Abstract
Objective
To evaluate the impact of ovarian cancer risk on the performance of the serum biomarkers mesothelin, HE4 and CA125.
Methods
We measured mesothelin, HE4 and CA125 levels from women with invasive ovarian cancer (n=143), benign gynecological conditions (n=124) and healthy women (n=344). Demographic, epidemiologic, reproductive, medical and family history data were collected using a standardized questionnaire. Pedigree and BRCA 1/2 test results were used to stratify women into average and high risk groups. The diagnostic accuracy of each biomarker was characterized using receiver operating characteristic (ROC) curve methods.
Results
Baseline characteristics did not vary by risk or case status. The distribution of stage and histology was similar in average and high risk women. All three markers discriminated ovarian cancer cases from risk-matched healthy and benign controls. Marker performance did not vary by risk status. The sensitivity at 95% specificity for discriminating cases from risk-matched healthy control women in the average and high risk groups respectively was 53.9% and 39.0% for mesothelin, 80.4% and 87.8% for HE4, and 79.4% and 82.9% for CA125. The performance of the markers was not as robust when cases were compared to benign controls. AUC values for cases vs. healthy and benign controls did not vary by risk status.
Conclusions
The ability of serum mesothelin, HE4 and CA 125 levels to discriminate ovarian cancer cases from healthy and benign controls is not influenced by risk status. Our findings support the pursuit of additional studies evaluating the early detection potential of these markers in high-risk populations.
Keywords: ovarian cancer screening, biomarkers, high-risk, molecular diagnosis and prognosis, molecular markers in prevention research, gynecological cancers: ovarian
INTRODUCTION
Ovarian cancer biomarker research is motivated by a strong desire to identify a panel of complementary biomarkers that will be useful for early detection of the disease. Diagnostic markers that can discriminate ovarian cancer cases at clinical diagnosis from cancer free controls with good sensitivity at high levels of specificity are candidate markers for early detection. Characterizing the performance of candidate early detection markers in women at increased ovarian cancer risk is of particular interest because women at high or elevated risk are ideal candidates for initial studies of new screening tests due to the low incidence of ovarian cancer in the general population.
The application of genomic and proteomic technologies has led to the identification of a number of ovarian cancer biomarkers that perform well when tested in the general population as diagnostic markers (1). Mesothelin and human epididymis protein 4 (HE4) are two of the most intensively studied of the novel biomarkers. Mesothelin is a 40-kDa polypeptide cell surface protein present on normal mesothelial lining cells. Mesothelin expression is increased in ovarian cancer tissues and a soluble form is detectable in blood (2–6). Elevated serum levels of mesothelin are detectable in 40–67% of patients with ovarian cancer (3). Mesothelin enhances the diagnostic performance of CA125, the only currently available and best validated ovarian cancer biomarker, at high levels of specificity that are relevant for early detection. In the general population, a composite marker that includes both CA125 and mesothelin increased the sensitivity at 98% specificity from 78.8% for CA 125 alone to 86.5% (7). HE4 is an 11-kDa protein that is a precursor to the epididymal secretory protein E4 and resides on human chromosome 20q12-13.1. HE4 is over expressed in 93% of serous epithelial ovarian cancers (8–10). In the general population, serum levels of HE4 are elevated in over 90% of women with ovarian cancer at diagnosis (11). Compared to CA125, HE4 is less frequently elevated in patients with benign gynecological disease (11). Because mesothelin and HE4 complement CA125 they are of particular interest for early detection.
Women at increased risk for developing ovarian cancer are ideal candidates for studies investigating novel early detection tests. Ovarian cancer is a relatively uncommon disease with an incidence of roughly 45 per 100,000 post menopausal women. The lifetime risk of developing ovarian cancer among unselected women in the US is roughly 1 in 65. A family history of ovarian cancer is the most important risk factor for developing the disease. Women with a single first-degree relative with ovarian cancer have a 5% lifetime risk for developing the disease. Approximately 7–10% of ovarian cancer cases occur in women with a strong family history of ovarian and/or breast cancer. A substantial proportion of the cancer prone phenotype in these high risk families is explained by mutations in the BRCA1 or BRCA2 genes. The lifetime risk for developing ovarian cancer for mutation positive women ranges from 15–40%, depending on the specific mutation. Interestingly, mutational testing of cohorts of ovarian cancer patients unselected for family history suggest that germline BRCA mutations contribute to ovarian cancer development in a larger proportion of cases than previously thought. Mutation rates as high as 11–15% have been observed in two recent population-based studies of unselected women with invasive ovarian cancer (12, 13). Furthermore it appears that germline mutations in BRCA1 and BRCA2 in unselected women confer a high risk of ovarian cancer similar to those women with multiple affected family members (14).
Tumors arising in women at increased genetic risk are molecularly distinct from sporadic ovarian cancer and have a different clinical behavior (15–18). Compared to sporadic cases, BRCA associated hereditary ovarian cancers are more likely to be serous subtype, of higher grade, contain more solid areas and to accumulate p53 (19). Even after controlling for clinical-pathologic features, BRCA-associated cancers have distinct gene expression profiles and respond better to standard therapies (20, 21). Differences in the clinical and molecular characteristics of sporadic and hereditary ovarian cancers raise the possibility that diagnostic performance of ovarian cancer biomarkers may differ in average and high risk women. We undertook this study to determine if there are differences in the diagnostic performance of CA125 and the novel candidate early detection markers HE4 and mesothelin in women at average and high risk for ovarian cancer.. Because our long term goal is applying these markers for use in early detection, we focused on testing the ability of the markers to discriminate ovarian cancer cases from healthy controls at high levels of specificity. We also included a benign ovarian tumor control group as benign tumors are a potential source of false positive screening tests.
