Abstract
Nucleoside reverse transcriptase inhibitors (NRTIs), such as zidovudine (AZT) and stavudine (d4T), cause toxicities to numerous tissues, including the liver and vasculature. While much is known about hepatic NRTI toxicity, the mechanism of toxicity in endothelial cells is incompletely understood. Human aortic endothelial and HepG2 liver cells were exposed to 1 μM AZT or d4T for up to 5 weeks. Markers of oxidative stress, mitochondrial function, NRTI phosphorylation, mitochondrial DNA (mtDNA) levels, and cytotoxicity were monitored over time. In endothelial cells, AZT significantly oxidized glutathione redox potential, increased total cellular and mitochondrial-specific superoxide, decreased mitochondrial membrane potential, increased lactate release, and caused cell death from weeks 3 through 5. Toxicity occurred in the absence of di- and tri-phosphorylated AZT and mtDNA depletion. These data show that oxidative stress and mitochondrial dysfunction in endothelial cells occur with a physiologically relevant concentration of AZT, and require long-term exposure to develop. In contrast, d4T did not induce endothelial oxidative stress, mitochondrial dysfunction, or cytotoxicity despite the presence of d4T-triphosphate. Both drugs depleted mtDNA in HepG2 cells without causing cell death. Endothelial cells are more susceptible to AZT-induced toxicity than HepG2 cells, and AZT caused greater endothelial dysfunction than d4T because of its pro-oxidative effects.
Keywords: Zidovudine, Stavudine, Oxidative stress, Endothelium, Toxicity, Mitochondria, mtDNA pol-γ
Introduction
Highly active antiretroviral therapy (HAART) has prolonged the life expectancy of human immunodeficiency virus (HIV)-infected patients and significantly reduced viral burden [1]. However, the development of severe side effects upon long-term treatment may overshadow therapeutic impact. Nucleoside reverse transcriptase inhibitors (NRTIs), which include zidovudine (AZT) and stavudine (d4T), constitute one of the largest classes of approved antiretroviral agents, and are the backbone of all HAART regimens [2]. Toxicity to NRTIs occurs in the heart, skeletal muscle, liver, blood, and vasculature [3–6]. Much is known about NRTI toxicity in the heart and liver, which occurs, at least in part, through inhibition of mitochondrial DNA polymerase-γ (mtDNA pol-γ), loss of mtDNA, and decreased mitochondrial function [7]. However, mechanisms of toxicity in endothelial cells are less completely understood despite the high incidence of vascular complications in HIV patients receiving antiretroviral therapy [8].
A recent clinical report [9] indicates that usage of NRTIs for less than 1 year is associated with 48.1% greater intima media thickness of the carotid bifurcation, an indicator of sub-clinical atherosclerosis, when compared to individuals who have never received an NRTI. Ample pre-clinical evidence also supports a role for NRTIs in endothelial cell dysfunction. For example, AZT promotes the production of the vasoconstrictor endothelin-1 [10], enhances cell proliferation [10], and induces intracellular gaps between endothelial cells [11]. Additionally, AZT treatment significantly reduces mitochondrial respiration, decreases ATP production, and impairs the mitochondrial membrane potential in cultured endothelial cells [12]. Our group has previously shown that AZT treatment of mice impairs maximum aortic endothelium-dependent relaxation by 34% [13]. Furthermore, a recent study determined that AZT treatment of rats impaired endothelial function without altering plasma triglycerides or total cholesterol levels [14], indicating that AZT, and potentially other NRTIs, may induce direct endothelial damage in vivo.
NRTI toxicity is believed to occur, at least in part, through inhibition of pol-γ and subsequent inhibition of mtDNA replication and/or repair [15]. Because NRTIs lack a 3′-OH group on their pentose rings, incorporation of the nucleoside analog prevents subsequent phosphodiester bond formation and terminates mtDNA chain elongation. mtDNA copy numbers decrease and mitochondrial-encoded genes involved in the electron transport chain are either inadequately expressed or harbor mutations, resulting in decreased mitochondrial number or defective oxidative phosphorylation. Termination of mtDNA synthesis by NRTIs requires the incorporation of its tri-phosphorylated nucleotide into the growing mtDNA strand. Various cellular deoxynucleotide kinases successively phosphorylate NRTIs to their monophosphate (−MP), diphosphate (−DP), and triphosphate (−TP) nucleotides [16]. The activity of these kinases is dependent upon the specific analog substrate, species, and mitotic state of the cell [17]. Many of the side effects of NRTI therapy including skeletal and cardiac myopathies, hyperlactatemia, and peripheral neuropathy are believed to originate from NRTI-induced mitochondrial toxicity in various cell types [18].
Current research is focused on NRTI toxicity to endothelial cells. Our group [13] and others [14] have previously shown that AZT treatment of mice and rats impairs endothelium-dependent relaxation in isolated aortas. Moreover, AZT-induced endothelial cell damage and dysfunction is associated with increased superoxide levels [12–14]. While AZT has been shown to promote reactive oxygen species (ROS) generation in cell-free systems and in non-endothelial cells [19–23], little is known regarding AZT- and d4T-induced ROS in endothelial cells. Furthermore, there is little direct evidence of the role of AZT-phosphorylation in endothelial cell toxicity. Herein, we describe the effects of a physiologically relevant concentration of AZT and d4T on oxidative stress and superoxide production in human aortic endothelial cells (HAECs). While endothelial cell toxicity is the main focus of this study, we also measured oxidative stress and mitochondrial damage in human hepatic HepG2 cells, as patients receiving HAART present with liver-associated side effects, the liver is the predominant site of NRTI metabolism, and HepG2 cells have relatively large numbers of mitochondria. Our data demonstrate that AZT and d4T have differential effects in terms of oxidative stress, mitochondrial integrity, and cytotoxicity in endothelial cells. We also examine the dependence of these effects upon AZT- and d4T-phosphorylation and mtDNA depletion.
Methods
Cell Lines and Drug Treatment
HAECs and human liver HepG2 cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and incubated at 5% CO2 and 37°C. HAECs were cultured in endothelial cell media-2 with the provided SingleQuot cytokines and growth factors (EGM2 bullet kit; Lonza, Basel, Switzerland) enhanced with 10% fetal bovine serum (FBS). HepG2 s were cultured in minimum essential medium (Eagle) adjusted with 10% FBS and 1% penicillin/streptomycin. The NRTIs, AZT, d4T, and zalcitabine (ddC), were kindly provided by Dr. Raymond Schinazi, and were used at a final concentration of 1 μM unless otherwise noted. Cell culture-grade sterile phosphate-buffered saline (PBS) was used to make NRTI stocks, and also used as vehicle in control cells. For long-term studies, cells were treated with fresh vehicle, NRTI, and medium every 48 h until completion of the study. Cells were trypsinized with 0.05% trypsin and 0.1% EDTA, and split once every week. Rotenone (5 μM for 1 h) and ddC (1 μM for 5 weeks) were used as positive controls for mitochondrial dysfunction and mtDNA depletion, respectively. Experiments were designed such that cells from all time points were harvested simultaneously and at the same passage number (i.e., P-8). Vehicle-treated control cells did not demonstrate any appreciable differences in the experimental outputs measured over time (data not shown); thus, the control values depicted represent data from cells treated with vehicle for 5 weeks. Cells were harvested when they reached ~85% confluence. Neither AZT nor d4T treatment for up to 5 weeks affected cell growth as determined by the MTT proliferation assay (data not shown). All other chemicals were obtained from Sigma (St. Louis, MO) unless otherwise noted.
