Abstract
Cognitively intact older individuals at risk for developing Alzheimer's disease frequently show increased functional magnetic resonance imaging (fMRI) brain activation presumably associated with compensatory recruitment, whereas mild cognitive impairment (MCI) patients tend not to show increased activation presumably due to reduced neural reserve. Previous studies, however, have typically used episodic memory activation tasks, placing MCI participants at a performance disadvantage relative to healthy elders. In this event-related fMRI study, we employed a low effort, high accuracy semantic memory task to determine if increased activation of memory circuits is preserved in amnestic MCI when task performance is controlled. Fifty-seven participants, aged 65–85 years, comprised three groups (n = 19 each): amnestic MCI patients; cognitively intact older participants at risk for developing Alzheimer's disease based on having at least one ApoE ε4 allele and a positive family history of Alzheimer's disease (At Risk); and cognitively intact participants without Alzheimer's disease risk factors (Control). fMRI was conducted on a 3T MR scanner while participants performed a famous name discrimination task. Participants also underwent neuropsychological testing outside the scanner; whole brain and hippocampal atrophy were assessed from anatomical MRI scans. The three groups did not differ on demographic variables or on fame discrimination performance (>87% correct for all groups). As expected, the amnestic MCI participants demonstrated reduced episodic memory performance. Spatial extent of activation (Fame—Unfamiliar subtraction) differentiated the three groups (Control = 0 ml, At Risk = 9.7 ml, MCI = 34.7 ml). The MCI and At Risk groups showed significantly greater per cent signal change than Control participants in 8 of 14 functionally defined regions, including the medial temporal lobe, temporoparietal junction, and posterior cingulate/precuneus. MCI participants also showed greater activation than Controls in two frontal regions. At Risk, but not MCI, participants showed increased activity in the left hippocampal complex; MCI participants, however, evidenced increased activity in this region when hippocampal atrophy was controlled. When performance is equated, MCI patients demonstrate functional compensation in brain regions subserving semantic memory systems that generally equals or exceeds that observed in cognitively intact individuals at risk for Alzheimer's disease. This hyperactivation profile in MCI is even observed in the left hippocampal complex, but only when the extent of hippocampal atrophy is taken into consideration.
Keywords: Area under the curve (AUC), fMRI, semantic memory, mild cognitive impairment, APOE ε4
Introduction
Objective biomarkers of Alzheimer's disease have the potential to make significant contributions to the timely detection and therapeutic management of the disease (Mayeux, 2004; Sunderland et al., 2006). Widely available, easily implemented, and cost-effective biomarkers may have their greatest utility in early, preclinical detection of Alzheimer's disease (Chong and Sahadevan, 2005; Chong et al., 2006). They may also play a substantial role in monitoring therapeutic response to interventions designed to slow disease progression (Chertkow and Black, 2007). Recently, functional magnetic resonance imaging (fMRI), based on blood oxygen dependent level (BOLD) contrast, has emerged as a candidate biomarker for detecting early changes in the central nervous system associated with Alzheimer's disease. Unlike anatomical imaging techniques, such as CT or MRI, or functional metabolic approaches, such as SPECT or PET (glucose and amyloid), fMRI examines the brain's response to cognitive challenges. This ‘cognitive stress test’ approach to imaging holds the promise of providing critically important data during the preclinical stage of Alzheimer's disease, especially if the selected task activates regions known to be affected by the disease, such as the hippocampus, posterior cingulate, and lateral temporoparietal regions (Petrella et al., 2003, 2007).
Relatively few studies have investigated the relationship between risk factors that may suggest susceptibility to the disease and potential biomarkers that would imply the presence of the disease (Schoonenboom et al., 2005). If fMRI is to be considered as a valid biomarker for Alzheimer's disease, it would be expected to show predictable patterns in the presence of known risk factors for the disease. In addition to age, two well-established risk factors for Alzheimer's disease are the presence of the ɛ4 allele of Apolipoprotein E (ApoE ɛ4) and a family history of Alzheimer's disease. A diagnosis of mild cognitive impairment (MCI), often considered a transitional stage between healthy ageing and Alzheimer's disease, confers even greater risk for developing the disease. A stable and sensitive fMRI biomarker of progression through the trajectory of early pathological changes would be expected to be influenced by the nature and number of risk factors. Such an indicator would be expected to be manifest in high-risk individuals, but it could also be instrumental in evaluating novel treatments designed to slow Alzheimer's disease progression.