Diagnostic performance is a necessary first step in evaluating candidate ovarian cancer early detection markers. However, assessment of their early detection potential requires validation in samples collected prior to clinical diagnosis when women are asymptomatic. This study provides evidence that the markers we studied have performance characteristics that support further testing in women at increased risk for ovarian cancer. This is the first study to report information on the relative diagnostic performance of these novel markers in average and high-risk populations. Our goal was to determine if these novel markers merit further study in screening programs designed for targeting women who are at high risk for ovarian cancer and to determine if findings from screening programs targeting high risk women are likely to be generalizable to women in the general population. Although high-risk women are ideal candidates for ovarian cancer screening trials and programs adequate performance in average risk women will be necessary to have a major impact on ovarian cancer mortality.
MATERIALS AND METHODS
Study Population
Women included in this report were enrolled between 1999 and 2003 in multiple IRB approved protocols associated with the Seattle-based Pacific Ovarian Cancer Research Consortium (POCRC), an NCI-funded Ovarian Cancer Specialized Program of Research Excellence (SPORE). All participants completed a standardized baseline questionnaire that queried women regarding a broad range of demographic, epidemiologic, and reproductive factors as well as personal or family cancer history. Risk for developing ovarian cancer was ascertained by self-report of personal and family history of cancer and BRCA gene mutation test results in study enrollment questionnaires. Women were specifically queried regarding the occurrence and age at diagnosis for breast, ovarian, colon, prostate and other cancers in first and second degree relatives. The criteria used to classify women as high risk for ovarian cancer are outlined in Table 1. We selected these criteria because they identify women with at least a 10% lifetime risk of developing ovarian cancer. Women with a less significant family history are classified as average risk. Specimen collection and processing protocols associated with each study are detailed below.
Table 1.
Criteria used to classify women as high-risk for ovarian cancer.
| • | The woman’s family contained at least two ovarian or breast cancer cases among the subject or her first or second degree relatives. This condition was satisfied by multiple primary cancers in the same person. In situations where breast cancer was used to meet this criterion, at least one breast cancer must have been diagnosed before menopause. If menopausal status was unknown, women under the age of 50 years were considered to be pre-menopausal; or |
| • | The women was of Ashkenazi Jewish ethnicity with one first-degree relative or two second-degree relatives with breast or ovarian cancer, or the woman was of Ashkenazi ancestry and had a personal history of breast cancer. As explained above, in situations where breast was used to meet this criterion, at least one breast cancer must have been diagnosed before menopause; or |
| • | The probability of carrying a BRCA I or BRCA II mutation given family pedigree of breast and ovarian cancer exceeded 20%. This was determined by the BRCAPRO 95% posterior probability interval. This criterion included women who tested positive for a BRCA I or BRCA II mutation and women who had a first or second degree relative with a BRCA I or BRCA II mutation. |
Ovarian cancer cases and women with benign gynecological disease (benign control group)
The POCRC Surgical Specimen Donation protocol identifies women undergoing gynecologic surgery for ovarian related conditions including benign and malignant disorders. These women are recruited and enrolled at the time of their pre-operative clinic visit. Up to 50 ml of blood is collected in the operating room after induction of anesthesia but prior to the onset of surgery. Serum is collected using Serum Separator Tubes (SST) (DB Vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ). The blood is allowed to coagulate at room temperature for up to four hours. The serum is then aliquoted and stored at −80° until analysis. The current study focuses on a representative sample of 143 women with invasive epithelial ovarian cancer and 124 women with benign ovarian tumors who were enrolled during the study interval. Diagnoses are confirmed by standardized review of medical records and examination of paraffin-embedded tissue by a research pathologist.
Healthy women
Healthy control women include participants in a local Seattle-based high-risk ovarian cancer screening program and women undergoing routine screening mammography at Swedish Medical Center - a large community-based hospital located in downtown Seattle. The ovarian cancer screening program focused on women at increased risk based on personal and family history of cancer and/or BRCA 1 and 2 gene mutation status as outlined in Table 1. Participants were screened quarterly with serum CA125 levels and transvaginal sonography (TVS) on an annual basis. Blood collection occurred in the outpatient setting at the time of ovarian cancer screening or mammography. Serum was collected and processed by the same staff and using an identical protocol as for Surgical Donation Protocol participants. All participants were determined to be free from all invasive cancers for a period of at least 5 years prior to and 2 years following the date of blood sample collection.
