Abstract
Partial seizures produce increased cerebral blood flow in the region of seizure onset. These regional cerebral blood flow increases can be detected by single photon emission computed tomography (ictal SPECT), providing a useful clinical tool for seizure localization. However, when partial seizures secondarily generalize, there are often questions of interpretation since propagation of seizures could produce ambiguous results. Ictal SPECT from secondarily generalized seizures has not been thoroughly investigated. We analysed ictal SPECT from 59 secondarily generalized tonic–clonic seizures obtained during epilepsy surgery evaluation in 53 patients. Ictal versus baseline interictal SPECT difference analysis was performed using ISAS (http://spect.yale.edu). SPECT injection times were classified based on video/EEG review as either pre-generalization, during generalization or in the immediate post-ictal period. We found that in the pre-generalization and generalization phases, ictal SPECT showed significantly more regions of cerebral blood flow increases than in partial seizures without secondary generalization. This made identification of a single unambiguous region of seizure onset impossible 50% of the time with ictal SPECT in secondarily generalized seizures. However, cerebral blood flow increases on ictal SPECT correctly identified the hemisphere (left versus right) of seizure onset in 84% of cases. In addition, when a single unambiguous region of cerebral blood flow increase was seen on ictal SPECT, this was the correct localization 80% of the time. In agreement with findings from partial seizures without secondary generalization, cerebral blood flow increases in the post-ictal period and cerebral blood flow decreases during or following seizures were not useful for localizing seizure onset. Interestingly, however, cerebral blood flow hypoperfusion during the generalization phase (but not pre-generalization) was greater on the side opposite to seizure onset in 90% of patients. These findings suggest that, with appropriate cautious interpretation, ictal SPECT in secondarily generalized seizures can help localize the region of seizure onset.
Keywords: epilepsy, cerebral blood flow, grand mal, surgery, nuclear medicine
Introduction
Patients with medically refractory epilepsy face many challenges. In selected cases, surgical treatment can offer the hope for permanent control of seizures. However, surgical treatment of epilepsy depends on precise knowledge of the region of seizure onset. It has long been recognized that focal seizure activity is associated with increased cerebral blood flow (CBF) in the involved region of cerebral cortex (Horsley, 1892; Penfield, 1933). Advances in neuroimaging now allow the non-invasive visualization of CBF changes in patients with epilepsy, which can be used as a clinical tool to identify the region of seizure onset. Although surgical treatment is aimed at the focal region of seizure onset, some seizures can secondarily generalize, which complicates the interpretation of CBF changes. The aim of the present study was to determine whether useful clinical information could be gleaned from CBF imaging performed in patients during partial seizures with secondary generalization.
Single photon emission computed tomography (SPECT) is currently the only practical method for imaging CBF during seizures. The advantage of SPECT is that the tracer is injected during the seizure (ictal SPECT), however, imaging can be done an hour or more later, when seizure motor activity has ended, thus avoiding movement artifact. This approach is possible because the tracer is rapidly taken up by the brain at the time of injection and does not significantly redistribute (Andersen, 1989; Devous et al., 1990). Since it was first introduced, ictal SPECT has come into widespread use in epilepsy centres performing pre-surgical evaluation for medically refractory epilepsy (O’Brien et al., 1998; Lee et al., 2000c, 2001; Knowlton, 2006; Kim et al., 2009). To improve sensitivity and specificity, ictal SPECT images are typically compared with a baseline SPECT scan in the same patient without seizures (interictal SPECT). Since the mid 1990s (Zubal et al., 1995; McNally et al., 2005), several methods have been developed to digitally coregister and subtract ictal–interictal SPECT images to obtain maps of ictal increases and decreases in CBF (Zubal et al., 1995; O’Brien et al., 1998; Spanaki et al., 1999; Lee et al., 2000a, b; Chang et al., 2002; McNally et al., 2005).
Many studies have been done to examine the clinical usefulness of ictal–interictal SPECT [reviewed in Kim et al. (2009)]. It has been shown that ictal SPECT is far more useful for correctly localizing seizure onset than interictal SPECT (Spencer et al., 1995; Devous et al., 1998; Kim et al., 2009). In addition, ictal–interictal SPECT difference imaging analysis yields better results than visual read of ictal SPECT alone (Zubal et al., 1995; O’Brien et al., 1998; Spanaki et al., 1999; Lee et al., 2000b; Koo et al., 2003). Another important determinant of the sensitivity and specificity of ictal SPECT is the time of tracer injection. Although at early injection times CBF increases are often confined to the region of seizure onset, later changes can be complicated, especially in the post-ictal period when both increases and decreases occur in different brain regions from seizure onset. When SPECT injection comes late, and especially when it occurs in the post-ictal period, the diagnostic yield of CBF increases for localizing seizure onset is very low (Rowe et al., 1991; Newton et al., 1994; Spencer, 1994; O’Brien et al., 1998; Avery et al., 1999; McNally et al., 2005). In addition, efforts to localize seizure onset based on SPECT CBF decreases, whether early or late, have not been very successful compared to ictal CBF increases (McNally et al., 2005; Kim et al., 2009), although some have reported good results in the post-ictal period (O’Brien et al., 1999).
Thus, ictal SPECT has emerged as a useful tool for pre-surgical localization of partial seizures, as long as the SPECT injection is performed during the seizure, and not post-ictally. One important unresolved question is how to interpret ictal SPECT in seizures that secondarily generalize. In secondarily generalized seizures, patients initially exhibit localized seizure manifestations such as focal limb movement, staring or automatisms, which is then followed by generalized tonic–clonic activity (Theodore et al., 1994; Jobst et al., 2001). On electroencephalography, localized changes are seen at onset, followed by propagation to involve widespread regions of cerebral cortex in secondarily generalized seizures (Rodin et al., 1969; Schindler et al., 2007). Over 70% of patients with localization-related epilepsy have occasional secondarily generalized seizures (Forsgren et al., 1996). Therefore, it is not uncommon for ictal SPECT injections to occur during partial seizures that secondarily generalize. A few studies have reported that ictal SPECT may still be useful in secondarily generalized seizures (Lee et al., 1987; O’Brien et al., 1998; Shin et al., 2002). However, this has not been rigorously investigated.
In the present study, we investigated the localizing value of ictal SPECT in a group of patients injected during or shortly after partial seizures with secondary generalization. In all cases, we evaluated whether a single region of increased CBF could be identified, and whether this agreed with overall seizure localization based on surgical outcome and other diagnostic tests. We found that ictal SPECT in secondarily generalized seizures often shows multiple regions of CBF increases. This makes the identification of a single unambiguous region of seizure onset more difficult than in partial seizures without secondary generalization. Nevertheless, we found that with careful interpretation, ictal SPECT in secondarily generalized seizures could be used to at least narrow down the region of seizure onset to a few regions or to one hemisphere. Thus, although it is preferable to obtain ictal SPECT in partial seizures without secondary generalization, if a secondarily generalized seizure occurs during ictal SPECT, the present work still enables clinically useful information to be obtained.
Methods
Patients
All procedures were in accordance with the Institutional Review Boards and NIH guidelines for human research. Inclusion and exclusion criteria were chosen to identify patients who had SPECT imaging during secondarily generalized tonic–clonic seizures. To obtain a sufficiently large sample, data were combined from three academic epilepsy centres (Yale New Haven Hospital, Columbia-Presbyterian Medical Center and University of Alabama, Birmingham). Inclusion criteria were ictal SPECT performed during video-EEG monitoring; SPECT injection performed during or immediately after a secondarily generalized tonic–clonic seizure based on video-EEG monitoring; and interictal SPECT performed at least 24 h after the most recent seizure. Exclusion criteria were diagnosis of primary generalized epilepsy; unavailable SPECT images; and inconclusive SPECT injection timing (e.g. poor video quality). We analysed a total of 59 interictal–ictal scan pairs in 53 patients (30 males, 23 females) with a mean age at time of SPECT injection of 33 years (age range 11–65 years).