Electron Spin Resonance (ESR) Measurement of Superoxide
Superoxide was determined by measuring the oxidation of the spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5, 5-tetramethylpyrrolidine (CMH, Alexis Biochemicals Corp., San Diego, CA) [24]. Cells were grown in 10 cm tissue culture plates and washed twice with warm deoxygenated Krebs–Hepes Buffer (KHB, 99 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2 × 2H2O, 1.2 mM MgSO4 × 7H2O, 25 mM NaHCO3, 1.0 mM KH2PO4, 5.6 mM glucose, 20 mM Na-HEPES, pH 7.4) containing the chelating agents DETC (5 μM) and deferoxamine (25 μM). Stock concentrations of chelating agents were made fresh daily in deoxygenated 0.9% saline. CMH was dissolved in fresh deoxygenated KHB with chelating agents at a final concentration of 0.5 mM, cells were washed with KHB, and CMH was added to cells for 1 h at 37°C. After incubation, the CMH solution was aspirated completely, cells were scraped with 500 μL CMH-free KHB, added to a 1 ml insulin syringe U-100 (BD Biosciences, San Jose, CA) and frozen in liquid N2. Cells were pre-incubated with 50 U/ml polyethylene-glycol superoxide dismutase (PEG-SOD) for 4 h before addition of CMH to measure SOD-inhibited superoxide. Values were normalized to total protein levels as determined by the Pierce (Rockford, IL) bicinchonic acid (BCA) assay. ESR measurements were done on the Bruker EMX ESR spectrometer with the following settings: microwave power, 10 mW; modulation frequency, 100 kHz; modulation amplitude, 5 G; field center, 3,340 G; sweep width, 80 G; microwave frequency, 9.39 GHz; conversion time, 655 ms; time constant, 5.24 s; number of scans, 2; sweep time, 60 s.
GSH/GSSG Measurement by HPLC
Reduced glutathione (GSH) and oxidized glutathione disulfide (GSSG) were derivatized as follows. Cells were washed twice with cold PBS, 500 μl of 5% perchloric acid/0.2 M boric acid/10 μM γ-glutamylglutamate solution were added, and cells were scraped into a 1.5 ml micro-centrifuge tube. Cells were centrifuged at 13,000 × g for 5 s, and 300 μl of supernatant was pipetted into new tubes. The protein pellets were dissolved in 1 M NaOH and diluted for determination of protein concentration using the BCA assay. Fresh stocks of iodoacetic acid (7.4 mg/ml in water) and dansyl chloride (20 mg/ml in acetone) were prepared. Iodoacetic acid (60 μl) was added to each 300 μl supernatant, the pH was adjusted to 9.0 ± 0.2 with 1 M KOH, and the tubes were incubated for 20 min at room temperature in the dark. Then 300 μl of dansyl chloride was added to each tube and incubated for a further 24 h before adding 500 μl of chloroform. High performance liquid chromatography (HPLC) analysis was done as previously described [25].
Mitochondrial Superoxide Measurement
Mitochondrial superoxide was measured using the Mito-SOX Red dye for live-cell imaging (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Cells were plated at a density of 10,000 cells/well in a white-walled 96-well plate. Cells were incubated with 5 μM working solution of MitoSOX for 10 min at 37°C, washed gently three times with warm Hank’s balanced salt solution (HBSS) with calcium and magnesium, and fluorescence was measured in a Wallac 1420 plate reader (Perkin Elmer, Boston, MA) with excitation/emission of 510/580 nm. Cells that were not incubated with MitoSOX were used as blanks. After reading the fluorescence, the relative fluorescence units (RFU) were normalized to the number of cells, and data were graphed as a percentage of control values.
Mitochondrial Membrane Potential (ΔΨmt)
Relative ΔΨmt was determined in cells incubated with a cationic mitochondrial membrane potential dye (Dual Censor MitoCasp, Cell Technology, Mountain View, CA) according to manufacturer’s instructions. After 60 min of incubation, cells were washed with PBS three times and fluorescence was measured in a Wallac 1420 plate reader. Excitation/emission wavelengths were 540/580 nm. After reading the fluorescence, RFU values were normalized to the number of cells, and data were graphed as a percentage of control values.
Lactate Release Measurement
Fresh medium, AZT, and d4T were added to cells grown in 10 cm plates 24 h prior to lactate measurement. Lactate standards and medium from cell cultures were added to 96-well plates at 10 μl/well. Stock medium was used as a blank. Lactate reagent (200 μl) was added to each well, the plates were incubated at 37°C for 5 min, and the absorbance was measured at 540 nm. Values were normalized to the total cellular protein using the BCA assay.
Phosphorylated NRTI Quantification
Levels of phosphorylated [3H]-AZT and [3H]-d4T metabolites were determined by HPLC as previously described [26].
mtDNA Quantification
Total DNA was isolated from cell cultures using the Wizard SV Genomic DNA Purification System by Promega (Madison, WI), and 10 ng of DNA were added to each well of a 96-well plate for amplification by real-time PCR (9700 HT Sequence Detection System, Applied Biosystems) for mtDNA- and nDNA-encoded genes (cytochrome oxidase II and 18 s ribosomal RNA, respectively) according to methods previously described [27]. All samples were performed in duplicate and repeated at least three times. During analysis, the amount of target (i.e., mtDNA gene) was normalized to the amount of an endogenous control (i.e., nDNA gene), and expressed relative to the vehicle-treated control.
Statistical Analysis
One-way ANOVA with Dunnett’s post-test was used for statistical analysis for each drug group. Significance was set at P < 0.05 and data were expressed as mean ± SEM. Experiments were repeated at least twice, and samples were run in duplicate or triplicate.