Laboratory Analysis
CA125 and HE4 serum levels were assessed using bead-based immunoassays performed as described by Scholler (9, 22). Anti-CA125 X52 mouse monoclonal antibody (mAb) and anti-HE4 3D8 mAb were biotinylated using the EZ-Link-sulfo-NHS-biotinylation kit (Pierce, Rockford, IL) according to the manufacturer’s instructions and dialyzed against Phosphate Buffered Saline (PBS) (Fisher BioReagents, Fair Lawn, NJ) using a dialysis slide (Slide-a-Lyzer 7kDa MWCO, Pierce). All incubations were carried out for 30 min, except as otherwise specified, in PBS supplemented with 1% Bovine Serum Albumin (PBS 1% BSA) (Sigma-Aldrich, St. Louis, MO). Washes were performed with PBS supplemented with 0.05% Tween-20 (PBST) (Sigma-Aldrich). Five µg/ml of anti-CA125 mAb X306 (Research Diagnostics, Inc, Flanders, NJ) was coupled to carboxy-coated beads. Antibody-coated beads were incubated with 4-fold diluted patient sera and captured antigen was detected with 2 µg/ml of biotinylated anti-CA125 mAb X52 (Research Diagnostics, Inc) followed by phycoerythrin-conjugated streptavidin (SA-PE) (Bio-Rad Laboratories Inc, Hercules, CA). Bead-based assays were carried out in 96 well MultiScreen®GV filter plates (Millipore Corporation, Billerica, MA) using a vacuum manifold (Millipore) to drain assay reagents. Plates were analyzed with the Bio-Plex Array reader (Bio-Rad). This procedure has been found to yield values that are strongly correlated (r >0.90) with the research standard CA125II RIA from Fujirebio Diagnostics, Inc. (FDI, Malvern, PA) (9).
Anti-HE4 mAbs 3D8 and 2H5 were kind gifts from Dr. Ingegerd Hellstrom. Anti-HE4 2H5 was coupled to beads at a concentration of 10 µg/ml with the following buffer modifications: bead activation buffer was made with 0.1 M Sodium Phosphate (NaH2PO4), pH 6.2 (Sigma-Aldrich). 1-ethyl-3-[3dimethylaminopropyl] carbodiimide hydrochloride (EDC) (Pierce, Rockford, IL) and N-hydroxysulfosuccinimide (S-NHS) (Pierce) were diluted to 38 mg/mL and to 109 mg/mL, respectively in activation buffer. The coupling buffer was made with 0.05 M 2[N-Morpholino] ethanesulfonic acid (MES), pH 5.0 (Sigma-Aldrich). PBS 1% BSA was used for bead blocking and storage buffers. Antibody-coated beads were incubated with 10-fold diluted sera. Captured antigens were detected with 2 µg/ml of biotinylated 3D8 followed by a 10 minute incubation with 1000-fold diluted SA-PE (Becton Dickinson Pharmingen, San Diego, CA).
Mesothelin serum levels were assessed using a novel bead-based immunoassay performed as described by Scholler (22). Briefly, carboxy-coated beads were conjugated to anti-mesothelin polyclonal antibody (pAb) (R&D Systems, Minneapolis, MN) at a concentration of 50 µg/ml. To capture antigen, antibody-coated beads were incubated with 5-fold diluted sera. Anti-mesothelin biobody (Bb) #7 at a concentration of 1 ug/ml (23) was preincubated with 2000-fold diluted PJ31S PhycoLink® Streptavidin-R-Phycoerythrin (Prozyme, San Leandro, CA) on ice and in the dark. Bead-captured antigens were detected with Bbs pre-incubated with PJ31S streptavidin. Buffers used for anti-mesothelin pAb bead conjugations were the same as the buffers used for anti-HE4 bead conjugations.
Statistical Analyses
Descriptive statistics were calculated for baseline characteristics of the healthy and benign control and ovarian cancer case groups for each risk category. Differences in the distribution of the baseline characteristics between the average and high risk women within each case-control group were compared using the Wilcoxon rank-sum test for continuous variables and chi-square for the categorical variables.
Serum levels of CA125, mesothelin and HE4 were log transformed and standardized to have mean 0 and standard deviation 1 in the average risk controls. This transformation induces the same measurement scale for all three markers, which promotes comparison among the three markers because their scales are the same (24).
The mean and standard deviation of the three biomarkers were calculated separately for the healthy controls, benign controls, and the cases. These values were also calculated by histology and stage within the cases. Student’s t-test was used to determine if there were statistically significant differences in the mean values of the markers between the average and high risk women.
The diagnostic accuracy of the three markers was assessed separately for the average risk and high risk women by estimating the ROC curves and area under the curve (AUC) statistics for cases versus healthy controls and cases versus benign controls (25). These receiver operating characteristic (ROC) curves display the misclassification rate (1-Specificity) in the controls, and the true classification rate in the cases (Sensitivity) (11). An AUC value of 1.0 represents perfect performance of the marker and 0.50 indicates a level of performance that is expected by chance alone. The aforementioned methods used to standardize the marker levels leave Receiver Operator Characteristic (ROC) curves and their p-values unchanged (7). We then compared the classification performance and equivalency of the three biomarkers by risk status using the non-parametric methods developed by DeLong and colleagues (26).
The STATA statistical software package (27) was used for these analyses. All statistical tests were two-sided and considered to be statistically significant at p≤0.05. No adjustments were made for multiple comparisons.
RESULTS
The baseline characteristics for the study population are presented in Table 2. The median age of cases was 58 and 57 for average and high-risk women respectively. Most features including age, BMI, or menopausal status did not vary by risk status in the either the case or control subgroups. Compared to average risk healthy controls, a larger proportion of high-risk healthy controls reported having ever used hormone replacement therapy (p<0.001). Average risk healthy controls were more likely to report their ethnicity as unknown (p<0.01).
Table 2.