Clinical data were obtained from patient records, and included results of magnetic resonance imaging (MRI), fluorodeoxyglucose positron emission tomography (FDG-PET), scalp and intracranial EEG seizure onset, site of surgical resection (when applicable), surgical pathology and seizure outcome after surgery. Overall seizure localization (see Supplementary Tables 1–3) was determined based on concordance of these data, not including SPECT imaging.
Behavioural and EEG review
Video and EEG of seizures for all SPECT injections were reviewed by two readers, blinded to the results of the imaging studies. Seizure onset was defined as the earliest EEG or clinical evidence of seizure activity. Seizure offset was defined as the last EEG or clinical evidence of seizure activity; usually this coincided with the last clonic jerk exhibited by the patient. Onset of generalization was defined based on head or eye version, vocalization or asymmetric tonic facial contraction, as in previous behavioural studies of generalized tonic–clonic seizures (Theodore et al., 1994; Jobst et al., 2001). SPECT injection time was defined as when the plunger of the syringe containing the radiopharmaceutical was fully depressed.
SPECT image acquisition
Ictal SPECT injections were performed during continuous scalp EEG and video recordings. Upon noting seizure onset, a nurse or technologist injected 20–40 mCi of Tc-99m labelled hexamethylpropylene-amine-oxime (HMPAO) (Amersham Healthcare, Arlington Heights, IL). Patients were asked to close their eyes during the injection. Inter-ictal injections were performed in these same patients after ≥24 h of no seizure activity, in a quiet room, while patients were at rest, awake, but with their eyes closed.
SPECT images were acquired within 90 min after injection. Projection data were obtained on a Picker PRISM 3000, 3000XP or Marconi/Philips IRIX three-headed scanner (Philips Medical Systems, Best, Netherlands) mounted with high resolution fan beam collimators. Transverse slices were reconstructed using standard low pass Butterworth filter and Chang attenuation correction as previously described (Zubal et al., 1995). SPECT image data were transferred to personal computer, and saved in Analyse format using ImageJ (http://rsb.info.nih.gov/ij/).
Image analysis
Ictal–interictal SPECT difference analysis was performed for each patient using methods described in detail previously (McNally et al., 2005) (http://spect.yale.edu/). This approach, referred to as ictal–interictal SPECT analysed by SPM (ISAS), calculates differences between ictal and interictal SPECT image pairs, and then determines which changes are statistically significant by comparison with image pairs in a normal database. Results are similar to conventional ictal–interictal difference imaging analysis (Zubal et al., 1995; O’Brien et al., 1998; Spanaki et al., 1999), but have the advantage of being more objective and providing a statistical significance level (Chang et al., 2002; McNally et al., 2005). Full details of the ISAS method, including free downloads of the software and normal database, are available at http://spect.yale.edu/. Briefly, each ictal–interictal scan pair was analysed using statistical parametric mapping (SPM2, Wellcome Department of Cognitive Neurology, London, UK http://fil.ion.ucl.ac.uk/spm/) on a MATLAB (The MathWorks, Inc. Natick, MA) platform. The patient scan pairs were compared with a group of 14 healthy normal scan pairs using a multi-group conditions and covariates design (McNally et al., 2005), (http://spect.yale.edu). Images were realigned, spatially normalized, masked and smoothed using a 16 × 16 × 16 mm Gaussian kernel. Global intensity normalization at an analysis threshold of 0.8 was performed to correct for differences in total brain counts among scan pairs (Friston et al., 1996; Acton and Friston, 1998). As a double check for problems in the spatial warping, smoothing or masking steps, especially due to extraneous signal from outside the brain, we also analysed images without these pre-processing steps using rview freeware (http://www.colin-studholme.net/software/software.html). Contrasts were set up in SPM looking at hyperperfusion and hypoperfusion throughout the entire brain for ictal minus interictal images. We used an extent threshold (k) of 125 voxels or 1 cc (voxel size 2 × 2 × 2 mm) because this is the approximate spatial resolution of SPECT in tissue. Height threshold (individual voxel-level significance) was set at P = 0.01 (Z-score 2.33), as determined by prior receiver operating characteristic analysis (McNally et al., 2005).
SPM identifies clusters of voxels with changes that exceed the previously defined thresholds. We further considered only clusters of voxels with significance level P < 0.05 (corrected cluster-level significance), which effectively corrects for multiple comparisons for the entire brain (Friston et al., 1996). ISAS results were interpreted as described previously (McNally et al., 2005). Briefly, SPECT ISAS localization was determined for each patient by the location of the most significant cluster of hyperperfusion voxels in the ictal–interictal comparison. If two or more lobes are equally involved, then localization is ambiguous and all involved lobes are listed. In partial seizures that do not secondarily generalize, this approach for localizing CBF increases has been shown to correctly and unambiguously identify a single region of seizure onset in most patients (McNally et al., 2005).
In addition to CBF increases, we also identified regions of CBF decreases using the same approach in ISAS. As reported previously for partial seizures, perfusion decreases may not be useful for detecting the lobe of seizure onset, but can help lateralize the hemisphere (left or right) of seizure onset (McNally et al., 2005). Therefore, we also calculated a hypoperfusion asymmetry index to determine which hemisphere had more extensive CBF decreases. The hypoperfusion asymmetry index was calculated based on the volume of significant voxels (at a voxel-level significance threshold P = 0.01, and cluster-level significance k = 125 as before) showing decreased perfusion in each hemisphere (McNally et al., 2005). Thus, we took the number of hypoperfusion voxels in the left hemisphere minus number of hypoperfusion voxels in the right hemisphere divided by the total number of hypoperfusion voxels [(kleftkright)/(kleft+kright)]. Binary images corresponding to each hemisphere were created in MRIcro (for detailed methods and downloadable binary masks, see http://spect.yale.edu/) and were used to determine kleft and kright using the small volume correction function in SPM.
Results
Although seizures were generalized, we observed focal CBF changes in all cases. Unlike partial seizures however, CBF increases during secondarily generalized seizures often involved several lobes. We studied a total of 59 secondarily generalized tonic–clonic seizures in 53 patients (six patients were injected twice on different days). Mean seizure duration was 126 ± 12 s (mean ± SEM), with mean duration of the partial phase prior to generalization 51 ± 11 s. Seizures were divided into three groups, based on SPECT injection time (see Methods section for definitions): (i) Pre-generalization: 12 seizures were injected during the partial phase prior to generalization; (ii) generalization: 12 seizures were injected after onset of generalization but prior to seizure end; and (iii) post-ictal: 35 seizures were injected after termination (Tables 1 and 2).
Table 1.
