Abstract
Brain atrophy measured by magnetic resonance structural imaging has been proposed as a surrogate marker for the early diagnosis of Alzheimer's disease. Studies on large samples are still required to determine its practical interest at the individual level, especially with regards to the capacity of anatomical magnetic resonance imaging to disentangle the confounding role of the cognitive reserve in the early diagnosis of Alzheimer's disease. One hundred and thirty healthy controls, 122 subjects with mild cognitive impairment of the amnestic type and 130 Alzheimer's disease patients were included from the ADNI database and followed up for 24 months. After 24 months, 72 amnestic mild cognitive impairment had converted to Alzheimer's disease (referred to as progressive mild cognitive impairment, as opposed to stable mild cognitive impairment). For each subject, cortical thickness was measured on the baseline magnetic resonance imaging volume. The resulting cortical thickness map was parcellated into 22 regions and a normalized thickness index was computed using the subset of regions (right medial temporal, left lateral temporal, right posterior cingulate) that optimally distinguished stable mild cognitive impairment from progressive mild cognitive impairment. We tested the ability of baseline normalized thickness index to predict evolution from amnestic mild cognitive impairment to Alzheimer's disease and compared it to the predictive values of the main cognitive scores at baseline. In addition, we studied the relationship between the normalized thickness index, the education level and the timeline of conversion to Alzheimer's disease. Normalized thickness index at baseline differed significantly among all the four diagnosis groups (P < 0.001) and correctly distinguished Alzheimer's disease patients from healthy controls with an 85% cross-validated accuracy. Normalized thickness index also correctly predicted evolution to Alzheimer's disease for 76% of amnestic mild cognitive impairment subjects after cross-validation, thus showing an advantage over cognitive scores (range 63–72%). Moreover, progressive mild cognitive impairment subjects, who converted later than 1 year after baseline, showed a significantly higher education level than those who converted earlier than 1 year after baseline. Using a normalized thickness index-based criterion may help with early diagnosis of Alzheimer's disease at the individual level, especially for highly educated subjects, up to 24 months before clinical criteria for Alzheimer's disease diagnosis are met.
Keywords: Early Alzheimer's disease, individual diagnosis, mild cognitive impairment, magnetic resonance imaging (MRI), cognitive reserve
Introduction
Clinical evaluation and neuropsychometry have shown their limits (Mueller et al., 2005; Venneri, 2007) for predicting evolution from the prodromal stage of Alzheimer's disease (i.e. mild cognitive impairment, MCI) (Petersen et al., 1999; Petersen, 2003) to the dementia stage. One of the major limits on prediction comes from the cognitive reserve, which acts as a confounding factor and may hide the early signs of the disease, especially for subjects with a high education level, who would be more successful at coping with greater brain damage (Mortimer et al., 2005; Stern, 2006; Roe et al., 2007). Alternatively, various types of neuroimaging have shown promising capacities for measuring the early onset of Alzheimer's disease, including MRI (Mungas et al., 2002; Jack et al., 2003) and cerebral glucose metabolism or beta amyloid imaging with positron emission tomography (Alexander et al., 2002; Engler et al., 2006). Moreover, some recent studies have investigated the interaction between the cognitive reserve factor and neuroimaging modes (Sole-Padulles et al., 2007; Hanyu et al., 2008; Kemppainen et al., 2008), and have shown that neuroimaging measurements may reflect the underlying pathology better than neuropsychometry since they are less affected by cognitive reserve, which, in turn, is linked to education level (Stern, 2006).
While brain metabolism or amyloid deposition may show higher sensitivity or specificity for the early detection of the disease (Small et al., 2006), structural MRI offers important advantages, such as greater availability, faster data acquisition, lower cost and the possibility of automatically deriving quantitative indices of regional atrophy (Mueller et al., 2006). Accordingly, the validation of structural MRI as a marker of Alzheimer's disease progression is the core project of the Alzheimer's Disease Neuroimaging Initiative (ADNI).
In this perspective, we have developed a fast (20 min per volume), robust and fully automated method for cortical thickness measurement. As an alternative to volumetric methods, cortical thickness measurement has given promising results while being less operator-dependent than hippocampal volume measurements and more suitable for quantification and localization (Lerch et al., 2005) than voxel-based morphometry (Ashburner and Friston 2000). Lerch et al. (2005) have also established a link between histopathologically confirmed pathological changes and cortical atrophy assessed through cortical thickness measurement. We tested the effectiveness of our method on cross-sectional data obtained from the ADNI database. We then assessed the power of cognitive testing and of cortical thickness to predict evolution from amnestic MCI to Alzheimer's disease at the individual level over a period of 24 months, with a particular emphasis on the possible impact of the cognitive reserve, using cortical thickness as a proxy for brain damage.
Methods
Study set-up and design
Data used in the preparation of this article were obtained from the ADNI database (www.loni.ucla.edu/ADNI). The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations as a $60 million, 5-year public–private partnership. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer's disease. Determination of sensitive and specific markers of very early Alzheimer's disease progression is intended to aid researchers and clinicians in the development of new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials (for more details, see Supplementary material).
Inclusion and diagnostic criteria
The general inclusion criteria were those of the ADNI (http://clinicaltrials.gov/show/NCT00106899). According to ADNI clinical procedures, diagnosis of Alzheimer's disease was made if the subject had a Mini Mental State Examination (MMSE) (Folstein et al., 1975) score between 20 and 26, a Clinical Dementia Rating scale (Morris, 1993) score of 0.5 or 1, and met NINCDS/ADRDA (McKhann et al., 1984) criteria for probable Alzheimer's disease.
Individuals were classified as single-domain amnestic MCI if they satisfied the following criteria: (i) score on the MMSE between 24 and 30; (ii) Clinical Dementia Rating scale = 0.5; (iii) reported memory complaint; (iv) objective memory loss measured by education-adjusted scores on Wechsler Memory Scale Logical Memory II (Wechsler, 1987); (v) absence of significant levels of impairment in other cognitive domains; (vi) preserved activities of daily life; and (vii) absence of dementia.
Healthy controls had an MMSE score between 24 and 30 and a Clinical Dementia Rating scale score of 0. Whatever the inclusion group, subjects had a Geriatric Depression Scale score of less than 6.