Healthy adults with a family history of Alzheimer's disease or an ApoE ɛ4 allele demonstrate altered patterns of fMRI brain activation in the absence of any performance deficits on neuropsychological tests (Smith et al., 1999; Bookheimer et al., 2000; Burggren et al., 2002; Bondi et al., 2005; Celone et al., 2006; Johnson et al., 2006b; Lind et al., 2006; Trivedi et al., 2006; Wishart et al., 2006). Differences in BOLD fMRI activation have also been seen in symptomatic MCI patients compared with healthy controls (Dickerson et al., 2005; Celone et al., 2006; Johnson et al., 2006a; Sandstrom et al., 2006; Vandenbulcke et al., 2007), primarily in brain regions typically affected by pathological changes in Alzheimer's disease, including the hippocampus, posterior cingulate, posterior lateral temporal and parietal cortices. However, the pattern of group differences have been inconsistent, with some studies showing increased activation in At Risk groups (Bookheimer et al., 2000; Bondi et al., 2005; Dickerson et al., 2005; Johnson et al., 2006b; Lind et al., 2006; Wishart et al., 2006), whereas others have found hypoactivation (Smith et al., 1999; Burggren et al., 2002; Johnson et al., 2006a; Trivedi et al., 2006; Wishart et al., 2006; Vandenbulcke et al., 2007).
The inconsistent findings involving MCI patients may be due to differences in study design and methodology, since most of these studies used effortful episodic memory tasks in which patient performance during imaging was typically worse than that of a healthy control group. When a patient group demonstrates poorer performance on a cognitive task during functional imaging, it is difficult to know whether differences in brain activation are due to alterations in the functional neuronal response or to motivational factors associated with lowered cognitive performance (Price and Friston, 1999). This issue is compounded by the use of blocked trial fMRI experimental designs that do not allow the elimination of errors from the generation of the haemodynamic response function (HRF) (Le et al., 2001; Hannula and Ranganath, 2008). Furthermore, whereas effortful episodic memory tasks may be valuable for demonstrating activation of memory circuits in presymptomatic At Risk groups, such tasks would be less useful for monitoring disease progression or treatment response in symptomatic patients (MCI, Alzheimer's disease), where performance is already at basal levels (Small et al., 2008). Thus, an fMRI biomarker using an episodic memory activation task would have limited applicability across the spectrum of disease severity. When less effortful memory tasks are used, significant relationships between cognitive performance and regional brain activity have emerged (Diamond et al., 2007). Therefore, the nature and complexity of the cognitive task performed during scanning is a critical consideration in the design of a useful fMRI biomarker.
In this event-related fMRI study, therefore, we used a low-effort, high-accuracy semantic memory task, which requires patients to discriminate famous from unfamiliar names, to compare activation in a group of MCI patients with that of healthy older participants with and without Alzheimer's disease risk factors. In our previous studies of cognitively intact older individuals, we have shown that this fame discrimination task activates a network consisting primarily of the medial temporal lobe (hippocampus), lateral temporoparietal and posterior cingulate regions (Douville et al., 2005; Nielson et al., 2006; Woodard et al., 2007). We predicted that when performance is equated, MCI patients would demonstrate ‘hyperactivation’ in memory circuits similar to that observed in At Risk healthy older adults.
Methods
Participants and procedure
Participants included 57 adults between the ages of 65 and 85 years divided equally into three groups: 19 patients diagnosed with amnestic MCI (‘MCI’ group), 19 cognitively intact individuals at risk for developing dementia by virtue of having at least one ApoE ɛ4 allele and a positive family history (‘At Risk’ group) and 19 cognitively intact individuals without ApoE ɛ4 or family history risk factors (‘Control’ group).
The cognitively intact participants were recruited from a larger sample of 459 community-dwelling adults who were recruited via newspaper advertisements. Following telephone screening, 92 participants met study inclusion and exclusion criteria, and 81 persons agreed to undergo ApoE genotyping from blood samples, a neuropsychological evaluation and an fMRI scanning session. Of these participants, individuals with both a positive family history and at least one ApoE ɛ4 allele (n = 20) or those with neither risk factor (n = 29) were included in this study. Family history was defined as a report of a clear clinical diagnosis of Alzheimer's disease or a reported history of gradual decline in memory and other cognitive functions, confusion, or judgement problems without a formal diagnosis of Alzheimer's disease prior to death in a first-degree relative. One participant reported a diagnosis of Alzheimer's disease in a second degree relative, with some mild cognitive changes noted in a parent prior to the parent's death.
Participants from the At Risk and Control groups were matched to the MCI participants on age, education and gender to form groups of equal size. The At Risk and Control groups (n = 19 each) were formed based on the presence/absence of at least one APOE ε4 allele and a family history of dementia. The At Risk group (FH + ε4) had a family history of dementia and one or both ε4 alleles (18 ε3/ε4; 1 ε4/ε4). The Control group did not have a family history of dementia and did not possess an APOE ε4 allele (1 ε2/ε3; 18 ε3/ε3).