Ictal SPECT increases: localization and lateralization of seizure onset
| Inj t (s) relative to |
|||||||
|---|---|---|---|---|---|---|---|
| Patienta | Seizure duration (s) | Onset | Gen | End | Overall localizationb | ISAS hyper-perfusion | Correct side? |
| Pre-generalization | |||||||
| 1 | 83 | 9 | −42 | −74 | LH | LT | Y |
| 2 | 171 | 10 | −33 | −161 | LH | LT, P, F | Y |
| 3 | 110 | 11 | −50 | −99 | RT NC | R P | Y |
| 4 | 154 | 16 | −19 | −138 | Unlocalized | BiF, Bi T | NA |
| 5 | 101 | 18 | −20 | −83 | LT versus RT NC | BiF, BiT, BiP | NA |
| 6 | 98 | 20 | −23 | −78 | LT | LT | Y |
| 7 | 95 | 28 | −33 | −67 | LT | Bi T | N |
| 8 | 161 | 36 | −57 | −125 | RH | RT | Y |
| 9 | 129 | 40 | −12 | −89 | LT | LT | Y |
| 10 | 210 | 55 | −64 | −155 | RH | RO, T, P | Y |
| 11 | 230 | 85 | −65 | −145 | Multifocal | LT | NA |
| 12 | 444 | 241 | −139 | −203 | RT NC | RT, O | Y |
| Generalization | |||||||
| 13 | 80 | 4 | 0 | −76 | LC (C3, P3) | LC | Y |
| 14 | 43 | 11 | 3 | −32 | LH | LF, T | Y |
| 15 | 83 | 12 | 4 | −71 | RH | RF, T, P | Y |
| 16 | 194 | 128 | 10 | −66 | L mesial T | LT | Y |
| 17 | 91 | 24 | 20 | −67 | RH | RP | Y |
| 18 | 133 | 81 | 31 | −52 | LH | LT | Y |
| 19 | 80 | 64 | 37 | −16 | Unlocalized | LT, P | NA |
| 20 | 68 | 57 | 47 | −15 | LH multifocal | RF, P | N |
| 21 | 72 | 58 | 53 | −14 | Unlocalized | RC | NA |
| 22 | 76 | 72 | 70 | −4 | RH | BiT | N |
| 23 | 150 | 118 | 78 | −32 | RH | RO | Y |
| 24 | 103 | 40 | NA | −63 | LH | LF, P | Y |
a Patients are listed in order of injection time relative to seizure onset (Pre-generalization group) or generalization (Generalization group; see Methods section for definitions of seizure onset, generalization and end). The following six patients were injected twice on different days, so appear in the Tables twice: Pt 12 = 25, 19 = 50, 20 = 33, 32 = 34, 37 = 41, 51 = 57. See also Table 2.
b Overall localization was based on concordance of MRI, PET, EEG, surgical pathology and outcome (see Supplementary Tables 1 and 2 online for details), and did not include SPECT results.
Inj = injection; ISAS = ictal–interictal SPECT analyzed by SPM; R = right; L = left; C = central; T = temporal; P = parietal; F = frontal; O = occipital; H = hemisphere; Bi = bilateral; NC = neocortical.
Table 2.
Post-ictal SPECT increases: localization and lateralization of seizure onset
| Inj t (s) relative to |
|||||||
|---|---|---|---|---|---|---|---|
| Patienta | Seizure duration (s) | Onset | Gen | End | Overall localizationb | ISAS hyper-perfusion | Correct side? |
| 25 | 128 | 129 | 69 | 1 | RT neocortical | RP, T, O | Y |
| 26 | 97 | 99 | 88 | 2 | LO | LF | Y |
| 27 | 90 | 93 | 63 | 3 | RH multifocal | RP | Y |
| 28 | 113 | 119 | 84 | 6 | BiO | RT, O | NA |
| 29 | 475 | 484 | 98 | 9 | Bi R > L T | RF | Y |
| 30 | 72 | 81 | 68 | 9 | LH | BiO | N |
| 31 | 101 | 103 | 75 | 12 | L mesial T | LT, P, F | Y |
| 32 | 108 | 120 | 87 | 12 | LT | None | N |
| 33 | 95 | 106 | 63 | 21 | LH multifocal | RO, T, F | N |
| 34 | 92 | 113 | 95 | 21 | LT | None | N |
| 35 | 89 | 103 | 88 | 24 | LH multifocal | RT, O | N |
| 36 | 81 | 111 | 91 | 30 | Unlocalized | RT | NA |
| 37 | 82 | 113 | NA | 31 | R H | None | N |
| 38 | 155 | 187 | 101 | 32 | Unlocalized | LT | NA |
| 39 | 90 | 129 | 110 | 39 | L T-P | BiT | N |
| 40 | 75 | 104 | 110 | 39 | R T-O | RF, T | Y |
| 41 | 61 | 101 | NA | 40 | RH | None | N |
| 42 | 486 | 530 | 110 | 44 | BiO | RP | NA |
| 43 | 107 | 151 | 110 | 44 | LH | LF, T | Y |
| 44 | 337 | 384 | 178 | 47 | RH | RH | Y |
| 45 | 94 | 142 | 105 | 48 | RT versus LT | RF, T | NA |
| 46 | 84 | 134 | 113 | 50 | L mesial T | LO | Y |
| 47 | 105 | 155 | 123 | 50 | RH | RO, T | Y |
| 48 | 179 | 233 | 113 | 54 | L P-O | LT, P | Y |
| 49 | 93 | 153 | 133 | 60 | L medial P | RT | N |
| 50 | 93 | 140 | 115 | 67 | Unlocalized | LP, O | NA |
| 51 | 72 | 109 | 97 | 72 | Unlocalized | None | NA |
| 52 | 61 | 135 | 141 | 74 | Unlocalized | LP | NA |
| 53 | 99 | 188 | 150 | 89 | R mesial T | None | N |
| 54 | 75 | 187 | 162 | 112 | RH | RT | Y |
| 55 | 85 | 198 | 168 | 113 | Unlocalized | LH | NA |
| 56 | 40 | 177 | 177 | 137 | RF | LF | N |
| 57 | 81 | 248 | 233 | 167 | Unlocalized | None | NA |
| 58 | 76 | 352 | 342 | 276 | Bi mesial T | None | NA |
| 59 | 107 | 399 | 359 | 292 | LH | BiT | N |
a Patients are listed in order of injection time relative to seizure end. Six patients in Tables 1 and 2 were injected twice (see Table 1 for details).
b Overall localization was based on concordance of MRI, PET, EEG, surgical pathology and outcome (see Supplementary Table 3 online for details), and did not include SPECT results.
Inj = injection; ISAS = ictal–interictal SPECT analysed by SPM; R = right; L = left; C = central; T = temporal; P = parietal; F = frontal; O = occipital; H = hemisphere; Bi = bilateral; NC = neocortical.
Ictal CBF increases can help localize seizure onset
The goal of ictal SPECT is to unambiguously localize a single lobe of seizure onset for surgical planning. When ictal SPECT shows equal involvement of multiple lobes, localization is ambiguous. We were interested in determining whether increases in CBF during secondarily generalized seizures would be useful to localize a single lobe of seizure onset. In addition, if multiple lobes were involved, we were interested in determining if this could at least help narrow down the possible side or lobes of seizure onset. We found that multiple lobes were involved in SPECT increases for 50% (12/24) of patients injected during seizures (Table 1). This was true, regardless of whether patients were injected pre-generalization (6/12), or during the generalization phase (6/12). By comparison, our recent study of partial seizures without generalization (McNally et al., 2005) found that CBF increases involved multiple lobes in only 15% (2/13) of patients injected during seizures. While comparisons between these studies should be interpreted cautiously since the patient composition could have differed in important ways, including of note the lower incidence of confirmed temporal lobe epilepsy in the present cohort, we did find significantly more involvement of multiple lobes in the present study (χ2 = 4.30; P = 0.04).
Although increases in multiple lobes cannot provide a single unambiguous region for epilepsy surgery, do they still provide some helpful localizing information? Comparison of ictal SPECT ISAS hyperperfusion regions to overall localization (Table 1) demonstrated that CBF increases during seizures correctly identified the side of seizure onset (left versus right hemisphere) in 84% of cases (16/19). Thus, for patients injected during the pre-generalization phase, regions of ISAS hyperperfusion correctly identified the side of seizure onset in nearly all cases (8/9) in which the side of onset was known based on other data (Table 1, see also Supplementary Tables). Even for patients injected during the generalization phase, the regions of CBF increases still correctly lateralized the side of seizure onset in most cases (8/10) (Table 1). In two of the cases where correct lateralization was not possible (Patients 7 and 22), this was because CBF increases were bilateral. These finding suggest that ictal SPECT during generalized tonic–clonic seizures often shows multiple areas of CBF increases, however, these can still be useful to at least narrow down the probable regions or side of seizure onset.