Results
AZT Treatment Increases Oxidative Stress in HAECs
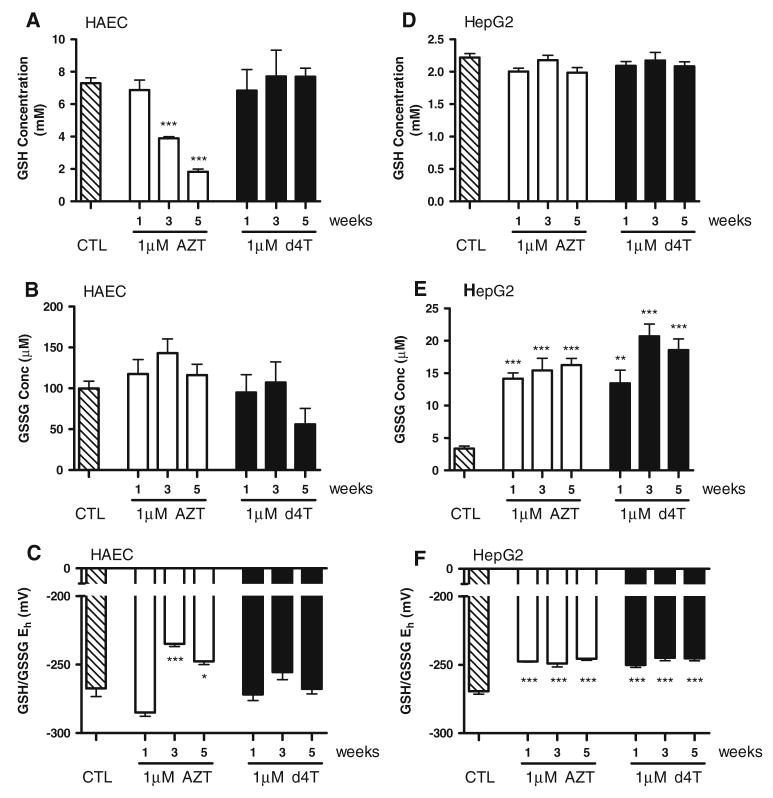
To monitor the induction of oxidative stress in endothelial cells, we treated HAECs with 1 μM AZT or d4T over a 5-week period, and measured levels of glutathione by HPLC. This concentration of NRTI falls within the range of steady-state NRTI plasma levels in HIV-patients receiving antiretroviral therapy [28, 29]. Exposure of HAECs to this concentration of AZT significantly lowered levels of GSH in a time-dependent manner (Fig. 1a). GSH concentrations dropped from 7.33 ± 0.32 mM in control cells to 3.88 ± 0.11 mM at week 3 and 1.83 ± 0.16 mM at week 5 of AZT treatment. Exposure of HAECs to 1 μM d4T, however, did not decrease GSH levels throughout the 5-week study. Neither AZT nor d4T significantly affected oxidized glutathione disulfide (GSSG, Fig. 1b). As shown in Fig. 1c, steady-state GSH/GSSG redox potential was concomitantly more oxidized in AZT-treated cells at weeks 3 and 5 (−234.9 ± 1.99 mV and −247.7 ± 2.48 mV, respectively) than in vehicle-treated control cells (−267.4 ± 5.96 mV), in correlation with the decreased GSH levels. No significant changes in GSH/GSSG redox potential were demonstrated with d4T.
Fig. 1.
Long-term exposure to a physiologically relevant concentration of AZT, but not d4T, oxidizes GSH/GSSG redox potential in HAECs. HAECs were treated with 1 μM AZT or d4T for the indicated time points, and glutathione was measured by HPLC. AZT time-dependently decreased levels of reduced glutathione (GSH) starting at week 3 and continuing until week 5 (a), but did not affect oxidized glutathione disulfide (GSSG) levels (b). c Redox potential, as calculated through the Nernst equation, was significantly more oxidized in AZT-treated cells from weeks 3 through 5. d4T did not affect GSH levels or redox potential at any time point in HAECs. HepG2 cells were treated with 1 μM AZT or d4T for the indicated time points, and GSH/GSSG was measured. AZT and d4T did not affect GSH levels (d), but raised GSSG levels (e) and oxidized redox potential (f) at all time points. n = 3–8. * P<0.05; *** P<0.001
AZT and d4T Increase Oxidative Stress in HepG2 Cells
HepG2 cells induced markers of oxidative stress, although in a slightly different manner than in HAECs. Unlike HAECs, HepG2 cells experienced no changes in reduced GSH levels throughout the study (Fig. 1d). However, HepG2 cells displayed significantly higher levels of GSSG with both AZT and d4T treatments at all time-points examined (Fig. 1e), resulting in a more oxidized redox potential in these cells (Fig. 1f).
AZT Increases Total Intracellular and Mitochondrial Superoxide in HAECs, but Only Mitochondrial Superoxide in HepG2 Cells
Chronic oxidative stress suggests an increase in superoxide. We, therefore, treated HAECs with either 1 μM AZT or d4T, and quantified total intracellular superoxide by measuring oxidation of the superoxide spin probe CMH using ESR spectroscopy (Fig. 2a). Compared to vehicle-treated control cells, long-term exposure of HAECs to 1 μM AZT significantly increased total superoxide 2.2-fold after 3 weeks and 1.8-fold after 5 weeks. Although not statistically significant, there was also a trend toward increased superoxide after only 1 week of exposure to AZT. Treatment with d4T failed to increase superoxide levels at any time point beyond control values in HAECs. Because mitochondria are believed to be the main source of NRTI-induced toxicity, we next measured mitochondrial-specific superoxide in HAECs using the MitoSOX dye (Fig. 2b). This well-characterized dye is rapidly and selectively targeted to the mitochondria in live cells, where it reacts with superoxide and fluoresces upon excitation [30–32]. Compared to vehicle-treated control cells, exposure of HAECs to 1 μM AZT increased mitochondrial-specific superoxide at all time-points with statistical significance occurring after weeks 3 and 5 (i.e., 1.5-fold increase), whereas d4T did not alter mitochondrial superoxide at any time-point. Rotenone, an inhibitor of mitochondrial complex I, was used as a positive control for mitochondrial superoxide production.
Fig. 2.
1 μM AZT increases superoxide production in HAECs. HAECs (a–b) and HepG2 cells (c–d) were exposed to 1 μM AZT or d4T for up to 5 weeks. a Total cellular superoxide levels were quantified by incubating HAECs with the superoxide spin probe CMH and measuring the CṀ product by ESR spectroscopy. Cells were incubated with 50 U/ml PEG-SOD, and the levels of SOD-inhibited superoxide were graphed as relative units (RU) per μg protein. n = 4–9. * P <0.05; ** P < 0.01. b Mitochondria-specific superoxide was detected by measuring the fluorescence of HAECs incubated with MitoSOX dye. Cells were treated with 5 μM rotenone (Rot) for 1 h as a positive control for mitochondrial superoxide generation. Treatment with d4T did not increase total or mitochondrial superoxide at any time point in HAECs. n = 3–9. ** P < 0.01; *** P < 0.001. c Neither AZT nor d4T significantly increased overall superoxide levels in HepG2 cells as measured by ESR. n = 4–5. d AZT, but not d4T, time-dependently increased mitochondrial superoxide in HepG2 cells. n = 4–5. * P < 0.05; *** P < 0.001
AZT and d4T did not significantly increase the CMH signal in HepG2 cells at any time-point examined (Fig. 2c). AZT, but not d4T, time-dependently increased mitochondrial superoxide in HepG2 cells (Fig. 2d).
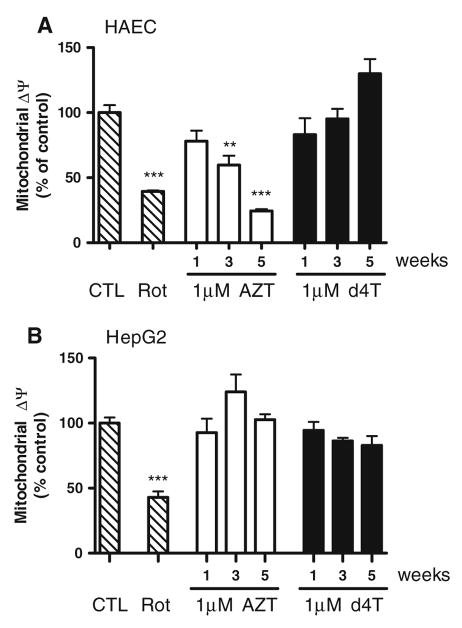
AZT Decreases Mitochondrial Membrane Potential in HAECs
To assess mitochondrial integrity and function, we analyzed changes in mitochondrial membrane potential (ΔΨmt) in AZT- and d4T-treated HAECs by measuring mitochondrial uptake of a fluorescent cationic dye (Fig. 3a). AZT time-dependently decreased ΔΨmt by ~40% after 3 weeks and ~76% after 5 weeks compared to vehicle-treated cells, while d4T did not have any effect. Rotenone was used as a positive control for ΔΨmt disruption. Neither AZT nor d4T decreased ΔΨmt in HepG2 cells (Fig. 3b).