Summary of the baseline characteristics for the average and high risk controls and cases.
| Characteristic | Healthy Controls (n=444) |
Benign Controls (n=124) |
Cases (n=143) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Average Risk (n=150) |
High Risk (n=294) |
p-value* | Average Risk (n=110) |
High Risk (n=14) |
p-value* | Average Risk (n=102) |
High Risk (n=14) |
p-value* | |
| Age, median (range) | 52 (30–75) | 50 (31–83) | 0.09 | 54 (27–83) | 51 (25–71) | 0.14 | 58 (35–86) | 57 (19–80) | 0.83 |
| BMI, median (range) | 24.5 (18.5–42.0) | 24.2 (16.2–43.9) | 0.82 | 25.8 (18.8–51.6) | 26.6 (17.8–50.6) | 0.92 | 25.6 (19.2–42.9) | 24.5 (20.6–53.7) | 0.90 |
| RACE | |||||||||
| White | 128 (85.3) | 269 (91.5) | 95 (86.4) | 13 (92.9) | 84 (82.4) | 37 (90.3) | |||
| Asian | 3 (2.0) | 3 (1.0) | 4 (3.6) | 0 | 5 (4.9) | 1 (2.4) | |||
| Black | 0 | 0 | 1 (0.9) | 0 | 0 | 1 (2.4) | |||
| Other† | 0 | 6 (2.0) | 3 (2.7) | 0 | 4 (3.9) | 0 | |||
| Unknown | 19 (12.7) | 16 (5.5) | 0.01 | 7 (6.4) | 1 (7.1) | 0.90 | 9 (8.8) | 2 (4.9) | 0.26 |
| MENOPAUSE STATUS, n (%) | |||||||||
| Pre-menopause | 53 (35.3) | 96 (32.7) | 17 (15.5) | 3 (21.4) | 13 (12.7) | 2 (4.9) | |||
| Post-menopause | 97 (64.6) | 198 (67.3) | 0.57 | 93 (84.5) | 11 (78.6) | 0.57 | 89 (87.3) | 39 (95.1) | 0.17 |
| HRT Use (ever), n (%) | |||||||||
| No | 32 (21.3) | 178 (60.6) | 48 (43.6) | 6 (42.9) | 42 (41.2) | 14 (34.2) | |||
| Yes | 45 (30.0) | 110 (37.4) | 53 (48.2) | 8 (57.1) | 47 (46.1) | 24 (58.5) | |||
| Unknown | 73 (48.7) | 6 (2.0) | <0.001 | 9 (8.2) | 0 | 0.51 | 13 (12.7) | 3 (7.3) | 0.36 |
p-values were obtained by Wilcoxon rank-sum test for continuous variables and chi-square for catergorical variables.
Other race includes American/Alaskan Indians and women who reported more than one race.
As shown in Table 3, the majority of the ovarian cancer cases were of serous histology and advanced stage. Only 36% of the average risk and 26% of the high risk cases were Stage I or II. The distribution of histologic subtypes and stage of ovarian cases was similar in the high and average risk groups.
Table 3.
Summary of the turnor characteistics for ovarian cancer cases by risk statys.
| Average Risk Cases (n=102) |
High Risk Cases (n=41) |
p-value* | |
|---|---|---|---|
| HISTOLOGY, n (%) | |||
| Clear Cell | 8 (7.8) | 2 (4.9) | |
| Endometrioid | 10 (9.8) | 4 (9.8) | |
| Mucinous | 6 (5.9) | 2 (4.9) | |
| Serous | 66 (64.7) | 24 (58.5) | |
| Other | 12 (11.8) | 9 (21.9) | p=0.62 |
| STAGE, n (%) | |||
| Stage 1 | 29 (29.3) | 6 (17.1) | |
| Stage 2 | 7 (7.1) | 3 (8.6) | |
| Stage 3 | 53 (53.5) | 22 (62.9) | |
| Stage 4 | 10 (10.1) | 4 (11.4) | p=0.58 |
p-value were obtained from chi-square
Missing data on stage for 3 average risk cases and 6 high risk cases
Log transformed and standardized mean levels of biomarkers for average and high risk cases and benign and healthy controls are presented in Table 4. Mean CA125 levels in high-risk healthy controls were lower than corresponding levels in average risk women (p<0.001). Although the total number of mucinous tumors was low, the mean HE4 and mesothelin levels in these tumors were higher in high risk women than in average risk women, 6.7 vs. 1.6 (p=0.006) for HE4 and 1.8 vs. −0.2 for mesothelin (p<0.001).
Table 4.