Only half (12/24) of the seizures showed a single unambiguous region of ictal CBF increase. Of these, an even smaller subset (5/12) had known localization to a single lobe based on other data (Patients 3, 6, 9, 13 and 16). However, in 80% (4/5) of these cases the single region of ictal CBF increase correctly localized seizure onset (Table 1). This compares favourably with partial seizures, where we recently found using the same methods that if a single unambiguous region of ictal CBF increase was found, it was correct in 10/10 patients (100%) meeting these criteria (McNally et al., 2005). In the other six patients with a single unambiguous CBF increase (Patients 8, 11, 17, 18, 21, 23), the precise localization was not known. However, as already discussed, the CBF increases correctly identified at least the hemisphere of onset in all of these patients in which the side of onset was known (Patients 8, 17, 18, 23).
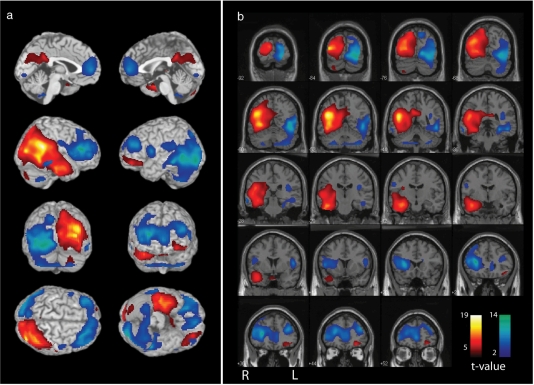
An example of an ictal SPECT injected during the pre-generalization period is shown in Fig. 1. In this patient (Patient 12, Table 1), ictal–interictal SPECT analysed using ISAS revealed CBF increases involving the right temporal and occipital lobes to an equal extent (with some lesser involvement of the right parietal lobe as well). It was, therefore, not possible to unambiguously localize the lobe of seizure onset based on the ictal SPECT in this patient. The increases in this patient did, however, include the right temporal lobe, which ultimately was confirmed by other data to be the region of seizure onset (Patient 12, Table 1, see also Supplementary Table 1). Thus, ictal SPECT correctly lateralized the seizure onset to the right hemisphere, and correctly identified a set of ‘candidate regions’ in this patient, but did not unambiguously identify a single lobe of seizure onset.
Figure 1.
Ictal SPECT injected during the pre-generalization period, with CBF increases including but not limited to the correct localization. Patient had right temporal neocortical epilepsy confirmed by intracranial EEG (Patient 12, Table 1, see also Supplementary Table 1). (A) Three dimensional rendering. (B) Coronal views with results superimposed on the SPM MRI template. Ictal SPECT scan was background subtracted using the patient's interictal SPECT, and the difference was then compared with a database of normal SPECT pairs using ISAS (see Methods section). CBF increases are shown as warm colours, and decreases are shown as cool colours; colour bars indicate t-values. The most significant hyperperfusion cluster was localized to the right temporal and occipital lobes (cluster-level significance P < 0.0001 corrected for multiple comparisons, Z-score of most significant voxel = 6.48, cluster size, k = 29 663 voxels). Extent threshold, k = 125 voxels (voxel dimensions 2 × 2 × 2 mm), voxel-level height threshold, P = 0.01.
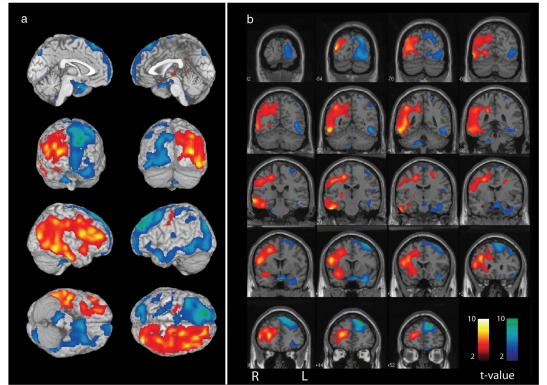
An example of an ictal SPECT injected during the generalization phase is shown in Fig. 2. This patient (Patient 15, Table 1) had equal involvement of the right frontal, parietal and temporal lobes based on CBF increases (with some lesser involvement of the right occipital lobe as well). Therefore, it was once again not possible to unambiguously identify a single lobe of seizure onset based on the ictal SPECT data in this patient. However, the ictal SPECT did lateralize the side of seizure onset correctly to the right hemisphere (Table 1, see also Supplementary Table 2).
Figure 2.
Ictal SPECT injected during the generalization period, with CBF increases involving multiple lobes in the hemisphere of seizure onset, and CBF decreases in the contralateral hemisphere. Patient had right hemisphere seizure onset (Patient 15, Table 1, see also Supplementary Table 2). (A) Three-dimensional rendering. (B) Coronal views with results superimposed on the SPM MRI template. The most significant hyperperfusion cluster was localized to the right frontal, temporal and parietal lobes (cluster-level significance P < 0.0001 corrected for multiple comparisons, Z-score of most significant voxel = 5.09, cluster size, k = 27 513 voxels). Hypoperfusion was greatest in the left hemisphere, contralateral to seizure onset (hypoperfusion asymmetry index = 0.91, Patient 15, Table 3). Ictal–interictal SPECT difference images were analysed using ISAS (see Methods section). CBF increases are shown as warm colours, and decreases are shown as cool colours; colour bars indicate t-values. Extent threshold, k = 125 voxels (voxel dimensions 2 × 2 × 2 mm), voxel-level height threshold, P = 0.01.
Post-ictal CBF increases are not helpful for localization
For injections in the post-ictal period, SPECT increases were not helpful for localizing or for lateralizing seizure onset. SPECT hyperperfusion regions agreed with the side of overall localization in 50% (12 of 24) of patients injected post-ictally in which side of onset was known (Table 2). Thus, for post-ictal injections, CBF increases lateralized to the correct side of seizure onset at chance levels. Interestingly, the lobe of seizure onset was usually not included in the regions of CBF increases for post-ictal injections. Thus, for patients with a single known lobe of seizure onset, this lobe was involved in only two (of nine) patients and these two occurred at relatively early post-ictal injection times (Patients 25, 31 in Table 2). Lobe of known seizure onset was not involved for other patients injected post-ictally (Patients 26, 32, 34, 46, 49, 53, 56 in Table 2).
For post-ictal injections, only 11 of 35 seizures showed a single unambiguous region of CBF increase (Patients 26, 27, 29, 36, 38, 42, 46, 49, 52, 54, 56 in Table 2). Of the remaining seizures, 16 showed CBF increases in multiple lobes, and eight showed no significant CBF increases in the post-ictal period (Table 2). Of the patients with a single unambiguous region of CBF increase, only six had known localization based on other data (Patients 26, 29, 42, 46, 49, 56 in Table 2, see also Supplementary Table 3). The single region of CBF increase correctly localized the lobe of seizure onset in none (0/6) of these cases. This was in agreement with our previous SPECT findings from partial seizures that did not secondarily generalize, which also showed very poor localization based on CBF increases in the post-ictal period (McNally et al., 2005).