Fig. 3.
AZT decreases mitochondrial membrane potential (ΔΨmt) in HAECs after 3 weeks exposure. a HAECs were incubated with a fluorescent cationic dye to assess mitochondrial membrane integrity. AZT, but not d4T, time-dependently decreased ΔΨmt starting at week 3 and continuing until week 5. n = 3–9. ** P < 0.01; *** P < 0.001. b Neither AZT nor d4T affected ΔΨmt in HepG2 cells. n = 3
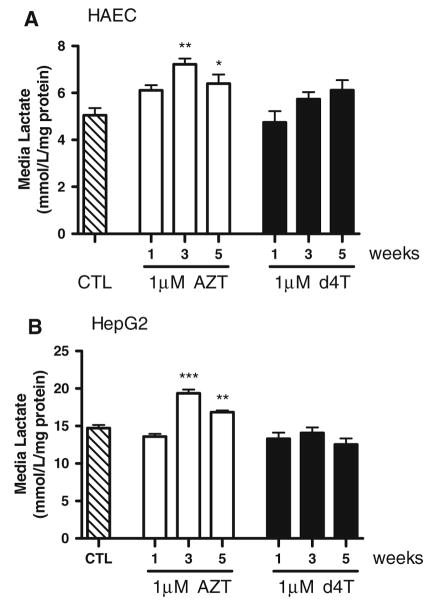
AZT Increases Lactate Release from HAECs and HepG2 Cells
Oxidative phosphorylation in mitochondria is the main source of energy production in cells. When mitochondrial function is impaired, cells derive their energy from cytosolic glycolysis, which produces lactate as a byproduct. Lactate release is therefore an indicator of mitochondrial dysfunction. AZT treatment significantly increased lactate release into the surrounding cell culture media at weeks 3 and 5 compared to vehicle in both HAECs (Fig. 4a) and HepG2 cells (Fig. 4b). Treatment with d4T did not enhance lactate release at any time-point in either cell line.
Fig. 4.
1 μM AZT, but not d4T, increases lactate release from HAECs and HepG2 cells after 3 weeks of treatment. Lactate was measured in the cell culture medium and normalized to total protein levels. Lactate release from HAECs (a) and HepG2 cells (b) was significantly higher at weeks 3 and 5 of AZT treatment, but did not change with d4T. n = 4–10. * P < 0.05; ** P < 0.01; *** P < 0.001
AZT-TP is not Detectable in HAECs or HepG2 Cells
Very little is known about the role of AZT or d4T phosphorylation in endothelial cell toxicity. In order to assess the degree of AZT- and d4T- phosphorylation, HAECs were incubated with 10 μM labeled drug for 2 and 6 h, and levels of phosphorylated metabolites were analyzed by HPLC (Table 1). AZT-MP was the only phosphorylated metabolite in HAECs. After 2 h exposure, AZT was present in either its non-phosphorylated state or as AZT-MP (145.0 ± 28.3 and 1,770 ± 175 pmol/106 cells, respectively). No AZT-DP or AZT-TP levels were detectable. After 6 h exposure of HAECs to AZT, levels of intracellular non-phosphorylated AZT and AZT-MP increased, yet no AZT-DP or AZT-TP levels were detectable.
Table 1.
Concentrations of phosphorylated AZT and d4T metabolites in HAEC and HepG2 cells
| Cellular concentration (pmol/106 cells) | ||||||
|---|---|---|---|---|---|---|
| Cell type | Cell line | Time (h) | AZT | AZT-MP | AZT-DP | AZT-TP |
| Aortic endothelium | HAEC | 2 | 145.0 ± 28.3 | 1770 ± 175 | ND | ND |
| 6 | 227.0 ± 50.3 | 2630 ± 159 | ND | ND | ||
| Liver | HepG2 | 2 | 14.62 ± 1.92 | 403.4 ± 28.7 | 2.69 ± 0.12 | ND |
| 6 | 31.89 ± 6.25 | 1009 ± 78.5 | 6.45 ± 0.68 | ND | ||
| Cell type | Cell line | Time (h) | d4T | d4T-MP | d4T-DP | d4T-TP |
|
| ||||||
| Aortic endothelium | HAEC | 2 | 11.69 ± 1.88 | 0.45 ± 0.17 | 1.17 ± 0.59 | 2.46 ± 1.16 |
| 6 | 19.16 ± 5.15 | 0.84 ± 0.06 | 1.75 ± 0.65 | 3.77 ± 1.56 | ||
| Liver | HepG2 | 2 | 38.62 ± 1.27 | 2.78 ± 0.09 | 1.31 ± 0.19 | 7.56 ± 0.43 |
| 6 | 26.88 ± 4.73 | 1.88 ± 0.34 | 0.75 ± 0.12 | 5.03 ± 1.06 | ||
Cells were treated with 10 μM labeled AZT or d4T for 2 or 6 h as indicated, and levels of phosphorylated metabolites were analyzed by HPLC. Results are presented as mean ± SEM. ND = not detected, n = 3 for each time point
Conversely, 2 h treatment of HAECs with d4T resulted in mainly non-phosphorylated intracellular d4T (11.69 ± 1.88 pmol/106 cells) with smaller amounts of d4T-MP, d4T-DP, and d4T-TP (0.45 ± 0.17, 1.17 ± 0.59, and 2.46 ± 1.16 pmol/106 cells, respectively). This same trend was observed in HAECs incubated with d4T for 6 h, although concentrations of each moiety were considerably higher.
HepG2 cells treated with AZT produced mainly AZT-MP, with smaller levels of non-phosphorylated AZT and AZT-DP. Levels of AZT-MP in HepG2 cells (403.4 ± 28.7 pmol/106 cells) were still considerably less than those in HAECs (1,770 ± 175 pmol/106 cells) at that same time-point. No AZT-TP was detectable in HepG2 cells. Levels of intracellular d4T in HepG2 cells followed a trend similar to those in HAECs, with non-phosphorylated d4T being the predominant metabolite and smaller levels of d4T-TP, d4T-MP, and d4T-DP detected.
AZT does not Alter mtDNA Levels in HAECs
mtDNA depletion is associated with mitochondrial toxicity due to inhibition of pol-γ. Real-time quantitative PCR analysis using probes against mtDNA- and nDNA-encoded genes show that neither 1 μM AZT nor d4T lowered mtDNA copy number in HAECs, even after 5 weeks of exposure (Table 2). Cells were treated with 1 μM ddC for 5 weeks as a positive control for mtDNA depletion. In contrast, AZT time-dependently decreased mtDNA levels in HepG2 cells with statistical significance occurring at 5 weeks. Treatment of HepG2 cells with d4T depleted mtDNA levels at all time points analyzed.