Marker levels for the controls and cases by risk status. The marker values have been log transformed and standardized to mean=0 and standard deviation=1 in the average risk controls.
| Average Rsik | High Risk | p-value* | |||
|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | ||
| HEALTHY CONTROLS | 150 | 294 | |||
| CA125 | 0 (1.0) | −0.8 (0.8) | <0.001 | ||
| Mesothelin | 0 (1.0) | 0.1 (1.0) | 0.63 | ||
| HE4 | 0 (1.0) | −0.2 (1.0) | 0.10 | ||
| BENIGN CONTROLS | 110 | 14 | |||
| CA125 | 0.7 (1.4) | 0.5 (1.1) | 0.67 | ||
| Mesothelin | 0.02 (1.2) | 0.1 (1.1) | 0.79 | ||
| HE4 | 0.43 (1.3) | 0.3 (1.3) | 0.95 | ||
| CASES | 102 | 41 | |||
| CA125 | 4.2 (2.6) | 3.6 (2.6) | 0.17 | ||
| Mesothelin | 2.6 (2.8) | 2.2 (2.8) | 0.38 | ||
| HE4 | 5.2 (3.9) | 5.5 (3.6) | 0.63 | ||
| CASES (by histology) | |||||
| Clear Cell | 8 | 2 | |||
| CA125 | 1.8 (2.0) | 0.6 (0.1) | 0.15 | ||
| Mesothelin | 0.3 (1.1) | −1.0 (0.5) | 0.09 | ||
| HE4 | 0.7 (1.3) | 2.1 (0.8) | 0.17 | ||
| Endometrioed | 10 | 4 | |||
| CA125 | 4.7 (3.1) | 3.5 (4.2) | 0.61 | ||
| Mesothelin | 1.2 (2.2) | 0.9 (3.2) | 0.85 | ||
| HE4 | 4.9 (3.4) | 5.7 (4.8) | 0.77 | ||
| Mucinous | 6 | 2 | |||
| CA125 | 3.0 (1.9) | 3.4 (1.5) | 0.80 | ||
| Mesothelin | −0.2 (0.6) | 1.8 (0.2) | <0.001 | ||
| HE4 | 1.6 (1.4) | 6.7 (0.8) | 0.006 | ||
| Serous | 66 | 24 | |||
| CA125 | 4.8 (2.4) | 4.4 (2.1) | 0.50 | ||
| Mesothelin | 3.7 (2.6) | 2.8 (2.6) | 0.16 | ||
| HE4 | 6.4 (3.8) | 6.3 (3.5) | 0.95 | ||
| Other | 12 | 9 | |||
| CA125 | 3.1 (2.9) | 2.1 (2.8) | 0.42 | ||
| Mesothelin | 1.0 (2.5) | 1.9 (3.3) | 0.50 | ||
| HE4 | 3.8 (3.5) | 3.9 (3.7) | 0.93 | ||
| CASES (by stage) | |||||
| Stage 1 | 29 | 6 | |||
| CA125 | 2.5 (2.6) | 2.8 (2.5) | 0.85 | ||
| Mesothelin | 0.4 (1.4) | 0.2 (1.7) | 0.82 | ||
| HE4 | 1.7 (2.0) | 3.9 (3.2) | 0.15 | ||
| Stage 2 | 7 | 3 | |||
| CA125 | 3.4 (2.3) | 2.8 (2.4) | 0.73 | ||
| Mesothelin | 1.0 (1.3) | 2.3 (3.7) | 0.62 | ||
| HE4 | 5.3 (3.0) | 6.2 (2.1) | 0.63 | ||
| Stage 3 | 53 | 22 | |||
| CA125 | 5.3 (2.1) | 4.4 (2.2) | 0.12 | ||
| Mesothelin | 3.8 (2.7) | 3.0 (2.9) | 0.26 | ||
| HE4 | 7.1 (3.5) | 6.8 (3.1) | 0.72 | ||
| Stage 4 | 10 | 4 | |||
| CA125 | 4.6 (2.8) | 5.8 (1.6) | 0.38 | ||
| Mesothelin | 3.4 (3.0) | 3.2 (2.6) | 0.88 | ||
| HE4 | 4.5 (3.3) | 7.9 (2.1) | 0.05 | ||
p-values were obtained from the Student's t-test.
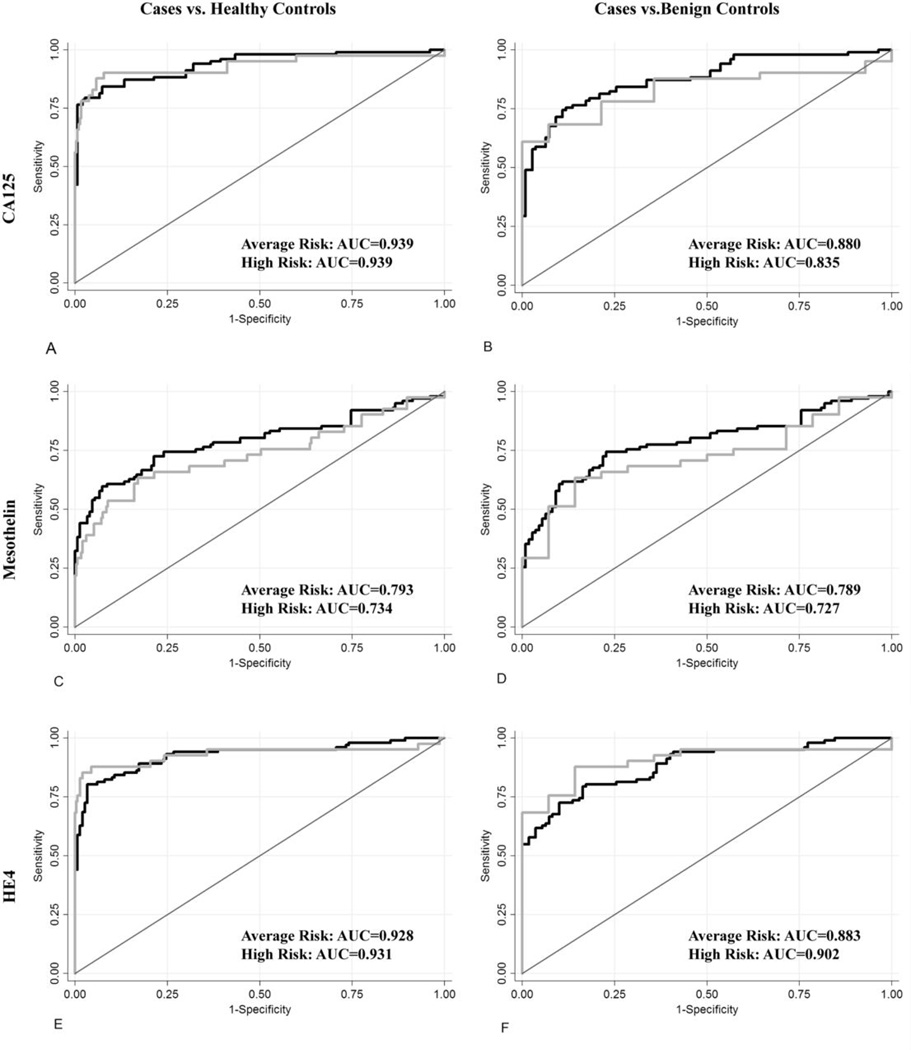
Receiver Operating Characteristic (ROC) curves which summarize for each marker the sensitivity across all levels of specificity for detecting ovarian cancer cases relative to healthy and benign controls in average and high risk women are presented in Figure 1. The area under the curve (AUC) numerically describes the overall performance of the marker, with an AUC of 1 indicating perfect sensitivity and specificity. Table 5 summarizes key features of the ROC curves including the AUC and marker performance at high levels of specificities that are relevant for early detection. Overall, the diagnostic performance of CA125 and HE4 was roughly equivalent and better than that of mesothelin. All markers performed better when cases were compared to healthy as opposed to benign tumor controls. There