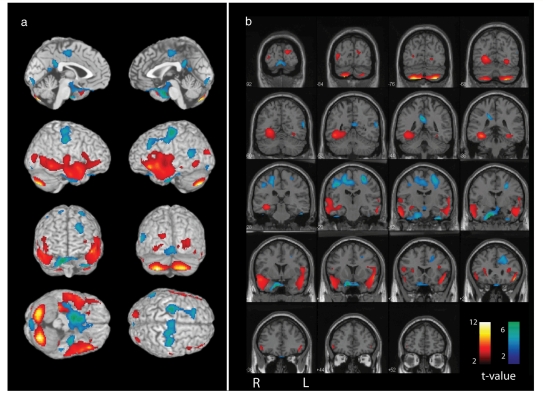
An example of a SPECT injection from the post-ictal period is shown in Fig. 3. In this patient (Patient 49, Table 2), ISAS analysis of CBF increases demonstrated that the most significant voxel cluster was located in the right temporal lobe. Of note, a second slightly less significant region of CBF increase was seen in the left temporal lobe as well (Fig. 3). However, the known region of seizure onset based on MRI, PET and surgical pathology was the left medial parietal cortex (Table 2, see also Supplementary Table 3). Thus, regions of CBF increase did not agree with the known region of seizure onset in this patient injected in the post-ictal period. Interestingly, bilateral CBF increases were also seen in the cerebellum in this patient injected in the post-ictal period (Fig. 3). Cerebellar changes in tonic–clonic seizures were analysed in greater detail in another recent study (Blumenfeld et al., 2009).
Figure 3.
Ictal SPECT injected during the post-ictal period was not useful for seizure localization. Patient had a left medial parietal localization based on MRI, PET and surgical pathology (Patient 49, Table 2, see also Supplementary Table 2). (A) Three-dimensional rendering. (B) Coronal views with results superimposed on the SPM MRI template. The most significant hyperperfusion cluster was localized to the right temporal lobe (cluster-level significance P < 0.0001 corrected for multiple comparisons, Z-score of most significant voxel = 4.32 cluster size, k = 6298 voxels), and a second large hyperperfusion cluster was present in the left temporal lobe (cluster-level significance P = 0.002 corrected for multiple comparisons, Z-score of most significant voxel = 4.80 cluster size, k = 2958 voxels). Hypoperfusion changes were also bilateral (hypoperfusion assymetery index = −0.03, Patient 49, Table 4). Ictal–interictal SPECT difference images were analysed using ISAS (see Methods section). CBF increases are shown as warm colours, and decreases are shown as cool colours; colour bars indicate t-values. Extent threshold, k = 125 voxels (voxel dimensions 2 × 2 × 2 mm), voxel-level height threshold, P = 0.01.
CBF decreases during generalization are contralateral to side of seizure onset
In addition to CBF increases, we also analysed the localizing and lateralizing value of CBF decreases during secondarily generalized tonic–clonic seizures (Tables 3 and 4). We found that CBF decreases during and after secondarily generalized tonic–clonic seizures were not useful for localizing the lobe of seizure onset. Thus, the regions of CBF decreases included the lobe of known seizure onset in none of the patients injected during seizures (Table 3), and in only four patients (Patients 46, 49, 53 and 56) injected post-ictally (Table 4). In agreement with this, a recent study of partial seizures (without secondary generalization), found that ictal and post-ical CBF decreases have poor localizing value for the specific lobe of seizure onset (McNally et al., 2005). It should be noted that some studies reported good localization of seizure onset based on post-ictal CBF decreases (Devous et al., 1998; O’Brien et al., 1999), which could reflect differences in analysis methods.
Table 3.
Ictal SPECT decreases: localization and lateralization of seizure onset
| Patienta | Overall Localizationb | ISAS Hypo- perfusion | L hemisphere hypoperfusion volumec | R hemisphere hypoperfusion volumec | Asymmetry Indexd | Side of greater hypoperfusion | Ipsi- or contra- lateral to localization |
|---|---|---|---|---|---|---|---|
| Pre-generalization | |||||||
| 1 | LH | LF | 7367 | 1342 | 0.69 | L | I |
| 2 | LH | RO | 10 772 | 23 805 | −0.38 | R | C |
| 3 | RT NC | RP, O | 2031 | 3530 | −0.27 | R | I |
| 4 | Unlocalized | RF, P | 10 075 | 15 218 | −0.20 | R | NA |
| 5 | LT versus RT NC | LF | 4690 | 1734 | 0.46 | L | NA |
| 6 | LT | LP | 2235 | 1940 | 0.07 | L | I |
| 7 | LT | BiF | 12 890 | 11 280 | 0.07 | L | I |
| 8 | RH | RP | 1348 | 4438 | −0.53 | R | I |
| 9 | LT | RF, P, T | 4956 | 23 453 | −0.65 | R | C |
| 10 | RH | LT, P | 7370 | 6358 | 0.07 | L | C |
| 11 | Multifocal | BiO | 20 647 | 17 345 | 0.09 | L | NA |
| 12 | RT NC | RF, LO | 17 519 | 10 538 | 0.25 | L | C |
| Generalization | |||||||
| 13 | LRo (C3, P3) | BiP, O | 4871 | 15 641 | −0.53 | R | C |
| 14 | LH | RP | 1110 | 17 331 | −0.88 | R | C |
| 15 | RH | LF, O | 14 753 | 674 | 0.91 | L | C |
| 16 | L mesial T | BiF | 18211 | 15 521 | 0.08 | L | I |
| 17 | RH | LF | 15 332 | 1850 | 0.78 | L | C |
| 18 | LH | BiF | 1560 | 2194 | −0.17 | R | C |
| 19 | Unlocalized | BiF,P | 13741 | 15 584 | −0.06 | R | NA |
| 20 | LH multifocal | LF | 10 136 | 10 726 | −0.03 | R | C |
| 21 | Unlocalized | RF, T | 3489 | 6286 | −0.29 | R | NA |
| 22 | RH | BiF,P | 26 728 | 22 833 | 0.08 | L | C |
| 23 | RH | LF, T, P | 21 020 | 4649 | 0.64 | L | C |
| 24 | LH | RP, O | 6594 | 14631 | −0.38 | R | C |
a Patients listed in order of injection time from seizure onset (Pre-generalization group), or generalization (Generalization group), as in Table 1.
b See Supplementary Tables 1 and 2 online for details.
c Volume expressed as number of significant voxels; voxel dimensions 2 × 2 × 2 mm (see Methods section).
d (L−R)/(L+R) See Methods section.
R = right; L = left; T = temporal; P = parietal; F = frontal; O = occipital; Ro = Rolandic; H = hemisphere; Bi = bilateral; NC = neocortical; I = ipsilateral to overall localization; C = contralateral.
Table 4.
Postictal SPECT decreases: Localization and lateralization of seizure onset
| Patienta | Overall Localizationb | ISAS Hypo- perfusion | L hemisphere hypoperfusion volumec | R hemisphere hypoperfusion volumec | Asymmetry Indexd | Side of greater hypoperfusion | Ipsi- or contra- lateral to localization |
|---|---|---|---|---|---|---|---|
| 25 | RT NC | BiF | 10 895 | 6855 | 0.23 | L | C |
| 26 | LO | RF, T, P | 5240 | 6712 | −0.12 | R | C |
| 27 | RH multifocal | NA | 2482 | 1386 | 0.28 | L | C |
| 28 | BiO | NA | 1086 | 329 | 0.53 | L | NA |
| 29 | Bi R > L T | RP, T | 17 215 | 2235 | 0.77 | L | C |
| 30 | LH | RF, T, P | 2603 | 16 448 | −0.73 | R | C |
| 31 | L mesial T | RP, T | 1958 | 4521 | −0.40 | R | C |
| 32 | LT | BiF | 5811 | 7114 | −0.10 | R | C |
| 33 | LH multifocal | BiP | 3148 | 3112 | 0.01 | L | I |
| 34 | LT | BiF | 9214 | 12 258 | −0.14 | R | C |
| 35 | LH multifocal | RF | 3994 | 4300 | −0.04 | R | C |
| 36 | Unlocalized | BiF, P | 34 579 | 31 058 | 0.05 | L | NA |
| 37 | RH | RP | 4120 | 9540 | −0.40 | R | I |
| 38 | Unlocalized | NA | 1588 | 827 | 0.32 | L | NA |
| 39 | L T-P | BiF, RP | 10 004 | 14 593 | −0.19 | R | C |
| 40 | R T-O | R P | 5764 | 8019 | −0.16 | R | I |
| 41 | RH | RT | 2497 | 9570 | −0.59 | R | I |
| 42 | BiO | LO | 4132 | 3089 | 0.14 | L | NA |
| 43 | LH | RP, O | 3265 | 5083 | −0.22 | R | C |
| 44 | RH | LF, T, P | 36 536 | 3873 | 0.81 | L | C |
| 45 | R versus L T | BiP | 3228 | 3768 | −0.08 | R | NA |
| 46 | L mesial T | LT | 1943 | 2307 | −0.09 | R | C |
| 47 | RH | BiF | 5127 | 1617 | 0.52 | L | C |
| 48 | L P-O | BiF | 1671 | 771 | 0.37 | L | I |
| 49 | L medial P | BiP | 2709 | 2851 | −0.03 | R | C |
| 50 | Unlocalized | RT | 1048 | 7402 | −0.75 | R | NA |
| 51 | Unlocalized | RF | 1459 | 7912 | −0.69 | R | NA |
| 52 | Unlocalized | BiF | 5647 | 4304 | 0.13 | L | NA |
| 53 | R mesial T | BiF, T, P | 11 540 | 19 173 | −0.25 | R | I |
| 54 | RH | BiF | 17 866 | 10 841 | 0.24 | L | C |
| 55 | Unlocalized | RP, F, T | 2351 | 32 534 | −0.87 | R | NA |
| 56 | RF | RF | 2893 | 5041 | −0.27 | R | I |
| 57 | Unlocalized | RF, T | 14 034 | 14 815 | −0.03 | R | NA |
| 58 | Bi mesial T | RF, P, O | 20 656 | 31 990 | −0.22 | R | NA |
| 59 | LH | BiF, P, O | 13 106 | 16 592 | −0.12 | R | C |
a Patients listed in order of injection time from seizure end, as in Table 2.