Table 2.
mtDNA levels in HAECs and HepG2 cells treated with 1 μM AZT or d4T for 5 weeks
| Cell line | Treatment | Time (weeks) | Ratio (mtDNA:nDNA) | n | P value |
|---|---|---|---|---|---|
| HAEC | Vehicle | 5 | 1.03 ± 0.10 | 7 | |
| ddC (+) | 5 | 0.42 ± 0.09 | 4 | < 0.05 | |
| AZT | 1 | 0.88 ± 0.09 | 7 | > 0.05 | |
| 3 | 1.10 ± 0.10 | 8 | > 0.05 | ||
| 5 | 1.06 ± 0.17 | 8 | > 0.05 | ||
| d4T | 1 | 0.89 ± 0.22 | 3 | > 0.05 | |
| 3 | 0.84 ± 0.07 | 5 | > 0.05 | ||
| 5 | 0.87 ± 0.07 | 8 | > 0.05 | ||
| HepG2 | Vehicle | 5 | 1.02 ± 0.10 | 4 | |
| ddC (+) | 5 | 0.08 ± 0.01 | 3 | < 0.001 | |
| AZT | 1 | 0.91 ± 0.13 | 4 | > 0.05 | |
| 3 | 0.70 ± 0.14 | 5 | > 0.05 | ||
| 5 | 0.51 ± 0.03 | 5 | < 0.01 | ||
| d4T | 1 | 0.45 ± 0.01 | 5 | < 0.001 | |
| 3 | 0.34 ± 0.09 | 4 | < 0.001 | ||
| 5 | 0.45 ± 0.05 | 4 | < 0.001 |
HAECs and HepG2 cells were treated for the indicated lengths of time with vehicle, 1 μM ddC (as a positive control for mtDNA depletion), 1 μM AZT or 1 μM d4T. The mitochondrial to nuclear DNA ratio (mtDNA:nDNA) was determined by quantitative PCR using primers against the mtDNA-encoded gene for cytochrome oxidase II and the nDNA-encoded gene for 18 S ribosomal RNA. A ratio of ~1 indicates no toxicity and a ratio <1 indicates mtDNA depletion. Values are presented as mean ± SEM, and statistics were obtained using one-way ANOVA and Dunnett’s post-test versus vehicle-treated cells
AZT Causes Cell Death in HAECs After 5 Weeks of Treatment
Overall cytotoxicity was assessed by measuring adenylate kinase release. The presence of adenylate kinase in the surrounding cell culture medium indicates a compromise in plasma membrane integrity, and is used as a marker of cell death. Using this approach, overall cell death was only found to occur in HAECs that had been exposed to 1 μM AZT for up to 5 weeks (Fig. 5a). No toxicity was observed in 1-week or 3-week AZT-treated HAECs, or at any time-point for d4T-treated cells. No cell death was observed in HepG2 cells treated with either drug (Fig. 5b).
Fig. 5.
AZT causes endothelial cell death after 5 weeks exposure. Cytotoxicity was determined by measuring the release of adenylate kinase into the cell culture medium and normalizing to total protein levels. a Cell death was only observed in AZT-treated HAECs after 5 weeks, and was never observed in d4T-treated HAECs. n = 4–5. *** P < 0.001. b Neither AZT nor d4T caused cell death in HepG2 cells. n = 5
Discussion
Antiretroviral drug regimens employing NRTIs are closely associated with the development of vascular complications in HIV patients [8]. While much has been learned about NRTI toxicity in other cell types, our understanding of NRTI toxicity in endothelial cells is limited. To replicate long-term exposure of endothelial cells to NRTIs, HAECs were treated with 1 μM AZT or d4T for up to 5 weeks. Our data show that a physiologically relevant concentration of AZT causes oxidative stress, mitochondrial dysfunction, and cell death in HAECs upon long-term exposure. Although there were some similarities between HAECs and the unrelated HepG2 hepatic cell line, HAECs eventually demonstrated enhanced total cellular superoxide levels, ΔΨmt disruption, and cell death in response to AZT while HepG2 cells did not. Our studies also reveal that AZT elicits its toxic effects in HAECs without depleting mtDNA, and that AZT causes more endothelial cell damage than d4T. Together, these observations highlight the susceptibility of endothelial cells to AZT-mediated toxicity, which is due, at least in part, to AZT’s pro-oxidative properties.
AZT and its Pro-Oxidative Effects in HAECs
Long-term AZT treatment of HAECs significantly increased markers of oxidative stress and mitochondrial damage. AZT altered GSH/GSSG redox potential, elevated total cellular and mitochondrial superoxide levels, caused ΔΨmt disruption, increased lactate release, and induced endothelial cell death. Moreover, these effects occurred without accumulation of AZT-TP and mtDNA depletion. The lack of detectable AZT-TP in our study and others [33–36], coupled with a lack of mtDNA depletion [37, 38], suggest that the pro-oxidative effects of AZT in certain cell types may arise from secondary targets or through AZT-specific redox properties, and not necessarily through mtDNA depletion or mutation.
The pro-oxidant properties of AZT-MP may contribute to generation of ROS in endothelial cells as AZT-MP is clearly the predominant metabolite in our study. AZT-MP does not transport out of the mitochondrial matrix after formation [36], and its dephosphorylation rate is only ~2% of that for endogenous thymidine-MP [39]. The continual accumulation of AZT-MP in the mitochondrion may lead to gradual enhancement of free radical production and oxidative damage over time. Others have postulated that AZT and AZT-MP competitively inhibit thymidine phosphorylation, which negatively affects mtDNA replication or repair [36, 40]. Additionally, other undefined secondary targets of AZT (or AZT-MP) may exist. AZT treatment has been suggested to inhibit cytochrome c oxidase and citrate synthase [38], poly-ADP-ribose polymerase [41], glycosphingolipid synthesis [42], and the ADP/ATP translocator [43]. Recently, Lund et al. [37] showed that AZT and AZT-MP directly interact with complex I of the mitochondrial electron transport chain and prevent its cAMP-dependent phosphorylation independently of pol-γ inhibition. These findings suggest that the pro-oxidative properties long associated with AZT treatment may be due to its interaction with a secondary target other than pol-γ in endothelial cells.
AZT is also unique among NRTIs in that it contains a 3′-azido group. Researchers have found that AZT and its azido-containing derivatives (AZT-MP, AZT-TP, and glucoronidated-AZT) have direct pro-oxidant activities in an in vitro cell-free chemical system, while non-azido-containing derivatives did not [19]. Furthermore, AZT and AZT-MP induce lipid peroxidation in membrane preparations devoid of cellular constituents and presumably most secondary targets [22]. Thus, AZT may have pro-oxidant properties inherent in its chemical structure. The azido group of AZT increases its lipophilicity and targets it to membranes, including those of the mitochondria [44]. This characteristic may partly explain the ability of AZT to cause lipid peroxidation in membrane preparations [22] and to interfere with cardiolipin molecules in the internal mitochondrial membrane [45]. Further exploration into AZT metabolism and redox chemistry, as well as its potential interaction with other mitochondrial targets, is necessary to determine its pro-oxidative mechanism of toxicity.