were no differences in the ability of any of the markers to discriminate cases from healthy or benign controls between risk groups. HE4 tended to perform better and mesothelin tended to perform worse in high risk women but these differences were not significant. The sensitivity at 98% specificity for discriminating ovarian cancer cases from healthy controls in average and high risk women respectively was 78.4% and 78.5% for CA 125, 44.1% and 31.7% for mesothelin and 68.6% and 82.9% for HE4.
Figure 1.
ROC curves for women at high-risk (grey line) and average-risk (black line) for ovarian cancer. Plot A: CA125 for cases vs. healthy controls, Plot B: CA125 for cases vs. benign controls, Plot C: Mesothelin for cases vs. healthy controls. Plot D: Mesothelin for cases vs. benign controls. Plot E: HE4 for cases vs. healthy controls. Plot F: HE4 for cases vs. beaign controls.
Table 5.
Diagnostic accuracy of the biomarkers for average and high risk women.
| Cases vs. Healthy Controls | Cases vs. Benign Controls | |||||
|---|---|---|---|---|---|---|
| AUC | Sensitivity at 95% Specificity |
Sensitivity at 95% Specificity |
AUC | Sensitivity at 95% Specificity |
Sensitivity at 98% Specificity |
|
| CA125 | ||||||
| Average Risk | 0.939 | 79.41 | 78.43 | 0.880 | 58.82 | 49.02 |
| High Risk | 0.939 | 82.93 | 78.05 | 0.835 | 63.41 | 60.98 |
| AUC differences p-value* | 0.98 | 0.45 | ||||
| Mesothelin | ||||||
| Average Risk | 0.793 | 53.92 | 44.12 | 0.789 | 43.14 | 37.25 |
| High Risk | 0.734 | 39.02 | 31.71 | 0.727 | 34.15 | 29.27 |
| AUC differences p-value* | 0.36 | 0.42 | ||||
| HE4 | ||||||
| Average Risk | 0.928 | 80.39 | 68.63 | 0.883 | 61.76 | 57.84 |
| High Risk | 0.931 | 87.80 | 82.93 | 0.902 | 75.61 | 70.73 |
| AUC differences p-value* | 0.94 | 0.69 | ||||
The AUC differences between the average risk and high risk women were compared for each biomarker using the non-parametric methods developed by DeLong29.
DISCUSSION
Mesothelin and HE4 are novel ovarian cancer biomarkers that may have utility for early detection. When tested as diagnostic markers using samples collected at clinical diagnosis from patients unselected for ovarian cancer risk both markers discriminate ovarian cases from healthy and benign controls at high levels of specificity. We undertook this study to evaluate if the diagnostic performance of CA125, mesothelin and HE4 was affected by the ovarian cancer risk status as high risk women are ideal candidates for early evaluation of new ovarian cancer screening tests. We recognize that although high performance in diagnostic samples is a necessary characteristic, the true utility of an early detection marker depends on its behavior prior to clinical diagnosis. We sought to determine if the markers were suitable for further testing in women at high risk since women who are at high risk for ovarian cancer are most likely to participate in a screening program for ovarian cancer. Our findings suggest that further studies evaluating early detection potential of the markers we tested in high risk women are warranted and that the findings from these studies may be generalizable to women in the general population.