b See Supplementary Table 3 online for details.
c Volume expressed as number of significant voxels; voxel dimensions 2 × 2 × 2 mm (see Methods section).
d (L−R)/(L+R) See Methods section. R = right; L = left; T = temporal; P = parietal; F = frontal; O = occipital; Ro = Rolandic; H = hemisphere; Bi = bilateral; NC = neocortical; I = ipsilateral to overall localization; C = contralateral.
Although post-ictal CBF decreases may have questionable value for localizing the lobe of seizure onset, we recently found that the hemisphere with greatest overall post-ictal CBF decreases usually corresponds to the side of seizure onset (McNally et al., 2005). Post-ictal CBF decreases in partial seizures without secondary generalization can thus be useful to at least lateralize the side of seizures onset. We were therefore interested in whether the side of overall greatest hypoperfusion would be useful for lateralizing the side of onset of partial seizures with secondary generalization. We found that for SPECT injections in the pre-generalization phase, the side of greater hypoperfusion was ipsilateral or contralateral to the side of onset with about equal frequency, and was therefore not clinically useful for lateralization (five ipsilateral and four contralateral, Table 3). Interestingly, for seizures injected during the generalization phase, hypoperfusion was greater in the hemisphere contralateral to seizure onset in 90% (9 of 10) of patients with known side of seizure onset (Table 3). For post-ictal injections, hypoperfusion remained greater in the hemisphere contralateral to onset in most cases (71%; 17 of 24 patients, Table 4). This was especially true for patients injected within 15 s of seizure end, where 100% (seven of seven) showed greater hypoperfusion contralateral to onset (Patients 25–32 in Tables 2 and 4). These findings suggest that while CBF decreases are not useful for localizing the lobe of seizure onset, CBF decreases during and shortly after generalization are usually greatest in the hemisphere contralateral to the side of onset. It is of interest that this is opposite to the pattern seen following partial seizures without generalization, where overall hypoperfusion is usually greatest in the hemisphere ipsilateral to side of onset (McNally et al., 2005).
Examples of CBF decreases during partial seizures with secondary generalization are shown in Figs 1–3. In the example shown during the pre-generalization period, CBF decreases were seen in multiple bilateral brain regions (Fig. 1), similar to those reported previously for temporal lobe seizures without generalization (Van Paesschen et al., 2003; Blumenfeld et al., 2004). In the example shown during generalization, CBF decreases were most prominent in the hemisphere contralateral to seizure onset (Fig. 2). During the late post-ictal period, in the example shown, CBF decreases were present bilaterally (Fig. 3).
Discussion
We found that ictal SPECT can provide clinically useful localizing information even when obtained during partial seizures with secondary generalization. Caution is necessary in interpreting ictal SPECT in secondarily generalized seizures, since multiple lobes are often involved in CBF increases. However, the side of ictal CBF increases correctly lateralizes the side of seizure onset 84% of the time. In addition, when focal CBF increases occur involving a single lobe this usually is the region of seizure onset, even in partial seizures with secondary generalization. These finding suggest that partial seizures with secondary generalization do not homogenously involve the whole brain, but rather affect some regions most intensely, while other areas are relatively spared even in so-called ‘generalized’ seizures.
A growing body of evidence supports the notion that ‘generalized’ seizures are not truly generalized. Even in primary generalized epilepsy where bilateral spike-wave discharges are seen, electrical mapping and neuroimaging studies show focal bilateral involvement of frontal and parietal cortex, while other regions are spared (Meeren et al., 2002; Archer et al., 2003; Salek-Haddadi et al., 2003; Aghakhani et al., 2004; Holmes et al., 2004; Nersesyan et al., 2004a,b; Berman et al., 2005; Berman et al., submitted for publication). In partial seizures with secondary generalization, there is also evidence that localized regions are most intensely involved, and that these regions are related to the site of seizure onset. For example, post-ictal Todd's paresis and other deficits are often localized to the region of seizure onset even following partial seizures with secondary generalization (Rolak et al., 1992; Blumenfeld et al., 2003b). Focal regional involvement with sparing of other areas in secondarily generalized seizures is also supported by intracranial EEG (Schindler et al., 2007) and by previous SPECT imaging studies in spontaneous and induced seizures (Lee et al., 1987; Green and Buchhalter, 1993; Koc et al., 1997; Shin et al., 2002; Blumenfeld et al., 2003a,b; Enev et al., 2007).
Prior investigations have also suggested that CBF increases during secondarily generalized seizures may be useful for localizing seizure onset (Lee et al., 1987; O'Brien et al., 1998; Shin et al., 2002). However, these studies were based on relatively few secondarily generalized seizures, and did not examine in detail how SPECT images during generalized seizures can be interpreted to yield clinically useful results. We found that SPECT increases during secondarily generalized seizures often involved multiple lobes, making it impossible to identify a single unambiguous lobe of seizure onset. However, our results enable the use of ictal SPECT increases in multiple lobes to at least narrow down the most likely hemisphere or group of regions for seizure onset, which can be helpful for planning additional presurgical studies. In addition, our results suggest that when a single lobe shows the greatest CBF increases even during secondarily generalized seizures, this lobe is often the correct localization for seizure onset.
Injections during both the pre-generalization and generalization periods were helpful for localization. However, injections during the post-ical period did not yield CBF increases that were helpful for seizure localization. This is in agreement with prior work from partial seizures, in which post-ictal CBF increases were very poor at correctly localizing seizure onset (McNally et al., 2005; Kim et al., 2009).