The inefficient ability of HAECs to phosphorylate AZT may partially explain the lack of mtDNA depletion. It is unclear why AZT-DP and AZT-TP are produced at such extremely low or undetectable levels in HAECs. Thymidylate kinase and pyrimidine nucleotide diphosphate kinase, the enzymes which produce AZT-DP and AZT-TP, respectively, are expressed in endothelial cells [46, 47]. It is equally puzzling considering that d4T uses the same deoxynucleotide kinases as AZT to achieve phosphorylation [48], yet d4T-DP and d4T-TP are readily detectable in our study. This observation does not go without precedent, however, as others have been unable to measure AZT-DP or AZT-TP in the perfused rat heart [33] and in mitochondria from rat heart and liver cells [34–36], suggesting that the lack of AZT tri-phosphorylation may be a tissue-specific phenomenon. Even in cells where AZT-TP is detectable, AZT-MP typically reaches low millimolar concentrations and greatly outnumbers AZT-TP by several orders of magnitude [48, 49]. Considering that AZT-TP has such poor affinity for pol-γ and that cells do not attain the high levels of AZT-TP necessary to inhibit the enzyme [50], our results demonstrating a lack of effect of AZT on mtDNA are consistent with little or no inhibition of pol-γ in endothelial cells. However, it is important to note that there are potential limitations in our phosphorylation data. We measured intracellular phosphorylated AZT and d4T metabolites after 6-h exposure to radiolabelled drug based upon the published in vitro half-lives and the fact that AZT is taken twice daily and the highest levels of the drug are found in the plasma several hours after treatment [51, 52]. A recent report by Olivero [53] has shown that the measurement of AZT incorporation into DNA may require up to 72-h exposure in cultured human mammary epithelial cells. Measurement of AZT incorporation into mtDNA in HAECs would be very informative; however, isolation of mitochondria from HAECs has been unsuccessful in our hands due to their extremely low number of mitochondria. In addition to these difficulties isolating mitochondria, measuring the levels of AZT is extremely difficult because AZT is a chain terminator and too few molecules are present to detect even with radioactive material. The phosphorylation of AZT also appears to be highly variable between individuals [53–55]. Thus, it is uncertain whether our commercially obtained HAECs are representative of enzymatic activity from the general population. Nevertheless, our phosphorylation and mtDNA:nDNA data from Tables 1 and 2 strongly suggest that AZT likely causes toxicity in HAECs quite differently than in HepG2 cells.
The current study extends upon observations by Jiang et al. [12] who demonstrated enhanced mitochondrial superoxide production and ΔΨmt disruption in human umbilical vein endothelial cells. They observed these effects in cells treated for <24 h with a slightly higher concentration of AZT than that used in our study. Despite the induction of oxidative stress and mitochondrial damage at week 3, we did not observe an overall increase in cell death until week 5. In the aforementioned study [12], apoptosis was not detected in AZT-treated cells at 24 h. AZT-induced oxidative stress and mitochondrial damage likely accumulate over time until a threshold level is reached, whereupon irreversible cytotoxicity and cell death occurs. The mitochondrion may have its own tissue-specific threshold capacity for electron transport chain inhibition until total mitochondrial output is affected [56]. Thus, for AZT-induced cell death to occur, endothelial cells may require longer exposure to drug (up to 5 weeks) for accumulated ROS to reach individual mitochondrion and total cell respiratory thresholds.
The Lack of d4T Toxicity in HAECs
AZT and d4T caused different effects in HAECs despite the fact that both drugs are thymidine analogues and belong to the same mechanistic class of antiretrovirals. While AZT increased oxidative stress and mitochondrial damage in a relatively time-dependent manner, d4T treatment never induced toxicity in HAECs. The lack of d4T toxicity in HAECs is not due to compromised activity of the drug as d4T treatment of HepG2 cells oxidized GSH/GSSG redox potential and depleted mtDNA. The lack of d4T-induced mtDNA depletion in HAECs is surprising given that d4T-TP has a relatively high affinity for pol-γ [57–59]. The low concentration of d4T, coupled with its high rate of intracellular metabolism (<3 h) [51], may prevent the accumulation of d4T-TP beyond the threshold level needed to inhibit pol-γ in HAECs. Cell type-specific differences in respiratory requirements, pol-γ activity, mitochondrial turnover, nucleotide transport, or other factors may exist in HAECs which could explain the lack of d4T toxicity in these cells.
The Unique Pro-Oxidative Effects of AZT in HAECs Versus HepG2 Cells
As mentioned previously, AZT did not decrease HAEC mtDNA levels at any time-point, yet AZT still produced severe oxidative stress, mitochondrial damage, and cell death. Although AZT eventually decreased mtDNA levels in HepG2 cells, AZT did not increase HepG2 total cellular superoxide levels as measured by ESR, nor did AZT cause ΔΨmt collapse or cell death. These differences indicate that AZT, and potentially other NRTIs, have cell-specific effects, and that AZT’s pro-oxidative properties contribute to the increased susceptibility of endothelial cells to AZT toxicity as determined by the ability of AZT to cause cell death in HAECs and not in HepG2 cells.
In conclusion, we have shown that GSH depletion, total cellular and mitochondrial-specific superoxide, mitochondrial dysfunction, and ultimately cytotoxicity develop in endothelial cells upon long-term exposure to AZT but not to d4T. These effects occurred in the absence of AZT-DP and AZT-TP, and without mtDNA depletion. Our findings also show that endothelial cells are extremely susceptible to the pro-oxidative effects of AZT, and that AZT eventually caused cell death in HAECs but not in an unrelated HepG2 cell line. Further exploration into how AZT exerts endothelial cell toxicity and promotes oxidative stress will benefit future drug development and reduce the risk of vascular complications in HIV patients.
Acknowledgments
This research was funded by NIH RO1 HL070892 to R.L.S.
References
- 1.Hung CC, Chang SC. Impact of highly active antiretroviral therapy on incidence and management of human immunodeficiency virus-related opportunistic infections. The Journal of Antimicrobial Chemotherapy. 2004;54(5):849–853. doi: 10.1093/jac/dkh438. [DOI] [PubMed] [Google Scholar]
- 2.Rathbun RC, Lockhart SM, Stephens JR. Current HIV treatment guidelines—An overview. Current Pharmaceutical Design. 2006;12(9):1045–1063. doi: 10.2174/138161206776055840. [DOI] [PubMed] [Google Scholar]
- 3.Day BJ, Lewis W. Oxidative stress in NRTI-induced toxicity: Evidence from clinical experience and experiments in vitro and in vivo. Cardiovascular Toxicology. 2004;4(3):207–216. doi: 10.1385/CT:4:3:207. [DOI] [PubMed] [Google Scholar]
- 4.Lewis W, Grupp IL, Grupp G, Hoit B, Morris R, Samarel AM, et al. Cardiac dysfunction occurs in the HIV-1 transgenic mouse treated with zidovudine. Laboratory Investigation. 2000;80(2):187–197. doi: 10.1038/labinvest.3780022. [DOI] [PubMed] [Google Scholar]