Participants
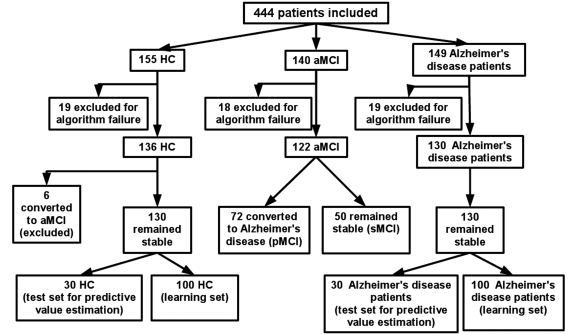
Four hundred and forty-four subjects from the ADNI database were included in our study (Fig. 1). These subjects were selected randomly, respecting roughly a 1:1:1 ratio (healthy controls: amnestic MCI: Alzheimer's disease patient). Fifty-six subjects (12.6%) were subsequently excluded because of unsuccessful cortical thickness measurement due to poor image quality.
Figure 1.
Inclusion diagram.
Every subject received a baseline clinical evaluation and was re-evaluated every six months after inclusion, over a total period of 24 months. After 24 months, 72 amnestic MCI had converted to Alzheimer's disease (hereafter referred to as progressive MCI, as opposed to stable MCI) and six healthy controls had converted amnestic MCI (and were thus excluded from the study). The resulting cross-sectional population was finally composed of 130 healthy controls, 50 stable MCI, 72 progressive MCI and 130 Alzheimer's disease patients, whose demographic data are presented in Table 1.
Table 1.
Demographic and clinical characteristics according to diagnosis group
| Variable | Diagnosis group |
P-value | |||
|---|---|---|---|---|---|
| Healthy controls (n = 130) | Stable MCI (n = 50) | Progressive MCI (n = 72) | Alzheimer's disease patients (n = 130) | ||
| Age (years) | 75.7 ± 5.2 | 75.6 ± 6.9 | 75.3 ± 7.0 | 75.0 ± 7.2 | 0.90 |
| ApoE (%)a | 27.0 | 42.0 | 63.9 | 70.6 | <0.001 |
| Education level (years) | 16.3 ± 2.4 | 15.9 ± 2.7 | 15.7 ± 2.8 | 14.8 ± 2.9 | 0.001 |
| Sex | 58M/42F | 34M/16F | 47M/25F | 58M/42F | 0.54 |
| Cognitive scores | |||||
| MMSE | 29.2 ± 0.9 | 27.4 ± 1.8 | 26.5 ± 1.8 | 23.1 ± 2.1 | <0.001 |
| ADAS-Cog, 10-word list delayed recall | 7.2 ± 1.7 | 4.1 ± 2.5 | 2.6 ± 1.9 | 1.2 ± 1.4 | <0.001 |
| AVLT delayed recall | 7.6 ± 3.7 | 3.5 ± 3.8 | 1.3 ± 1.9 | 0.5 ± 1.3 | <0.001 |
| TMTB time to complete (s) | 80.7 ± 29 | 101.2 ± 52.2 | 152.1 ± 79.4 | 194.3 ± 96.5 | <0.001 |
Plus–minus values are means ± SD. P-values correspond to the differences between the four diagnosis groups.
a The given percentage indicates the proportion of individuals carrying at least one allele E4.
For the cognitive reserve analysis, all the subjects were dichotomized in two groups: the Lower Education Group consisted of the subjects with an education level lower than the mean education level of the amnestic MCI sample, i.e. subjects with ≤15 years of education, and the Higher Education Group consisted of the subjects with an education level equal to or >16 years of education. To assess the predictive value of our methodology on a test set, a subset consisting of 30 healthy controls and 30 Alzheimer's disease patients, who did not differ in terms of age, education, gender or MMSE score from the remaining 100 healthy controls and 100 Alzheimer's disease patients, was randomly selected.
Neuropsychological scores
The delayed recall performance in the Auditory Verbal Learning Test (Rey, 1964) and in the Alzheimer's disease Assessment Scale score (ADAS-Cog) 10-Word list (Rosen et al., 1984) were selected from the cognitive measures included in the ADNI database because delayed recall has been shown to be a strong predictor of Alzheimer's disease (Estevez-Gonzalez et al., 2003; Rountree et al., 2007). Complementary to memory performance, executive functions were evaluated by Trail Making Test B (time to complete) and a measure of global functioning was provided by the MMSE score.
Information on conversion from healthy controls to amnestic MCI and from amnestic MCI to Alzheimer's disease was used as given in the ADNI database (after review by the Central Review Committee, according to the ADNI procedures).
MRI volumes
MRI volumes at baseline were downloaded from the ADNI database. All the volumes were acquired on a 1.5 Tesla scanner. However, as ADNI is a multicentre project, other acquisition parameters were specific to the centres concerned.
Method of cortical thickness measurement
All the procedures described below (segmentation, cortical thickness measurement, cortical thickness map parcellation) were implemented in-house. A validation procedure was carried out using publicly available simulated population data (Lerch and Evans, 2005). More details on this validation are provided in the supplementary data.
Volumes were resampled to a 1 × 1 × 1 mm resolution, then segmented using an in-house reimplemented segmentation algorithm, which followed the procedure described by Ashburner and Friston (1997). Using the grey matter, white matter and cerebrospinal fluid maps given by this algorithm, cortical thickness was computed on the whole cortical ribbon in the native space of the subject's brain, using a Laplace's-equation-based algorithm as described by Jones et al. (2000). To take into account partial volume effects at the white matter/grey matter and cerebrospinal fluid/grey matter interfaces, a boundary cortical thickness was derived by using a fuzzy distance method (Bloch, 2004), which led to a sub-millimetric measurement of cortical thickness.
By computing cortical thickness for the entire cortical ribbon, a 3D cortical thickness map was obtained, which was then registered in the standardized MNI space (Evans and Collins, 1993) using nine-parameter rigid registration (ITK Software©, Ixico, London) (Studholme et al., 1999).
