appeared strongest in those without diabetes, hypertension, or other traditional HF risk factors.
Our findings had several important clinical implications. First, demonstration of the prognostic value of MPO in predicting incident HF has provided further support for the inflammatory hypothesis of disease progression in patients with HF. A wide range of inflammatory markers has been associated with incident HF in non-MI populations.19–23 Most have observed heightened risk independent of myocardial ischemia, although some reports identified variable predictive risks in men and women. Second, our data indicated that the presence of underlying pathophysiologic processes highlighted by increased MPO may predispose to the development of HF beyond traditional cardiac risk factors, history of MI, and even subclinical evidence of cardiovascular diseases. This came as an unexpected finding and emphasized the importance of biomarker assessment of underlying physiologic mechanisms rather than inferring disease processes with the presence of co-morbidities. Future studies are needed to validate our findings, and if replicated in alternative cohorts, there is a need for prospective trials to evaluate the best interventions in patients with increased MPO for the prevention of HF development. In this regard, it was particularly interesting that MPO inhibition as a target for cardiovascular therapeutics was receiving increasing attention.
The strength of this analysis was the carefully characterized patient cohort of an apparently healthy elderly population in a prospective longitudinal follow-up. Nevertheless, several limitations should also be discussed. MPO was measured at a single time, and therefore variability of MPO over time was not available. There was a lack of other cardiac biomarkers for HF, such as natriuretic peptide. Another limitation was the lack of careful characterization of other co-morbid conditions (especially underlying inflammatory diseases) either at baseline or developed in the interim that may contribute to the development of HF.
Acknowledgments
A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
This work was supported by Grants No. P01 HL076491-055328, HL077107-050004, and P01 HL087018-020001 from the National Institutes of Health, Bethesda, Maryland, and contracts previously supporting development of the Cardiovascular Health Study, including N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland, with additional contribution from the National Institute of Neurological Disorders and Stroke, Bethesda, Maryland. Drs. Hazen and Tang were supported by Grant No. 1UL1RR024989 from the National Institutes of Health. Dr. Tang reports having received research grant support and honorarium from Abbott Laboratories. Dr. Hazen is named as co-inventor on pending and issued patents filed by the Cleveland Clinic that relate to the use of biomarkers in inflammatory and cardiovascular disease; reports he is the scientific founder of PrognostiX Inc.; has received speaking honoraria from Pfizer, AstraZeneca, Merck, Merck Schering Plough, Bio-Site, Lilly, Wyeth, and Abbott; has received research grant support from Abbott Diagnostics, Pfizer, Merck, PrognostiX Inc., Hawaii Biotech, ArgiNOx, Sanofi, and Takeda; and has received consulting fees from Abbott Laboratories, Pfizer, PrognostiX Inc., Wyeth, BioPhysical, and AstraZeneca.
References
- 1.Vita JA, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH, Penn MS, Keaney JF, Jr, Hazen SL. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation. 2004;110:1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 3.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 4.Cavusoglu E, Ruwende C, Eng C, Chopra V, Yanamadala S, Clark LT, Pinsky DJ, Marmur JD. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am J Cardiol. 2007;99:1364–1368. doi: 10.1016/j.amjcard.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 5.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 6.Meuwese MC, Stroes ESG, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, Wareham NJ, Luben R, Kastelein JJP, Khaw KT, Boekholdt SM. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals; the EPIC-Norfolk prospective population study. J Am Coll Cardiol. 2007;50:159–165. doi: 10.1016/j.jacc.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM, Winterbourn CC. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol. 2007;49:1993–2000. doi: 10.1016/j.jacc.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Tang WH, Brennan ML, Philip K, Tong W, Mann S, Van Lente F, Hazen SL. Plasma myeloperoxidase levels in patients with chronic heart failure. Am J Cardiol. 2006;98:796–799. doi: 10.1016/j.amjcard.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, Jasper S, Hazen SL, Klein AL. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–2370. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 10.Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197:615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 13.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 14.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Pearte CA, Furberg CD, O’Meara ES, Psaty BM, Kuller L, Powe NR, Manolio T. Characteristics and baseline clinical predictors of future fatal versus nonfatal coronary heart disease events in older adults: the Cardiovascular Health Study. Circulation. 2006;113:2177–2185. doi: 10.1161/CIRCULATIONAHA.105.610352. [DOI] [PubMed] [Google Scholar]
- 17.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 18.Ng LL, Pathik B, Loke IW, Squire IB, Davies JE. Myeloperoxidase and C-reactive protein augment the specificity of B-type natriuretic peptide in community screening for systolic heart failure. Am Heart J. 2006;152:94–101. doi: 10.1016/j.ahj.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Vasan RS, Beiser A, D’Agostino RB, Levy D, Selhub J, Jacques PF, Rosenberg IH, Wilson PW. Plasma homocysteine and risk for congestive heart failure in adults without prior myocardial infarction. JAMA. 2003;289:1251–1257. doi: 10.1001/jama.289.10.1251. [DOI] [PubMed] [Google Scholar]
- 20.Vasan RS, Sullivan LM, D’Agostino RB, Roubenoff R, Harris T, Sawyer DB, Levy D, Wilson PW. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- 21.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D’Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 22.Kardys I, Knetsch AM, Bleumink GS, Deckers JW, Hofman A, Stricker BH, Witteman JC. C-Reactive protein and risk of heart failure. The Rotterdam Study. Am Heart J. 2006;152:514–520. doi: 10.1016/j.ahj.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 23.van Vark LC, Kardys I, Bleumink GS, Knetsch AM, Deckers JW, Hofman A, Stricker BH, Witteman JC. Lipoprotein-associated phospholipase A2 activity and risk of heart failure: The Rotterdam Study. Eur Heart J. 2006;27:2346–2352. doi: 10.1093/eurheartj/ehl230. [DOI] [PubMed] [Google Scholar]