- 5.Nolan D, Mallal S. Complications associated with NRTI therapy: Update on clinical features and possible pathogenic mechanisms. Antiviral Therapy. 2004;9(6):849–863. [PubMed] [Google Scholar]
- 6.Velsor LW, Kovacevic M, Goldstein M, Leitner HM, Lewis W, Day BJ. Mitochondrial oxidative stress in human hepatoma cells exposed to stavudine. Toxicology and Applied Pharmacology. 2004;199(1):10–19. doi: 10.1016/j.taap.2004. 03.005. [DOI] [PubMed] [Google Scholar]
- 7.Lewis W, Kohler JJ, Hosseini SH, Haase CP, Copeland WC, Bienstock RJ, et al. Antiretroviral nucleosides, deoxynucleotide carrier and mitochondrial DNA: Evidence supporting the DNA pol gamma hypothesis. AIDS (London, England) 2006;20(5):675–684. doi: 10.1097/01.aids.0000216367.23325.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr A. Cardiovascular risk factors in HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes. 2003;34(Suppl 1):S73–S78. doi: 10.1097/00126334-200309011-00011. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz MW, Stephan C, Harmjanz A, Staszewski S, Buehler A, Bickel M, von Kegler S, Ruhkamp D, Steinmetz H, Sitzer M. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2007;196:720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Hebert VY, Crenshaw BL, Romanoff RL, Ekshyyan VP, Dugas TR. Effects of HIV drug combinations on endothelin-1 and vascular cell proliferation. Cardiovascular Toxicology. 2004;4(2):117–131. doi: 10.1385/CT:4:2:117. [DOI] [PubMed] [Google Scholar]
- 11.Fiala M, Murphy T, MacDougall J, Yang W, Luque A, Iruela-Arispe L, et al. HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovascular Toxicology. 2004;4(4):327–337. doi: 10.1385/CT:4:4:327. [DOI] [PubMed] [Google Scholar]
- 12.Jiang B, Hebert VY, Li Y, Mathis JM, Alexander JS, Dugas TR. HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production, but not apoptosis. Toxicology and Applied Pharmacology. 2007;224:60–71. doi: 10.1016/j.taap.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Sutliff RL, Dikalov S, Weiss D, Parker J, Raidel S, Racine AK, et al. Nucleoside reverse transcriptase inhibitors impair endothelium-dependent relaxation by increasing superoxide. American Journal of Physiology Heart and Circulatory Physiology. 2002;283(6):H2363–H2370. doi: 10.1152/ajpheart.00151.2002. [DOI] [PubMed] [Google Scholar]
- 14.Jiang B, Hebert VY, Zavecz JH, Dugas TR. Antiretrovirals induce direct endothelial dysfunction in vivo. Journal of Acquired Immune Deficiency Syndromes. 2006;42(4):391–395. doi: 10.1097/01.qai.0000228790.40235.0c. [DOI] [PubMed] [Google Scholar]
- 15.Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nature Reviews Drug Discovery. 2003;2(10):812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- 16.Furman PA, Fyfe JA, Clair MH, Weinhold K, Rideout JL, Freeman GA, et al. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(21):8333–8337. doi: 10.1073/pnas.83.21. 8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clinical Therapeutics. 2000;22(6):685–708. doi: 10.1016/S0149-2918(00)90004–3. [DOI] [PubMed] [Google Scholar]
- 18.Pinti M, Salomoni P, Cossarizza A. Anti-HIV drugs and the mitochondria. Biochimica et Biophysica Acta. 2006;1757(5–6):700–707. doi: 10.1016/j.bbabio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Komarov AM, Hall JM, Weglicki WB. Azidothymidine promotes free radical generation by activated macrophages and hydrogen peroxide-iron-mediated oxidation in a cell-free system. Biochimica et Biophysica Acta. 2004;1688(3):257–264. doi: 10.1016/j.bbadis.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-de-la-Asuncion J, Gomez-Cambronero LG, Del Olmo ML, Pallardo FV, Sastre J, Vina J. Vitamins C and E prevent AZT-induced leukopenia and loss of cellularity in bone marrow. Studies in mice. Free Radical Research. 2007;41(3):330–334. doi: 10.1080/10715760600868537. [DOI] [PubMed] [Google Scholar]
- 21.de la Asuncion JG, Del Olmo ML, Gomez-Cambronero LG, Sastre J, Pallardo FV, Vina J. AZT induces oxidative damage to cardiac mitochondria: protective effect of vitamins C and E. Life Sciences. 2004;76(1):47–56. doi: 10.1016/j.lfs.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Mak IT, Nedelec LF, Weglicki WB. Pro-oxidant properties and cytotoxicity of AZT-monophosphate and AZT. Cardiovascular Toxicology. 2004;4(2):109–115. doi: 10.1385/CT: 4:2:109. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Katoh I, Kurata S. Azidothymidine causes functional and structural destruction of mitochondria, glutathione deficiency and HIV-1 promoter sensitization. European Journal of Biochemistry. 2002;269(11):2782–2788. doi: 10.1046/j.1432-1033.2002.02954.x. [DOI] [PubMed] [Google Scholar]
- 24.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49(4):717–727. doi: 10.1161/01.HYP.0000258594. 87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DP. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods in Enzymology. 2002;348:93–112. doi: 10.1016/S0076-6879(02)48630–2. [DOI] [PubMed] [Google Scholar]
- 26.Gustafson EA, Schinazi RF, Fingeroth JD. Human herpesvirus 8 open reading frame 21 is a thymidine and thymidylate kinase of narrow substrate specificity that efficiently phosphorylates zidovudine but not ganciclovir. Journal of Virology. 2000;74(2):684–692. doi: 10.1128/JVI.74.2.684-692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuyver LJ, Lostia S, Adams M, Mathew JS, Pai BS, Grier J, et al. Antiviral activities and cellular toxicities of modified 2′, 3′-dideoxy-2′, 3′-didehydrocytidine analogues. Antimicrobial Agents and Chemotherapy. 2002;46(12):3854–3860. doi: 10.1128/AAC.46.12.3854–3860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakuda TN, Page LM, Anderson PL, Henry K, Schacker TW, Rhame FS, et al. Pharmacological basis for concentration-controlled therapy with zidovudine, lamivudine, and indinavir. Antimicrobial Agents and Chemotherapy. 2001;45(1):236–242. doi: 10.1128/AAC.45.1.236-242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyer TP, Temesgen Z, Enger R, Estes L, Charlson J, Oliver L, et al. Drug monitoring of antiretroviral therapy for HIV-1 infection: Method validation and results of a pilot study. Clinical Chemistry. 1999;45(9):1465–1476. [PubMed] [Google Scholar]