Cortical thickness map parcellation
The registered cortical thickness map was parcellated into 96 areas (48 Brodmann areas × 2 hemispheres) using the Brodmann area 3D map given by the MRIcro package (http://www.sph.sc.edu/comd/rorden/mricro.html). To reinforce the robustness of the measures, these areas were then grouped into 22 zones (11 zones × 2 hemispheres) defined by three neurologists (J.P., J-F.D., M.P.) following pathophysiological criteria (see Supplementary data, Table 1). A mean cortical thickness was computed for each zone of each subject.
Normalized thickness index
We developed a Normalized Thickness Index (NTI) that measured the severity of brain atrophy, for a single subject, on a continuum between healthy state and Alzheimer's disease. The NTI was designed to take age into account since cortical thickness decreases with age (Salat et al., 2004), and to provide a single, normalized measure instead of a set of raw measurements obtained from different zones.
The NTI computation is based on a supervised learning approach. The first paragraph below describes the generic process to compute a parametrical NTI, which depends on the subset of zones used for the computation among the 22 available. The optimal subset of zones that best discriminated stable MCI subjects from progressive MCI subjects was then chosen according to a procedure that we describe in the second paragraph.
First, to compute the NTI corresponding to any subset of n zones among the 22 zones of a subject S, 50 healthy controls and 50 Alzheimer's disease patients were randomly selected from their respective groups (involving the 100 healthy controls and 100 Alzheimer's disease patients who constituted the learning sets). These subsets were used as a training sample. If the considered subject S (for whom the NTI was to be computed) belonged to the healthy controls or the Alzheimer's disease groups, he/she was discarded so as not to be included in the training sample. A normalized linear discriminant function was computed, taking age and the mean cortical thickness values of the n zones into account. The function was normalized by centring the learning set of healthy controls on 1 and that of Alzheimer's disease patients on −1. This function was then applied to the subject S and gave a temporary NTI. To prevent the NTI from being dependent on the learning sets, the whole process was repeated a hundred times (always by choosing the learning sets at random). The final NTI for the subject S was the average of the hundred values obtained.
We then used an automatic procedure to determine the optimal subset of zones among the 22, which was led by our main goal of best discriminating the stable MCI from the progressive MCI. For each amnestic MCI subject, we computed an NTI for every possible subset of zones regardless of their number and spatial normalization. For each possible NTI, a Receiver Operating Curve (ROC) was computed and the Area under the ROC was stored. We finally selected the subset whose NTI yielded the best Area under the ROC between stable MCI and progressive MCI. As a result of the procedure, the optimal combination of zones included the right posterior cingulate, the right medial temporal zone and the left lateral temporal zone. Additionally, a cross-validation process was used to test whether this optimal subset of zones depended on the amnestic MCI sample (for more details, see Supplementary material).
In what follows, the term ‘NTI’ will refer to the NTI computed with this optimal subset of zones.
Statistical analysis
Global analysis on cortical thickness
For each of the 22 zones, mean cortical thicknesses were compared between diagnosis groups by a multiple analysis of covariance with three intergroup factors (Gender, Diagnosis and ApoE) and two covariates (Age and Education), to correct for their significant effects across diagnosis groups (Table 1). The diagnosis factor had four levels: ‘Healthy Controls’, ‘progressive MCI’, ‘stable MCI’ and ‘Alzheimer's disease’, and the ApoE factor had two levels (no E4 allele, and at least one E4 allele).
The NTI was globally validated by testing its power to discriminate between healthy controls and Alzheimer's disease patients. A first step computed the optimal threshold to separate the 100 healthy controls from the 100 Alzheimer's disease patients of the learning set, using 10-fold cross validation. This optimal threshold was then applied to the test set (30 healthy controls and 30 Alzheimer's disease patients).
Cognitive reserve analysis
The NTI was compared between the two education groups by a multiple analysis of variance with two inter-group factors (Diagnosis and Education Level Group). The same analysis scheme was used to compare the MMSE scores between the two groups.
Predictive value analysis
We tested the power of the NTI and of neuropsychological scores to discriminate progressive MCI from stable MCI using ROC. Area under the ROC values (used to appraise the discriminant power of each parameter) were compared between each other using a non-parametric method for correlated samples (DeLong et al., 1988). The NTI and the neuropsychological scores were tested independently by submitting each of them to a one-level decision tree. Discriminant power was measured through the misclassification cost, computed by a 10-fold cross validation as provided by Matlab® Statistics Toolbox.
Our index was combined with the neuropsychological scores using a linear classification tree. The discriminant power was measured as explained above, and the pruning level of the tree was chosen so as to obtain the lowest cross validated misclassification cost.
Finally, we chose the optimal threshold of the NTI given by the one-level decision tree analysis described above as the best criterion for detecting Alzheimer's disease at its prodromal stage. We hypothesized that there might be a time difference between the observation of an NTI below the threshold at baseline and the effective conversion to Alzheimer's disease. To test this hypothesis, we applied the optimal threshold to the whole set of amnestic MCI subjects, thus separating them into two groups: amnestic MCI subjects having an NTI below the threshold (referred to as anatomically demented) and amnestic MCI subjects having an NTI above the threshold (referred to as anatomically healthy). At each clinical re-evaluation, we reported the clinical outcome (stable MCI versus progressive MCI) of anatomically healthy and anatomically demented subjects by means of a 2 × 2 contingency table (NTI threshold versus clinical outcome).
MANCOVA was performed using Statistica (version 6.1); decision trees and ROCs were computed using Matlab (version 7.5).
Results
Global analysis
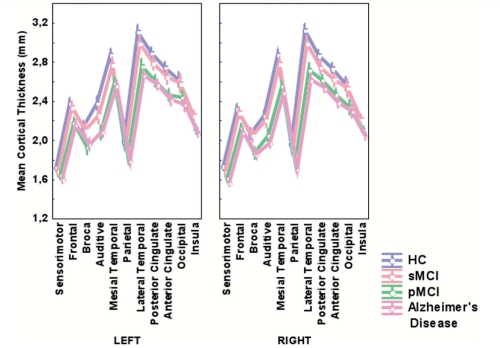
Cortical thickness differed significantly between the diagnosis groups (P < 0.001) (Table 2, Fig. 2).