We used self-report of personal and family cancer history to a standardized questionnaire to obtain information for risk classification. Women were asked an extensive series of questions about diagnosis and age at diagnosis of ovarian, breast, colon and other cancers in first and second degree family members. We also asked women if they had been tested for BRCA 1 and 2 mutations and if a deleterious mutation or variant of uncertain significance was identified. However, only 48 (7%) of the women included herein reported they had been tested for the mutations and 28 of these women (58%) reported having a positive test.
A limitation of our study is that we were not able to validate self-report of information used to characterize risk status. It is possible that either direct interview or review of personal and family medical records might have provided more complete or accurate information and better risk classification. Misclassification could have occurred if a participant believed a relative’s cancer was ovarian when in fact it arose in another site. It is also possible women may have been inappropriately classified as being average risk if they chose to withhold information about genetic testing because of concerns about confidentiality.
The classification system we used to characterize women at high risk identifies a heterogeneous group with respect to their true risk of ovarian cancer. Ovarian cancer risk in this group spans from roughly a 10% lifetime risk for developing ovarian cancer for women meeting minimal criteria to perhaps as high as 60% lifetime risk for BRCA1 mutation carriers. A more homogeneous population, particularly one limited to mutation carriers, might be more informative. However our criteria for identifying women as high risk are generally accepted clinically and consistent with eligibility criteria used to select women for enrollment into large multi-center, prospective ovarian cancer screening studies targeting women at high risk (28, 29).
The average and high risk groups in this study are well balanced for most of the baseline demographic information that was collected. Self-report of use of hormone replacement therapy (HRT) among healthy control women did vary by risk status (p <0.01). The difference remained significant even after women responding “unknown” to having ever used HRT were removed from the analysis, suggesting most of the difference is related to a higher proportion of high risk women reporting having ever used HRT. More frequent reported use of HRT among the high risk women is probably explained by the fact that a greater proportion of these women were post-menopausal (67% vs. 35%; p=.53). We also identified differences in ethnicity between high and average risk healthy controls however these differences were not significant when women who reported their race as unknown were removed from the analysis.
Interestingly, mean levels of CA125 were lower in high risk as compared to average risk healthy controls. Mean CA125 levels fall substantially after menopause (30), and the lower CA levels in high risk controls may be explained by the high proportion of post-menopausal women in the high- risk group. In a prior report we noted that CA125 levels in healthy post-menopausal high risk women were affected by ovarian cancer risk factors including talc use and parity although the effects were minor; these parameters were not evaluated in the current report (31). Pauler reported that CA125 levels in healthy women vary based on personal characteristics including age, race, smoking, caffeine intake, age at menarche and menopause status (30). Factors evaluated in this report including age and race did not vary by risk group.
We found that for mucinous ovarian cancer cases mesothelin and HE4 levels were higher in high risk as compared to average risk women. This result could be spurious as the total number of mucinous cancers in the cohort was small (n=6); and there were only 2 mucinous cancers in the high risk group. Differences in marker levels could not be explained by either tumor volume or stage as these factors vary between the two groups (data not shown).
The overall diagnostic performance of the markers estimated using the area under the ROC curve did not vary by risk status. When ovarian cancer cases were compared to healthy controls the AUC values for average and high risk women were nearly identical (.939 vs. .939 for CA 125; .793 vs. .734 for mesothelin; and .928 and .931 for HE4). The findings were similar although the AUC values were lower when cases were compared to benign controls. The reduction in AUC is not surprising as some benign tumors are known to emit low levels of the markers. At high levels of specificity mesothelin appeared to perform better in average risk women and HE4 performed better in high risk women although the differences were relatively minor, not statistically significant, and may be influenced by the sparseness of the data in this portion of the curve. For mesothelin the sensitivity at 98% specificity for ovarian cases vs. healthy controls was 44.1 % and 31.71% for average and risk women respectively. For HE4 the values were 68.3% for average and 82.9% for high risk women. The direction and magnitude of the differences did not change when the analysis was limited to serous cancers (data not shown). We chose not to evaluate marker combinations because we believe that optimizing marker panels using diagnostic samples collected from symptomatic patients is not likely to be relevant for early detection. However based on our data it is unlikely that the diagnostic performance of a combination marker that includes CA125, mesothelin and/or HE4 will vary by patient risk status.