We found that CBF decreases during the generalization period and early post-ictal periods were usually greatest in the hemisphere contralateral to seizure onset. This was quantified using a hypoperfusion asymmetry index, and provides another useful method for interpreting ictal SPECT during secondarily generalized seizures to glean clinically useful information. It is interesting that in partial seizures without generalization, we found that post-ictal CBF decreases were greatest in the hemisphere ipsilateral to onset (McNally et al., 2005), whereas during and shortly following generalized seizures the present study shows greater CBF decreases contralateral to seizure onset. It is likely that the CBF decreases occur through different mechanisms in these two situations. It is known that partial seizures often cause post-ictal CBF decreases both in the region of seizures (Newton et al., 1992; Zubal et al., 1999) and in surrounding regions (Avery et al., 2000; Blumenfeld et al., 2004; Englot et al., 2008), which together may explain the larger post-ictal CBF decreases on the side of seizure onset with partial seizures (McNally et al., 2005). In contrast, secondarily generalized seizures, often show large CBF increases involving multiple lobes on the side of seizure onset (e.g. see Fig. 2), which may cause a relative decrease in CBF in the contralateral hemisphere either through long-range neuronal network mechanisms (Blumenfeld et al., 2004; Englot et al., 2008) or vascular mechanisms. We also cannot exclude the possibility that the intensity normalization used in image processing to correct for differences in total brain counts between ictal and interictal scans may artificially depress the relative SPECT signal in the contralateral hemisphere when increases in the ipsilateral hemisphere are large. Further investigation will be needed to determine the mechanisms, but in any case, contralateral SPECT signal decreases remain a useful clinical sign for lateralizing the side of onset of secondarily generalized tonic–clonic seizures.
Additional investigation of the cortical and subcortical changes in secondarily generalized tonic–clonic seizures may also provide important insights into pathophysiology of this seizure type. For example, regions showing the most intense changes could be related to pathological damage caused by extreme increases in neuronal electrical activity and metabolism (Schridde et al., 2008). Regions of CBF decreases may also contribute to impaired cerebral function during and following seizures (Blumenfeld et al., 2004; Englot et al., 2008). In addition, late increases in cerebellar activity, which we often observed during and following generalized tonic–clonic seizures (e.g. Fig. 3) could be important for mechanisms of seizure termination or post-ictal suppression (Salgado-Benitez et al., 1982). Group analysis of neuroimaging data, to determine the regions most commonly affected before, during, and after secondarily generalized tonic-seizures may help elucidate some of these fundamental questions, and were the subject of another recent study (Blumenfeld et al., 2009).
Conclusions
We found that ictal and post-ictal SPECT from secondarily generalized tonic–clonic seizures demonstrate regional CBF changes that are clinically useful for localizing seizure onset. Although partial seizures without generalization provide more focal changes, even secondarily generalized seizures do not involve the whole brain homogeneously. When CBF increases are observed in multiple regions during the pre-generalization and generalization periods, these regions usually include the correct lobe and are greatest in the hemisphere of seizure onset. In addition, when a single region shows the greatest CBF increase, even during generalized tonic–clonic seizures, this is most often the correct lobe of seizure onset. CBF decreases do not identify the specific lobe of seizure onset, but are usually greatest in the hemisphere contralateral to onset during the generalization period and shortly afterwards. These patterns of CBF changes allow SPECT obtained from secondarily generalized tonic–clonic seizures to be used for presurgical localization in patients with medically refractory epilepsy. Therefore, although partial seizures are preferred, if secondarily generalized seizures occur during ictal SPECT, careful interpretation can provide clinically useful results.
Funding
National Institutes of Health (R01 NS055829); Donaghue Investigator Award; Betsy and Jonathan Blattmachr family.
Supplementary Material
Acknowledgements
We thank Sarah Doernberg and Kathryn Davis for initial patient identification and video/EEG review, and Matthew DeSalvo for helpful comments on the manuscript.
References
- Acton PD, Friston KJ. Statistical parametric mapping in functional neuroimaging: beyond PET and fMRI activation studies. Euro J Nucl Med. 1998;25:663–7. [PubMed] [Google Scholar]
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, et al. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–44. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- Andersen AR. 99mTc-D,L-hexamethylene-propyleneamine oxime (99mTc-HMPAO): basic kinetic studies of a tracer of cerebral blood flow. Cerebrovasc Brain Metab Rev. 1989;1:288–318. [PubMed] [Google Scholar]
- Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–22. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Avery RA, Spencer SS, Spanaki MV, Corsi M, Seibyl JP, Zubal IG. Effect of injection time on postictal SPET perfusion changes in medically refractory epilepsy. Eur J Nucl Med. 1999;26:830–6. doi: 10.1007/s002590050456. [DOI] [PubMed] [Google Scholar]
- Avery RA, Zubal IG, Stokking R, Studholme C, Corsi M, Seibyl JP, et al. Decreased cerebral blood flow during seizures with ictal SPECT injections. Epilepsy Res. 2000;40:53–61. doi: 10.1016/s0920-1211(00)00109-1. [DOI] [PubMed] [Google Scholar]
- Berman R, Negishi M, Constable RT, Novotny EJ, Levy S, Blumenfeld H. Combined EEG and fMRI during typical childhood absence seizures at 3T (AES abstracts) Epilepsia. 2005 [Google Scholar]
- Berman R, Negishi M, Spann M, Chung M, Bai X, Purcaro M, et al. Simultaneous EEG, fMRI, and behavioral testing in typical childhood absence seizures. (Submitted for publication) [DOI] [PMC free article] [PubMed]
- Blumenfeld H, McNally KA, Ostroff R, Zubal IG. Targeted prefrontal cortical activation with bifrontal ECT. Psychiat Res Neuroimag. 2003a;123:165–170. doi: 10.1016/s0925-4927(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, et al. Positive and negative network correlations in temporal lobe epilepsy. Cerebral Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese G, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009. (in press) [DOI] [PMC free article] [PubMed]
- Blumenfeld H, Westerveld M, Ostroff RB, Vanderhill SD, Freeman J, Necochea A, et al. Selective frontal, parietal and temporal networks in generalized seizures. Neuroimage. 2003b;19:1556–66. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- Chang DJ, Zubal IG, Gottschalk C, Necochea A, Stokking R, Studholme C, et al. Comparison of statistical parametric mapping and SPECT difference imaging in patients with temporal lobe epilepsy. Epilepsia. 2002;43:68–74. doi: 10.1046/j.1528-1157.2002.21601.x. [DOI] [PubMed] [Google Scholar]
- Devous MD, Sr, Leroy RF, Homan RW. Single photon emission computed tomography in epilepsy. Semin Nucl Med. 1990;20:325–41. doi: 10.1016/s0001-2998(05)80237-5. [DOI] [PubMed] [Google Scholar]
- Devous MD, Sr, Thisted RA, Morgan GF, Leroy RF, Rowe CC. SPECT brain imaging in epilepsy: a meta-analysis. J Nucl Med. 1998;39:285–93. [PubMed] [Google Scholar]