Table 2.
Magnitude of effects of various factors on mean cortical thickness values
| Effect | P-value | Level | Value |
|---|---|---|---|
| Diagnosis | <0.001 | Healthy controls | 2.46 ± 0.03 |
| Stable MCI | 2.33 ± 0.03 | ||
| Progressive MCI | 2.26 ± 0.04 | ||
| Alzheimer's disease | 2.17 ± 0.04 | ||
| Gender | 0.68 | Male | 2.3 ± 0.03 |
| Female | 2.31 ± 0.03 | ||
| Apoe | 0.04 | Carrier | 2.31 ± 0.03 |
| Non-carrier | 2.37 ± 0.03 | ||
| Age | <0.001 | NA | −0.40 ± 0.04 |
| Education | 0.001 | NA | −0.12 ± 0.04 |
For main effects (Diagnosis, Sex, Apoe), plus–minus values are means of cortical thickness (in mm) ± SD. For covariates (Age, Education), plus–minus values are the estimators of the β coefficient ± SD, i.e. regression slopes calculated on centred scaled values.
Figure 2.
Baseline mean cortical thickness values according to the diagnosis groups. Normalized values obtained after controlling for age, education, sex and ApoE. Vertical bars represent the 95% confidence interval for the mean estimators. For each of the 22 areas, mean cortical thickness was highest in the healthy controls group and lowest in the Alzheimer's disease group.
Moreover, a significant-differences Newman–Keuls post hoc analysis showed that all the diagnosis groups differed from each other in terms of cortical thickness. Cortical thickness significantly decreased with age (P < 0.001), while people with a high level of education showed a significantly thinner cortex (P = 0.001). Subjects carrying at least one ApoE4 allele had a significantly thinner cortex than those carrying none (P = 0.04).
Results of the 10-fold cross validation for the healthy controls/Alzheimer's disease patients classification showed that the NTI correctly classified 85% of the subjects. The optimal threshold was found at NTI = 0. This threshold was used on the test sample of 30 healthy controls and 30 Alzheimer's disease patients, a sample which was completely independent of that used to compute NTI threshold; this procedure correctly classified these subjects again at the rate of 85%.
Cognitive reserve analysis
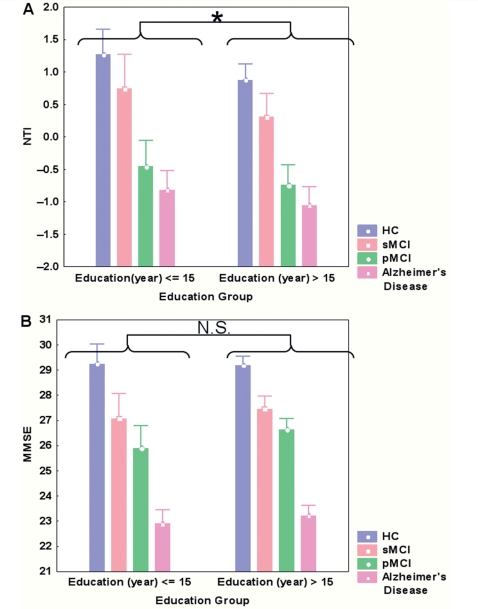
Education level differed significantly (P < 104) between the Higher Education group (mean 17.4 years ± 0.2) and the Lower Education group (mean 12.6 years ± 0.3). There was no significant difference (P = 0.15) in terms of MMSE (Fig. 3B) between Higher Education and Lower Education subjects, but a significant main effect of Diagnosis (P < 10−4). A Newman–Keuls post hoc test led to four homogeneous groups: {MMSEHC} > {MMSEsMCI} >{MMSEpMCI} > {MMSEAlzheimer's disease}. No interaction was found between the Diagnosis and Education Group (P = 0.70), suggesting that, for all the four diagnosis groups, Higher Education and Lower Education subjects had the same cognitive level.
Figure 3.
Comparison of NTI (A) and MMSE (B) between Education Groups, according to their Diagnosis Groups HC, Healthy Control; sMCI, stable MCI; pMCI, progressive MCI; NS, Not Significant. (A) A significant main effect of Education on the NTI (*P = 0.002). The NTI also differs significantly across diagnosis groups (P < 10−4), with no interaction between Education and Diagnosis (P = 0.85). (B) No main effect of education on the MMSE (NS, P = 0.15). The MMSE differs significantly across diagnosis groups (P < 10−4), with no interaction between Education and Diagnosis (P = 0.70). For more details, see post hoc tests in the results section.
As expected, there was a significant effect of diagnosis (P < 10−4) on the NTI. A Newman–Keuls post hoc test led to three homogeneous groups: {NTIHC} > {NTIsMCI} >{NTIpMCI∼NTIAlzheimer's disease} (Fig. 3A), which indicates that progressive MCI subjects and Alzheimer's disease patients cannot be distinguished in terms of NTI. There also was an Education Level Group effect (P = 0.002), with subjects of higher education level presenting a lower NTI. No interaction between diagnosis and education Level was found (P = 0.848).
Discrimination stable MCI/progressive MCI
NTI power to discriminate stable MCI from progressive MCI was compared with the discriminant power of cognitive scores, age and education. Age (Area under the ROC = 0.52 ± 0.04) and education (Area under the ROC = 0.53 ± 0.04) classified subjects nearly at random and had a significantly lower Area under the ROC than the NTI and the cognitive scores. NTI had a significantly higher Area under the ROC (0.76 ± 0.04) with respect to Auditory Verbal Learning Test (0.67 ± 0.04), ADAS-cog 10-word list delayed recall (0.67 ± 0.04), and MMSE (0.64 ± 0.04), but there was no significant difference with the Trail Making Test B (0.72 ± 0.04).
Misclassification costs computed for the one-level decision trees were of the same order of magnitude as the Area under the ROC (see Supplementary data, Table 2). As for the healthy controls/Alzheimer's disease patients discrimination, the NTI optimal threshold for the discrimination between stable MCI and progressive MCI was again found at NTI = 0.
Combining the cognitive scores, age and education gave a correct classification for 61% of the subjects. None of the possible combinations of NTI and cognitive scores improved the cross-validated predictive value of the one-level decision tree with the NTI alone.