- 30.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochemical and Biophysical Research Communications. 2007;358(1):203–208. doi: 10.1016/j.bbrc. 2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(41):15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. American Journal of Physiology Heart and Circulatory Physiology. 2007;293(1):H610–H619. doi: 10.1152/ajpheart.00236. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Susan-Resiga D, Bentley AT, Lynx MD, LaClair DD, McKee EE. Zidovudine inhibits thymidine phosphorylation in the isolated perfused rat heart. Antimicrobial Agents and Chemotherapy. 2007;51(4):1142–1149. doi: 10.1128/AAC. 01227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynx MD, Bentley AT, McKee EE. 3′-Azido-3′-deoxythymidine (AZT) inhibits thymidine phosphorylation in isolated rat liver mitochondria: a possible mechanism of AZT hepatotoxicity. Biochemical Pharmacology. 2006;71(9):1342–1348. doi: 10.1016/j.bcp.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynx MD, McKee EE. 3′-Azido-3′-deoxythymidine (AZT) is a competitive inhibitor of thymidine phosphorylation in isolated rat heart and liver mitochondria. Biochemical Pharmacology. 2006;72(2):239–243. doi: 10.1016/j.bcp.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKee EE, Bentley AT, Hatch M, Gingerich J, Susan-Resiga D. Phosphorylation of thymidine and AZT in heart mitochondria: Elucidation of a novel mechanism of AZT cardiotoxicity. Cardiovascular Toxicology. 2004;4(2):155–167. doi: 10.1385/CT:4:2:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lund KC, Peterson LL, Wallace KB. Absence of a universal mechanism of mitochondrial toxicity by nucleoside analogs. Antimicrobial Agents and Chemotherapy. 2007;51(7):2531–2539. doi: 10.1128/AAC.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan-Zhou XR, Cui L, Zhou XJ, Sommadossi JP, Darley-Usmar VM. Differential effects of antiretroviral nucleoside analogs on mitochondrial function in HepG2 cells. Antimicrobial Agents and Chemotherapy. 2000;44(3):496–503. doi: 10.1128/AAC.44.3.496-503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzon C, Rampazzo C, Scaini MC, Gallinaro L, Karlsson A, Meier C, et al. Cytosolic and mitochondrial deoxyribonucleotidases: Activity with substrate analogs, inhibitors and implications for therapy. Biochemical Pharmacology. 2003;66(3):471–479. doi: 10.1016/S0006-2952(03)00290-9. [DOI] [PubMed] [Google Scholar]
- 40.Song S, Wheeler LJ, Mathews CK. Deoxyribonucleotide pool imbalance stimulates deletions in HeLa cell mitochondrial DNA. The Journal of Biological Chemistry. 2003;278(45):43893–43896. doi: 10.1074/jbc.C300401200. [DOI] [PubMed] [Google Scholar]
- 41.Szabados E, Fischer GM, Toth K, Csete B, Nemeti B, Trombitas K, et al. Role of reactive oxygen species and poly-ADP-ribose polymerase in the development of AZT-induced cardiomyopathy in rat. Free Radical Biology & Medicine. 1999;26(3–4):309–317. doi: 10.1016/S0891-5849(98)00199-3. [DOI] [PubMed] [Google Scholar]
- 42.Yan JP, Ilsley DD, Frohlick C, Steet R, Hall ET, Kuchta RD, et al. 3′-Azidothymidine (zidovudine) inhibits glycosylation and dramatically alters glycosphingolipid synthesis in whole cells at clinically relevant concentrations. The Journal of Biological Chemistry. 1995;270(39):22836–22841. doi: 10.1074/jbc.270.39.22836. [DOI] [PubMed] [Google Scholar]
- 43.Valenti D, Barile M, Passarella S. AZT inhibition of the ADP/ATP antiport in isolated rat heart mitochondria. International Journal of Molecular Medicine. 2000;6(1):93–96. doi: 10.3892/ijmm.6.1.93. [DOI] [PubMed] [Google Scholar]
- 44.Robins MJ, Wood SG, Dalley NK, Herdewijn P, Balzarini J, De Clercq E. Nucleic acid related compounds. 57. Synthesis, x-ray crystal structure, lipophilic partition properties, and antiretroviral activities of anomeric 3′-azido-2′,3′-dideoxy-2,6-diaminopurine ribosides. Journal of Medicinal Chemistry. 1989;32(8):1763–1768. doi: 10.1021/jm00128a017. [DOI] [PubMed] [Google Scholar]
- 45.Cazzalini O, Lazze MC, Iamele L, Stivala LA, Bianchi L, Vaghi P, et al. Early effects of AZT on mitochondrial functions in the absence of mitochondrial DNA depletion in rat myotubes. Biochemical Pharmacology. 2001;62(7):893–902. doi: 10.1016/S0006-2952(01)00713-4. [DOI] [PubMed] [Google Scholar]
- 46.Jansson O, Bohman C, Munch-Petersen B, Eriksson S. Mammalian thymidine kinase 2. Direct photoaffinity labeling with [32P]dTTP of the enzyme from spleen, liver, heart and brain. European Journal of Biochemistry. 1992;206(2):485–490. doi: 10.1111/j.1432-1033.1992.tb16951.x. [DOI] [PubMed] [Google Scholar]
- 47.Pearson JD, Carleton JS, Gordon JL. Metabolism of adenine nucleotides by ectoenzymes of vascular endothelial and smooth-muscle cells in culture. The Biochemical Journal. 1980;190(2):421–429. doi: 10.1042/bj1900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lavie A, Schlichting I, Vetter IR, Konrad M, Reinstein J, Goody RS. The bottleneck in AZT activation. Nature Medicine. 1997;3(8):922–924. doi: 10.1038/nm0897-922. [DOI] [PubMed] [Google Scholar]
- 49.Frick LW, Nelson DJ, St Clair MH, Furman PA, Krenitsky TA. Effects of 3′-azido-3′-deoxythymidine on the deoxynucleotide triphosphate pools of cultured human cells. Biochemical and Biophysical Research Communications. 1988;154(1):124–129. doi: 10.1016/0006-291X(88)90659-6. [DOI] [PubMed] [Google Scholar]
- 50.Sales SD, Hoggard PG, Sunderland D, Khoo S, Hart CA, Back DJ. Zidovudine phosphorylation and mitochondrial toxicity in vitro. Toxicology and Applied Pharmacology. 2001;177(1):54–58. doi: 10.1006/taap.2001.9288. [DOI] [PubMed] [Google Scholar]
- 51.Ho HT, Hitchcock MJ. Cellular pharmacology of 2′, 3′-dideoxy-2′, 3′-didehydrothymidine, a nucleoside analog active against human immunodeficiency virus. Antimicrobial Agents and Chemotherapy. 1989;33(6):844–849. doi: 10.1128/aac.33.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian M, Bui T, Ho RJ, Unadkat JD. Metabolism of 3′-azido-3′-deoxythymidine (AZT) in human placental trophoblasts and Hofbauer cells. Biochemical Pharmacology. 1994;48(2):383–389. doi: 10.1016/0006-2952(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 53.Olivero OA. Relevance of experimental models for investigation of genotoxicity induced by antiretroviral therapy during human pregnancy. Mutation Research. 2008;658(3):184–190. doi: 10.1016/j.mrrev.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng Q, Olivero OA, Fasco MJ, Bellisario R, Kaminsky L, Pass KA, et al. Plasma and cellular markers of 3′-azido-3′-dideoxythymidine (AZT) metabolism as indicators of DNA damage in cord blood mononuclear cells from infants receiving prepartum NRTIs. Environmental and Molecular Mutagenesis. 2007;48(3–4):307–321. doi: 10.1002/em.20298. [DOI] [PubMed] [Google Scholar]
- 55.Olivero OA, Shearer GM, Chougnet CA, Kovacs AA, Landay AL, Baker R, et al. Incorporation of zidovudine into leukocyte DNA from HIV-1-positive adults and pregnant women, and cord blood from infants exposed in utero. AIDS (London, England) 1999;13(8):919–925. doi: 10.1097/0000 2030-199905280-00007. [DOI] [PubMed] [Google Scholar]
- 56.Davey GP, Canevari L, Clark JB. Threshold effects in synaptosomal and nonsynaptic mitochondria from hippocampal CA1 and paramedian neocortex brain regions. Journal of Neurochemistry. 1997;69(6):2564–2570. doi: 10.1046/j.1471-4159.1997.69062564.x. [DOI] [PubMed] [Google Scholar]
- 57.Johnson AA, Ray AS, Hanes J, Suo Z, Colacino JM, Anderson KS, et al. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. The Journal of Biological Chemistry. 2001;276(44):40847–40857. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- 58.Lim SE, Copeland WC. Differential incorporation and removal of antiviral deoxynucleotides by human DNA polymerase gamma. The Journal of Biological Chemistry. 2001;276(26):23616–23623. doi: 10.1074/jbc.M101114200. [DOI] [PubMed] [Google Scholar]
- 59.Martin JL, Brown CE, Matthews-Davis N, Reardon JE. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrobial Agents and Chemotherapy. 1994;38(12):2743–2749. doi: 10.1128/aac.38.12.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]