- Enev M, McNally KA, Varghese G, Zubal IG, Ostroff RB, Blumenfeld H. Imaging onset and propagation of ECT-induced seizures. Epilepsia. 2007;48:238–44. doi: 10.1111/j.1528-1167.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008 doi: 10.1523/JNEUROSCI.2014-08.2008. 28: 9066–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren L, Bucht G, Eriksson S, Bergmark L. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia. 1996;37:224–9. doi: 10.1111/j.1528-1157.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–35. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Green C, Buchhalter JR. Ictal SPECT in a 16-day-old infant. Clin Nucl Med. 1993;18:768–70. doi: 10.1097/00003072-199309000-00009. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Brown M, Tucker DM. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45:1568–79. doi: 10.1111/j.0013-9580.2004.23204.x. [DOI] [PubMed] [Google Scholar]
- Horsley V. An address on the origin and seat of epileptic disturbance. Br Med J. 1892;1:693–6. doi: 10.1136/bmj.1.1631.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst BC, Williamson PD, Neuschwander TB, Darcey TM, Thadani VM, Roberts DW. Secondarily generalized seizures in mesial temporal epilepsy: clinical characteristics, lateralizing signs, and association with sleep-wake cycle. Epilepsia. 2001;42:1279–87. doi: 10.1046/j.1528-1157.2001.09701.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Zubal IG, Blumenfeld H. Epilepsy localization by ictal and interictal SPECT. In: Van Heertum RL, Ichise M, Tikofsky RS, editors. Functional cerebral SPECT and PET imaging. Lippincott: Williams & Wilkins; 2009. [Google Scholar]
- Knowlton RC. The role of FDG-PET, ictal SPECT, and MEG in the epilepsy surgery evaluation. Epilepsy Behav. 2006;8:91–101. doi: 10.1016/j.yebeh.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Koc E, Serdaroglu A, Kapucu O, Atalay Y, Gucuyener K, Atasever T. Ictal and interictal SPECT in a newborn infant with intractable seizure. Acta Paediatrica. 1997;86:1379–81. doi: 10.1111/j.1651-2227.1997.tb14918.x. [DOI] [PubMed] [Google Scholar]
- Koo CW, Devinsky O, Hari K, Balasny J, Noz ME, Kramer EL. Stratifying differences on ictal/interictal subtraction SPECT images. Epilepsia. 2003;44:379–86. doi: 10.1046/j.1528-1157.2003.29402.x. [DOI] [PubMed] [Google Scholar]
- Lee HW, Hong SB, Tae WS. Opposite ictal perfusion patterns of subtracted SPECT. Hyperperfusion and hypoperfusion. Brain. 2000a;123:2150–9. doi: 10.1093/brain/123.10.2150. [DOI] [PubMed] [Google Scholar]
- Lee JD, Kim HJ, Lee BI, Kim OJ, Jeon TJ, Kim MJ. Evaluation of ictal brain SPET using statistical parametric mapping in temporal lobe epilepsy. Eur J Nucl Med. 2000b;27:1658–65. doi: 10.1007/s002590000364. [DOI] [PubMed] [Google Scholar]
- Lee SK, Lee SH, Kim SK, Lee DS, Kim H. The clinical usefulness of ictal SPECT in temporal lobe epilepsy: the lateralization of seizure focus and correlation with EEG. Epilepsia. 2000c;41:955–62. doi: 10.1111/j.1528-1157.2000.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Lee DS, Lee SK, Lee MC. Functional neuroimaging in epilepsy: FDG PET and ictal SPECT. J Korean Med Sci. 2001;16:689–96. doi: 10.3346/jkms.2001.16.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BI, Markand ON, Wellman HN, Siddiqui AR, Mock B, Krepshaw J, et al. HIPDM single photon emission computed tomography brain imaging in partial onset secondarily generalized tonic-clonic seizures. Epilepsia. 1987;28:305–11. doi: 10.1111/j.1528-1157.1987.tb04223.x. [DOI] [PubMed] [Google Scholar]
- McNally KA, Paige AL, Varghese G, Zhang H, Novotny EJ, Spencer SS, et al. Localizing value of ictal-interictal SPECT analyzed by SPM (ISAS) Epilepsia. 2005;46:1450–64. doi: 10.1111/j.1528-1167.2005.06705.x. [DOI] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–95. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow Metab. 2004a;24:1057–68. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- Nersesyan H, Hyder F, Rothman D, Blumenfeld H. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004b;24:589–99. doi: 10.1097/01.WCB.0000117688.98763.23. [DOI] [PubMed] [Google Scholar]
- Newton MR, Berkovic SF, Austin MC, Rowe CC, McKay WJ, Bladin PF. Postictal switch in blood flow distribution and temporal lobe seizures. J Neurol, Neurosurg Psychiatry. 1992;55:891–4. doi: 10.1136/jnnp.55.10.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton MR, Berkovic SF, Austin MC, Rowe CC, McKay WJ, Bladin PF. Ictal postictal and interictal single-photon emission tomography in the lateralization of temporal lobe epilepsy. Eur J Nucl Med. 1994;21:1067–71. doi: 10.1007/BF00181061. [DOI] [PubMed] [Google Scholar]
- O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50:445–54. doi: 10.1212/wnl.50.2.445. [DOI] [PubMed] [Google Scholar]
- O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Jack CR, Jr, et al. Subtraction SPECT co-registered to MRI improves postictal SPECT localization of seizure foci. Neurology. 1999;52:137–46. doi: 10.1212/wnl.52.1.137. [DOI] [PubMed] [Google Scholar]
- Penfield W. The evidence for a cerebral vascular mechanism in epilepsy. Ann Int Med. 1933;7:303–10. [Google Scholar]
- Rodin E, Onuma T, Wasson S, Porzak J, Rodin M. Nonfocal grand mal seizures as seen through high frequency recordings. Electroencephalogr Clinical Neurophysiol. 1969;27:697. doi: 10.1016/0013-4694(69)91333-9. [DOI] [PubMed] [Google Scholar]
- Rolak LA, Rutecki P, Ashizawa T, Harati Y. Clinical features of Todd's post-epileptic paralysis. J Neurol, Neurosurg Psychiatry. 1992;55:63–4. doi: 10.1136/jnnp.55.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Berkovic SF, Austin MC, McKay WJ, Bladin PF. Patterns of postictal cerebral blood flow ni temporal lobe epilepsy: qualitative and quantitative analysis. Neurology. 1991;41:1096–1013. doi: 10.1212/wnl.41.7.1096. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53:663–7. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- Salgado-Benitez A, Briones R, Fernandez-Guardiola A. Purkinje cell responses to a cerebral penicillin-induced epileptogenic focus in the cat. Epilepsia. 1982;23:597–606. doi: 10.1111/j.1528-1157.1982.tb05074.x. [DOI] [PubMed] [Google Scholar]
- Schindler K, Leung H, Lehnertz K, Elger CE. How generalised are secondarily “generalised” tonic clonic seizures? J Neurol Neurosurg Psychiatry. 2007;78:993–6. doi: 10.1136/jnnp.2006.108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow J, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cerebral Cortex. 2008;18:1814–27. doi: 10.1093/cercor/bhm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin WC, Hong SB, Tae WS, Kim SE. Ictal hyperperfusion patterns according to the progression of temporal lobe seizures. Neurology. 2002;58:373–80. doi: 10.1212/wnl.58.3.373. [DOI] [PubMed] [Google Scholar]
- Spanaki MV, Spencer SS, Corsi M, MacMullan J, Seibyl J, Zubal IG. Sensitivity and specificity of quantitative difference SPECT analysis in seizure localization. J Nucl Med. 1999;40:730–6. [PubMed] [Google Scholar]
- Spencer SS. The relative contributions of MRI, SPECT, and PET imaging in epilepsy. Epilepsia. 1994;35(Suppl 6):S72–89. doi: 10.1111/j.1528-1157.1994.tb05990.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Theodore WH, Berkovic SF. Clinical applications: MRI, SPECT, and PET. Magn Reson Imaging. 1995;13:1119–24. doi: 10.1016/0730-725x(95)02021-k. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Porter RJ, Albert P, Kelley K, Bromfield E, Devinsky O, Sato S. The secondarily generalized tonic-clonic seizure: A videotape analysis. Neurology. 1994;44:1403–7. doi: 10.1212/wnl.44.8.1403. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W, Dupont P, Van Driel G, Van Billoen H, Maes A. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 2003;126:1103–11. doi: 10.1093/brain/awg108. [DOI] [PubMed] [Google Scholar]
- Zubal IG, Spanaki MV, MacMullan J, Corsi M, Seibyl JP, Spencer SS. Influence of technetium-99m-hexamethylpropylene amine oxime injection time on single-photon emission tomography perfusion changes in epilepsy. Eur J Nucl Med. 1999;26:12–17. doi: 10.1007/s002590050353. [DOI] [PubMed] [Google Scholar]
- Zubal IG, Spencer SS, Imam K, Seibyl J, Smith EO, Wisniewski G, et al. Difference images calculated from ictal and interictal technetium-99m-HMPAO SPECT scans of epilepsy. J Nucl Med. 1995;36:684–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.