NTI criterion: prediction of the clinical outcome
The NTI criterion detected 77 anatomically demented out of the 122 amnestic MCI. Among these 77 anatomically demented, 16 were progressive MCI at 6 months (21%), 36 were progressive MCI at 12 months (47%), 51 were progressive MCI at 18 months (66%) and 58 were progressive MCI at 24 months (75%) (Table 3).
Table 3.
Time shift between detection of atrophy and clinical conversion
| pMCI | sMCI | |
|---|---|---|
| At 6 months | ||
| aD | 16 | 61 |
| aH | 3 | 42 |
| Total | 19 | 103 |
| At 12 months | ||
| aD | 36 | 41 |
| aH | 11 | 34 |
| Total | 47 | 75 |
| At 18 months | ||
| aD | 51 | 26 |
| aH | 12 | 33 |
| Total | 63 | 59 |
| At 24 months | ||
| aD | 58 | 19 |
| aH | 14 | 31 |
| Total | 72 | 50 |
At baseline, 77 subjects were classified as aD and 45 as aH. Therefore, the sums of the figures across the lines remain constant at 6, 12, 18 and 24 months. In contrast, the total of sMCI decreased over time as the total of pMCI increased, reflecting disease's progression throughout the study period. The four panels show the cumulative occurrences of conversion for the aD and aH subjects at 6, 12, 18 and 24 months.
aH = anatomically Healthy (NTI ≥ 0), aD = anatomically Demented (NTI < 0).
The NTI criterion detected 45 anatomically healthy. Among these 45 anatomically healthy, 42 were stable MCI at 6 months (93%), 34 were stable MCI at 12 months (76%), 33 were stable MCI at 18 months (73%) and 31 were stable MCI at 24 months (69%). Thus, the sensitivity of the NTI rose from 21% at 6 months to 75% at 24 months, and its specificity evolved from 93% to 69%. The resulting accuracy of this criterion rises, therefore, from 48% at 6 months to 73% at 24 months.
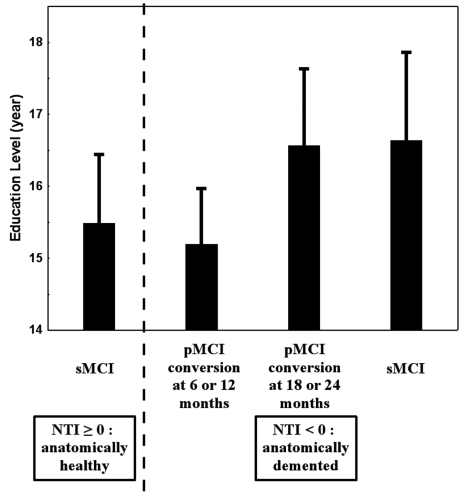
From a practical point of view, NTI detected 80.6% (58/72) of the patients who were to be clinically diagnosed as patients with Alzheimer's disease 24 months later. The cognitive reserve may have an impact on the timeline of conversion to Alzheimer's disease. Therefore, even after anatomical signs of brain damage are observed, the time to clinical conversion may be modulated according to the education level of the patient. To test this hypothesis, we compared the education level among four groups. The first group, for sake of comparison, included stable MCI subjects who had a positive NTI at baseline. The second group included progressive MCI subjects who converted to Alzheimer's disease at 6 or 12 months after baseline, the third group included the progressive MCI subjects who converted to Alzheimer's disease at 18 or 24 months, and the fourth group included the stable MCI with an NTI negative score (an anatomical pattern similar to that of Alzheimer's disease patients) (Fig. 4). There was a significant difference of education level among these four groups (P = 0.04), with the fourth group having the highest education level. Planned comparison between the second group (converters at 6 or 12 months) and the third group (converters at 18 or 24 months) gave a nearly significant difference of education level (P = 0.06).
Figure 4.
Effect of the education level on the timeline of conversion to Alzheimer's disease. Progressive MCI subjects who converted at 6 or 12 months had a lower education level than progressive MCI subjects who converted at 18 or 24 months. In addition, stable MCI subjects who were detected at baseline as having an NTI negative score (thus showing a pathological anatomical pattern) had an education level slightly higher than that of progressive MCI subjects who converted at more than a year after baseline, suggesting that they may soon convert to Alzheimer's disease. For comparison, stable MCI subjects with a positive NTI were included in the model, and showed a ‘moderate’ education level when compared to stable MCI subjects with an NTI negative score.
The effect of educational level on the prediction of conversion according to the NTI was achieved by comparing the education level (in years) between amnestic MCI subjects that were properly classified by the NTI and amnestic MCI that were misclassified by the NTI [i.e. anatomically healthy subjects that had converted to Alzheimer's disease during the study period (false negative) or anatomically demented subjects that had still not converted at 24 months after baseline (false positive)]. No significant difference was found (P = 0.97), showing that the global reliability of the method was not affected by education level. Additionally, we investigated whether there was an effect of education on misclassification with respect to the diagnosis group (stable MCI or progressive MCI). Amnestic MCI subjects were classified into four groups according to their education level (‘lower than or equal to 15 years’ or ‘higher than 15 years’) and their diagnosis groups (‘sMCI’ or ‘pMCI’). A contingency table was built (Table 4) and a Fisher exact test was done on this table, which revealed significance (P = 0.04), indicating that a higher proportion of misclassified subjects were stable MCI subjects with a high level of education.
Table 4.
Contingency table of amnestic MCI subjects misclassified by the NTI
| sMCI | pMCI | |
|---|---|---|
| Educational level 15 or less | 4 | 8 |
| Educational level more than 15 | 15 | 6 |
Discussion
To evaluate the capacity of MRI to improve Alzheimer's disease early diagnosis at the individual level, we designed a fast, automated method for assessing whether the MRI measurement of cortical thickness could predict progression to Alzheimer's disease from its prodromal stage at individual level. Applying our cortical thickness based Normalized Index (NTI) to a large sample of patients from the ADNI database, we found evidence that structural MRI can be used to detect subtle structural changes that help predict the subject's outcome up to 24 months before clinical diagnosis. We also investigated the cognitive reserve hypothesis on our population and showed that subjects with a higher education level had a significantly thinner cortex than less educated subjects with the same level of cognitive performance. For these subjects, structural imaging may thus appear more sensitive to the incipient disease than cognitive testing.