Acknowledgments
Funding Source:
Pacific Ovarian Cancer Research Consortium (POCRC)/SPORE in Ovarian Cancer, (NIH/NCI P50 CA83636), Seattle, WA
Northwest Cancer Genetics Network: CGN Ovarian Cancer Screening Pilot Trial in High Risk Women, (NIH/NCI U24 CA78164)
The Marsha Rivkin Center for Ovarian Cancer Research, Seattle, WA
Quality of Life Effects of Risk Education and Screening for Ovarian Cancer, (NIH/NCI 1 R01 CA75494)
Use of Novel Technologies to Identify and Investigate Molecular Markers for Ovarian Cancer Screening and Prevention, (DAMD17-98-1-8649)
REFERENCES
- 1.Bast RC, Jr, Badgwell D, Lu Z, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005 Nov–Dec;15 Suppl 3:274–281. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 2.Creaney J, van Bruggen I, Hof M, et al. Combined CA125 and mesothelin levels for the diagnosis of malignant mesothelioma. Chest. 2007 Oct;132:1239–1246. doi: 10.1378/chest.07-0013. [DOI] [PubMed] [Google Scholar]
- 3.Hassan R, Remaley AT, Sampson ML, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006 Jan 15;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 4.Huang CY, Cheng WF, Lee CN, et al. Serum mesothelin in epithelial ovarian carcinoma: a new screening marker and prognostic factor. Anticancer Res. 2006 Nov–Dec;26:4721–4728. [PubMed] [Google Scholar]
- 5.Rosen DG, Wang L, Atkinson JN, et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005 Nov;99:267–277. doi: 10.1016/j.ygyno.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Yen MJ, Hsu CY, Mao TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006 Feb 1;12:827–831. doi: 10.1158/1078-0432.CCR-05-1397. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh MW, Drescher C, Karlan B, et al. Combining CA 125 and SMR serum markers for diagnosis and early detection of ovarian carcinoma. Gynecol Oncol. 2004 Oct;95:9–15. doi: 10.1016/j.ygyno.2004.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drapkin R, von Horsten HH, Lin Y, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005 Mar 15;65:2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 9.Scholler N, Crawford M, Sato A, et al. Bead-based ELISA for validation of ovarian cancer early detection markers. Clin Cancer Res. 2006 Apr 1;12:2117–2124. doi: 10.1158/1078-0432.CCR-05-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schummer M, Ng WV, Bumgarner RE, et al. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene. 1999 Oct 1;238:375–385. doi: 10.1016/s0378-1119(99)00342-x. [DOI] [PubMed] [Google Scholar]
- 11.Hellstrom I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003 Jul 1;63:3695–3700. [PubMed] [Google Scholar]
- 12.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005 Dec 15;104:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 13.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001 Mar;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003 May;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. Jama. 1997 Mar 26;277:997–1003. [PubMed] [Google Scholar]
- 16.Hogg R, Friedlander M. Biology of epithelial ovarian cancer: implications for screening women at high genetic risk. J Clin Oncol. 2004 Apr 1;22:1315–1327. doi: 10.1200/JCO.2004.07.179. [DOI] [PubMed] [Google Scholar]
- 17.Levine DA, Federici MG, Reuter VE, Boyd J. Cell proliferation and apoptosis in BRCA-associated hereditary ovarian cancer. Gynecol Oncol. 2002 Jun;85:431–434. doi: 10.1006/gyno.2002.6646. [DOI] [PubMed] [Google Scholar]
- 18.Shaw PA, McLaughlin JR, Zweemer RP, et al. Histopathologic features of genetically determined ovarian cancer. Int J Gynecol Pathol. 2002 Oct;21:407–411. doi: 10.1097/00004347-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Lakhani SR, Manek S, Penault-Llorca F, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clin Cancer Res. 2004 Apr 1;10:2473–2481. doi: 10.1158/1078-0432.ccr-1029-3. [DOI] [PubMed] [Google Scholar]
- 20.Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. Jama. 2000 May 3;283:2260–2265. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 21.Jazaeri AA, Yee CJ, Sotiriou C, Brantley KR, Boyd J, Liu ET. Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers. J Natl Cancer Inst. 2002 Jul 3;94:990–1000. doi: 10.1093/jnci/94.13.990. [DOI] [PubMed] [Google Scholar]
- 22.Scholler N, Lowe KA, Bergan L, et al. Use of yeast-secreted in vivo biotinylated recombinant antibodies (biobodies) in bead-based ELISA. Clin Cancer Res. 2008 May 1;14:2647–2655. doi: 10.1158/1078-0432.CCR-07-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergan L, Gross JA, Nevin B, Urban N, Scholler N. Development and in vitro validation of anti-mesothelin biobodies that prevent CA125/Mesothelin-dependent cell attachment. Cancer Lett. 2007 Oct 8;255:263–274. doi: 10.1016/j.canlet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Pepe MS, Longton G. Standardizing diagnostic markers to evaluate and compare their performance. Epidemiology. 2005 Sep;16:598–603. doi: 10.1097/01.ede.0000173041.03470.8b. [DOI] [PubMed] [Google Scholar]
- 25.Bamber D. The area above the ordinal dominance graph and the area below the receiver operating characteristic graph. J Math Psych. 1975;12:387–415. [Google Scholar]
- 26.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 Sep;44:837–845. [PubMed] [Google Scholar]
- 27.Stata Statistical Software. College Station, TX: StataCorp LP; [Google Scholar]
- 28.Menon U, Burnell M, Sharma A, et al. Decline in use of hormone therapy among postmenopausal women in the United Kingdom. Menopause. 2007 May-Jun;14:462–467. doi: 10.1097/01.gme.0000243569.70946.9d. [DOI] [PubMed] [Google Scholar]
- 29.Skates S, Horick N, Finkelstein D, Lu K, editors. Prospective multicenter ovarian cancer screening trial for women at high risk: Preliminary results from the first 2,200 women. Risk Prediction Workshop; May 20–21; Washington D.C.. 2004. [Google Scholar]
- 30.Pauler DK, Menon U, McIntosh M, Symecko HL, Skates SJ, Jacobs IJ. Factors influencing serum CA125II levels in healthy postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2001 May;10:489–493. [PubMed] [Google Scholar]
- 31.Lowe KA, Shah C, Wallace E, et al. Effects of personal characteristics on serum CA125, mesothelin, and HE4 levels in healthy postmenopausal women at high-risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2008 Sep;17:2480–2487. doi: 10.1158/1055-9965.EPI-08-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]