The preliminary validation process we used showed that our methodology was reliable. The cortical values measured in this study are consistent with reported measurements of cortical thickness, in particular regarding the significant effects of age and ApoE genotype, or the non-significant effect of gender, as well as the differences in cortical thickness regional patterns observed between healthy controls, amnestic MCI and Alzheimer's disease patients (Salat et al. 2004; Lerch and Pruessner, 2005; Singh et al., 2006; Shaw et al., 2007; Teipel et al., 2007). Interestingly, our results in terms of regional atrophy between healthy controls, amnestic MCI and Alzheimer's disease patients are very similar to those of other studies based on the ADNI data (Fan et al., 2008; Hua et al., 2008). The NTI may also be considered as an accurate and robust index of Alzheimer's disease, since it yielded a high cross-validated accuracy (85%) for distinguishing Alzheimer's disease patients from healthy controls on a set of subjects that was distinct from the one used for the NTI computation.
For the construction of the NTI, we retained the right posterior cingulate, the right medial temporal zone and the left lateral temporal zone as the optimal combination of zones. However, this choice of zones is not critical regarding the predictive accuracy, since numerous combinations can lead to the same accuracy as illustrated in the supplementary Table. This can be explained by the fact that widely spread cortical atrophy was observed in the progressive MCI population, which may indicate that these subjects were already at an advanced stage of the disease from an anatomical point of view, despite the fact that they had still not been clinically diagnosed as Alzheimer's disease patients. Had these patients been at an earlier stage of the disease, a more focal atrophy might have been observed, resulting in a smaller subset of discriminative zones and therefore a smaller number of optimal combinations. Further studies on other amnestic MCI populations (or possibly on a preclinical population) are still required to analyse the respective predictive accuracies of these combinations at earlier stages of the disease.
We found that the NTI was able to accurately predict evolution from amnestic MCI to Alzheimer's disease up to 24 months before clinical criteria of Alzheimer's disease are met, with a cross-validated predictive value of 76%. Results from the literature give varied assessments of the ability of structural imaging and of cognitive scores (alone or combined) to predict conversion to Alzheimer's disease. Korf et al. (2004) showed that atrophy in the medial temporal lobe could predict conversion to Alzheimer's disease with a global accuracy of 69%. Sarazin et al. (2007) showed that cognitive scores could predict future conversion to Alzheimer's disease with high accuracy (94%), but the age difference alone between their two groups classified 72% of the individuals. Devanand et al. (2007) found that a combination of cognitive scores and hippocampal and entorhinal cortex volumes could predict conversion to Alzheimer's disease with an accuracy of 87.7%; however, age alone correctly classified 71.9% of the subjects, and their follow-up period (5 years in average) was much longer than ours. Visser et al. (2002) showed that a combination of cognitive scores, hippocampal and parahippocampal volumes and visually assessed atrophy of the medial temporal lobe could predict conversion to Alzheimer's disease for 81% of the subjects, but age on its own classified 78% of the subjects. In our study, age was not a relevant predictor, since we showed that it classified subjects nearly randomly (Area under the ROC = 0.52). More importantly, our present study has the crucial advantage of using a cross-validation procedure that guarantees higher validity of the estimation of predictive values than those reported by other studies which tested predictive values within a single sample of subjects. Cross validation, which usually gives lower predictive values than those obtained on the learning sets, is the recommended way of evaluating the predictive accuracy of a given marker at the individual level. Our results may thus better reflect the true predictive value of structural imaging on a 24-month period. With regards to this predictive capacity, it is important to acknowledge, first, that the conversion rate over 2 years in our amnestic MCI sample was very high, reaching 59% (72/122). Such high annual conversion rates of about 27–30% in amnestic MCI subjects recruited within the framework of clinical settings such as the ADNI study have been reported recently, reaching 28% in the study by Schmidtke and Hermeneit (2008) and 34% in the study by Rozzini et al. (2007). Such high rates are likely to result from the way subjects are recruited and the diagnosis criteria used for the enrolment of amnestic MCI subjects. It is interesting to compare these rates to those derived from population cohorts or community-based studies: the annual conversion rate observed in the population cohort studied by Lopez et al. (2007) was 18% for probable MCI, and the annual rate reported in the community-based study by Fischer et al. (2007) was 19%. Thus, the conversion rate in the ‘population’ of MCI subjects could be considered to be about 20% per year, compared with 30% in MCI samples ‘enriched’ with patients with early Alzheimer's disease as recruited for clinical studies.
The influence of such a difference on the accuracy of the NTI used to predict conversion to Alzheimer's disease in the general population of amnestic MCI can be evaluated by comparing the positive and negative predictive values (PPV and NPV) of the NTI using the Alzheimer's disease prevalence observed at 24 months in our ADNI amnestic MCI group (59%) and in the general amnestic MCI population (∼40%) (Fischer et al., 2007; Lopez et al., 2007). The computed positive predictive value on the ADNI sample is 83% compared with 69% in the general amnestic MCI population, whereas the negative predictive value increases from 71% in the ADNI population to 85% in the amnestic MCI population. The values obtained in the general amnestic MCI population supports the notion that the NTI still conveys useful information in clinical practice for the prediction of conversion from the amnestic MCI stage to Alzheimer's disease.
As suggested by Stern (2006), clinical evaluation alone may prove insufficient for the early diagnosis of Alzheimer's disease. Indeed, it suffers from the confounding effect of the cognitive reserve and cannot directly reveal the underlying pathology. Stern stressed the fact that it would be wise to focus on the development of surrogate markers such as neuroimaging, which may be less affected by the cognitive reserve. Our results strongly support this suggestion: indeed, we showed that cognitive testing, even when combined with education level, could not predict the evolution to Alzheimer's disease as accurately as the NTI. Besides, using education as a proxy for cognitive reserve and the NTI as a proxy for Alzheimer's disease pathological burden, we showed that highly educated subjects had a significantly decreased NTI whereas their MMSE was not affected. This result indicates that the NTI, being less affected by the cognitive reserve than an index of global functioning such as the MMSE, may prove more sensitive for the early diagnosis of Alzheimer's disease, in particular in highly educated subjects. This notion is confirmed by the post hoc analyses, which revealed no differences between progressive MCI subjects and Alzheimer's disease patients in terms of NTI, but a significant difference in terms of MMSE. We also obtained further consistent results by testing the effect of education level on the timeline of conversion to Alzheimer's disease: progressive MCI subjects who converted to Alzheimer's disease earlier than 12 months after baseline had a significantly lower level of education than those who converted later than 12 months after baseline. This result supports the cognitive reserve hypothesis within the progressive MCI group, since it indicates that individuals with a higher degree of education have less apparent symptoms for a longer time. Moreover, our results show that stable MCI subjects with an NTI negative score had a high education level, even slightly higher than that of the progressive MCI subjects who converted to Alzheimer's disease later than 12 months after baseline. Such a high education level may have prevented them from showing clinical signs of dementia 24 months after baseline, while having an advanced Alzheimer's disease pathology. Study of the clinical status 36 months after baseline will indicate if, as predicted by the NTI, most of these subjects eventually converted to Alzheimer's disease.
We therefore believe that NTI could be very useful to predict conversion to Alzheimer's disease, especially for subjects with a high level of education. The cognitive reserve is particularly confounding for these subjects because it prevents cognitive testing from being able to efficiently detect their underlying disease at an early stage. On the other hand, cognitive reserve seems less confounding with regard to structural changes in these subjects. Therefore, the NTI is able to detect the disease up to 24 months before signs of overt dementia are readily observed.
The cognitive reserve hypothesis has already been investigated for the Alzheimer's disease population in recent studies (Sole-Padulles et al., 2007; Garibotto et al., 2008; Hanyu et al., 2008; Kemppainen et al., 2008), which found that, for a given level of cognitive burden, Alzheimer's disease patients with a higher education level had a lower regional cerebral blood flow (Hanyu et al., 2008), a higher Pittsburgh Compound B uptake (Kemppainen et al., 2008), a lower glucose metabolic rate (Garibotto et al., 2008; Kemppainen et al., 2008) and a lower brain volume (Sole-Padulles et al., 2007) than Alzheimer's disease patients with lower levels of education. Solé Padullés et al. (2007) extended these results to a small amnestic MCI group, while Garibotto et al. (2008) showed that MCI converters with a higher education level had a relatively lower glucose metabolism. To the best of our knowledge, our study is the first to investigate the relation between cognitive reserve, structural changes and the timeline of evolution to Alzheimer's disease.
The relationships between cortical thickness and education level might be more complex, however, than the cognitive reserve hypothesis suggests. We observed the same effects in healthy controls as in the amnestic MCI group, subjects with a higher degree of education having a lower NTI. Similar results were found by Coffey et al. (1999), who concluded that highly educated healthy subjects managed to remain healthy while having more age-related atrophy. Im et al. (2006) studied the links between cortical thickness, level of education and fractal dimension of the cortical surface for young healthy subjects, and found that high education level was associated with a high fractal dimension of the cortical surface, which itself was associated with small cortical thickness. Their results may lead to the conclusion that a high education level is associated with low cortical thickness, even for young healthy subjects. On the other hand, Solé-Padullés et al. (2007) found a positive correlation between education level and brain volume on a small population of healthy elderly subjects. It remains unclear whether the effect of education that we observed is related to the pathology at a preclinical phase or whether cortical thickness is directly affected by education level. Future work on younger populations may be required to address this issue.
One limitation of our study comes from the high education level of our population, and the effect of education on people with a low education level (education level ≤10 years) could not really be accounted for. This issue may need further study, including younger healthy subjects. However, the main results of our study were only slightly affected by this limitation since we emphasize that studying anatomical changes may be beneficial, particularly for highly educated subjects.
Another limitation comes from the novelty of our methodological approach. A comparative validation may be required, for example, by comparing our results in terms of predictive value with those of other studies on the ADNI population. However, we have given evidence that our method is reliable, since it had a high predictive value on the Alzheimer's disease population, and an encouraging predictive value on the amnestic MCI population. Also, our results on the cognitive reserve are in accordance with those of the current literature.
Using a new diagnostic criterion based on the NTI, we have highlighted the fact that Alzheimer's disease may be characterized by structural changes that can be detected up to 24 months before the current clinical criteria for Alzheimer's disease are fulfilled. This result supports a recent proposal (Dubois et al., 2007) that emphasizes the need to revise the Neurological Disorders and Stroke-Alzheimer Disease and Related Disorders criteria in order to improve the accuracy of Alzheimer's disease diagnosis and to make the diagnosis at the earliest stages of the disease. The authors propose new criteria which include one or more biomarkers, among which is MRI structural neuroimaging. They however point out that there is still no MRI-based methodology that has been completely validated as suitable for integration in clinical routine. Our study shows that, among subjects meeting MCI criteria, the NTI can anticipate the diagnosis of Alzheimer's disease 2 years before the clinical criteria are fulfilled. The NTI has shown promising results at the individual level on a large population and is an operator independent, fully automated and quick method based on a single 1.5 T MRI scan. It can therefore be easily integrated into clinical routine to improve the early diagnosis of Alzheimer's disease.
Supplementary material
Supplementary material is available at Brain online.
Funding
Alzheimer's disease Neuroimaging Initiative (ADNI; Principal Investigator: Michael Weiner; National Institutes of Health grant U01 AG024904); ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering; and through contributions from the following: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer's Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the U.S. Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles.
Supplementary Material
Acknowledgements
The authors thank Susan Becker for her careful checking of English language, and the reviewers for their very constructive suggestions. The Image Registration Toolkit was used under Licence from Ixico Ltd.
Glossary
Abbreviations:
- aD
anatomically demented
- ADAS-Cog
Alzheimer's disease Assessment Scale score
- ADNI
Alzheimer's disease Neuroimaging Initiative
- aH
anatomically healthy
- aMCI
amnestic Mild Cognitive Impairment
- HC
Healthy Control
- MMSE
Mini Mental State Examination
- MRI
Magnetic Resonance Imaging
- NTI
Normalized Thickness Index
- pMCI
progressive amnestic Mild Cognitive Impairment
- ROC
Receiver Operating Curve
- sMCI
stable amnestic Mild Cognitive Impairment
References
- Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: A potential outcome measure in Alzheimer's disease treatment studies. Am J Psychiatry. 2002;159:738–45. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Multimodal image coregistration and partitioning–a unified framework. Neuroimage. 1997;6:209–17. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bloch I. Fuzzy spatial relationships for image processing and interpretation: a review. Image Vis Comput. 2004;23:89–110. [Google Scholar]
- Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53:189–96. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain. 2006;129(Pt 11):2856–66. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- Estevez-Gonzalez A, Kulisevsky J, Boltes A, Otermin P, Garcia-Sanchez C. Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer's disease: comparison with mild cognitive impairment and normal aging. Int J Geriatr. Psychiatry. 2003;18:1021–8. doi: 10.1002/gps.1010. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL. A 305-member MRI-based stereotactic atlas for CBF activation studies. In Proceedings of the 40th the 40th Annual Meeting of the Society for Nuclear Medicine.1993. [Google Scholar]
- Fan Y, Batmanghelich N, Clark CM, Davatzikos C. Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. Neuroimage. 2008;39:1731–43. doi: 10.1016/j.neuroimage.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–91. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garibotto V, Borroni B, Kalbe E, Herholz K, Salmon E, Holtoff V, et al. Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology. 2008;71:1342–9. doi: 10.1212/01.wnl.0000327670.62378.c0. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Shimizu S, Kanetaka H, Iwamoto T, Koizumi K. The effect of education on rCBF changes in Alzheimer's disease: a longitudinal SPECT study. Eur J Nucl Med Mol Imaging. 2008;35:2182–90. doi: 10.1007/s00259-008-0848-4. [DOI] [PubMed] [Google Scholar]
- Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, et al. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer's disease: an MRI study of 676 AD, MCI, and normal subjects. Neuroimage. 2008;43:458–69. doi: 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Yoon U, Shin YW, Hong SB, Kim IY, et al. Fractal dimension in human cortical surface: Multiple regression analysis with cortical thickness, sulcal depth, and folding area. Hum Brain Mapp. 2006;27:994–1003. doi: 10.1002/hbm.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Slomkowski M, Gracon S, Hoover TM, Felmlee JP, Stewart K, et al. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology. 2003;60:253–60. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Buchbinder BR, Aharon I. Three-dimensional mapping of cortical thickness using Laplace's equation. Hum Brain Mapp. 2000;11:12–32. doi: 10.1002/1097-0193(200009)11:1<12::AID-HBM20>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Ann Neurol. 2008;63:112–8. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–73. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC. Focal decline of cortical thickness in Alzheimer's disease identified by computational neuroanatomy. Cereb Cortex. 2005;15:995–1001. doi: 10.1093/cercor/bhh200. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH, Becker JT, Dulberg C, Sweet RA, Gach HM, et al. Incidence of dementia in mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol. 2007;64:416–20. doi: 10.1001/archneur.64.3.416. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Borenstein AR, Gosche KM, Snowdon DA. Very early detection of Alzheimer neuropathology and the role of brain reserve in modifying its clinical expression. J Geriatr Psychiatry Neurol. 2005;18:218–23. doi: 10.1177/0891988705281869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Weiner MW. Evaluation of treatment effects in Alzheimer's and other neurodegenerative diseases by MRI and MRS. NMR Biomed. 2006;19:655–68. doi: 10.1002/nbm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, et al. Ways toward an early diagnosis in Alzheimer's disease: The Alzheimer's Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Jagust WJ, DeCarli C, Mack WJ, Kramer JH, et al. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59:867–73. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment clinical trials. Nat Rev Drug Discov. 2003;2:646–53. doi: 10.1038/nrd1155. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Rey A. Presses universitaires de France; 1964. L’examen clinique en psychologie. [Google Scholar]
- Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68:223–8. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Rountree SD, Waring SC, Chan WC, Lupo PJ, Darby EJ, Doody RS. Importance of subtle amnestic and nonamnestic deficits in mild cognitive impairment: prognosis and conversion to dementia. Dement Geriatr Cogn Disord. 2007;24:476–82. doi: 10.1159/000110800. [DOI] [PubMed] [Google Scholar]
- Rozzini L, Chilovi BV, Conti M, Bertoletti E, Delrio I, Trabucchi M, et al. Conversion of amnestic Mild Cognitive Impairment to dementia of Alzheimer type is independent to memory deterioration. Int J Geriatr Psychiatry. 2007;22:1217–22. doi: 10.1002/gps.1816. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69:1859–67. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- Schmidtke K, Hermeneit S. High rate of conversion to Alzheimer's disease in a cohort of amnestic MCI patients. Int Psychogeriatr. 2008;20:96–108. doi: 10.1017/S1041610207005509. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Singh V, Chertkow H, Lerch JP, Evans AC, Dorr AE, Kabani NJ. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer's disease. Brain. 2006;129(Pt 11):2885–93. doi: 10.1093/brain/awl256. [DOI] [PubMed] [Google Scholar]
- Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–63. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- Sole-Padulles C, Bartres-Faz D, Junque C, Vendrell P, Rami L, Clemente IC, et al. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.10.008. (Epub ahead of print, November 27, 2007) [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Studholme C, Hill DLG, Hawkes DJ. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recog. 1999;32:71–86. [Google Scholar]
- Teipel SJ, Born C, Ewers M, Bokde AL, Reiser MF, Moller HJ, et al. Multivariate deformation-based analysis of brain atrophy to predict Alzheimer's disease in mild cognitive impairment. Neuroimage. 2007;38:13–24. doi: 10.1016/j.neuroimage.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Venneri A. Imaging treatment effects in Alzheimer's disease. Magn Reson Imaging. 2007;25:953–68. doi: 10.1016/j.mri.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Verhey FR, Hofman PA, Scheltens P, Jolles J. Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry. 2002;72:491–7. doi: 10.1136/jnnp.72.4.491. PMID: 11909909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.