The majority of MCI participants were recruited from the Memory Disorders Clinic at the Medical College of Wisconsin. To be included in the MCI group, participants met Petersen criteria (Petersen et al., 2001): (i) memory complaint preferably corroborated by an informant; (ii) objective memory impairment by neuropsychological testing (see below); (iii) normal general cognitive functioning; (iv) intact activities of daily living; and (v) not demented. MCI participants were also evaluated by a neurologist with expertise in dementia to rule out other possible bases for the memory impairment. All MCI participants obtained a modified Hachinski ischemia score below 4. At Risk and Control participants were required to perform within normal limits on neuropsychological testing (see below).
Of the MCI group (n = 19), 59% were ApoE ɛ4 positive (6 ε3/ε4; 4 ε4/ε4) and 63% were family-history positive; two of the 19 MCI patients were taking cholinesterase inhibitors at the time of evaluation. Informed consent was obtained according to the Declaration of Helsinki and consistent with institutional guidelines established by the Medical College of Wisconsin Human Subjects Review Committee; all participants received financial compensation.
Any prospective participant was excluded if he/she reported any previous or current history of neurological disease, major psychiatric disturbance meeting DSM-IV Axis I criteria, substance abuse meeting DSM-IV Axis I criteria, current use of psychoactive medications, or any disturbance of activities of daily living. Any participant with an anomaly on high resolution anatomical MRI scans also was excluded. Additional exclusion criteria related to fMRI scanning included pregnancy, weight inappropriate for height, ferrous objects within the body, low visual acuity and a history of claustrophobia. A blood chemistry screen (TSH, homocysteine, vitamin B12, folate and creatinine) was not found to be clinically significant in any of the participants.
Neuropsychological testing and the fMRI scanning were conducted on the same day. Participants were asked to refrain from alcohol use 24 h and caffeine use 12 h prior to testing. The neuropsychological test battery consisted of the Mini-Mental State Examination (Folstein et al., 1975), Mattis Dementia Rating Scale-2 (DRS-2); (Mattis, 1988; Jurica et al., 2001), Rey Auditory Verbal Learning Test (RAVLT) (Rey, 1958), Geriatric Depression Scale (GDS) (Yesavage et al., 1983) and Lawton Activities of Daily Living (LADL) (Lawton and Brody, 1969). ApoE genotype was determined using a PCR method (Saunders et al., 1996). DNA was isolated with Gentra Systems Autopure LS for Large Sample Nucleic Acid Purification.
To evaluate memory performance, local norms were collected from 91 healthy older adult subjects to establish cutoff scores for the delayed recall and long-term percentage recall (LTPR) indices from the Rey Verbal Learning Test. Separate cutoff scores were established for men and women as there were significant sex differences in the local group performance on the RAVLT. Using a criterion corresponding to a performance of 1.5 standard deviation below the mean, delayed recall of five words or lower for women and four words or lower for men, and per cent retention scores below 60% were the established cutoff scores used to identify the MCI group. All MCI subjects scored below both these cutoff scores while all healthy controls (see below) scored above these cutoff scores. In addition, age and education corrected Mayo Older American Normative Studies (MOANS) (Lucas et al., 1998) scaled scores of five or more on the DRS-2 subscales (other than memory) were required for the diagnosis of MCI, indicating an absence of dementia. All MCI subjects obtained MMSE scores above 23. A score of <16 for controls and <20 for MCI participants on the GDS was required for inclusion in the study in order to rule out moderate to severe depressive symptoms. Finally, all MCI subjects scored in the normal range on a measure of activities of daily living (LADL). Whenever possible, a collateral reviewed participant responses.
Control and At Risk participants did not show deficits on the Rey Auditory Verbal Learning Test (RAVLT; Rey, 1958) defined as performance below 1.5 SD from their age and gender-adjusted mean performance on the delayed recall and long-term per cent retention measures. Similarly, Control and At Risk participants showed no deficits on the Mattis Dementia Rating Scale-2, defined as performance below 2 SD from their age- and education-adjusted mean.
Groups were balanced for gender, age and education (Table 1). One-way ANOVAs revealed no significant differences in activities of daily living (LADL) or the Construction and Conceptualization portions of the DRS-2 global cognition battery. Inherent in the diagnosis of MCI, expected group differences were seen on the MMSE, RAVLT and Initiation/Perseveration, Attention and Memory portions of the DRS-2. The only unanticipated group difference was on the GDS (higher score in the MCI group; no participants, however, were clinically depressed).
Table 1.
Group demographics, neuropsychological test results and fMRI task performance
| Variables | Control (n = 19) |
At Risk (n = 19) |
MCI (n = 19) |
|||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | P | η2 | |
| Demographics | ||||||||
| Age | 75.1 | 5.9 | 72.1 | 3.9 | 75.4 | 6.9 | NS | 0.070 |
| Education | 14.0 | 2.4 | 15.3 | 2.7 | 14.1 | 2.4 | NS | 0.055 |
| Gender (female/male) | 15/4 | 15/4 | 15/4 | NS | – | |||
| Global cognition (DRS-2) | ||||||||
| Total | 139.8 | 3.6 | 140.5 | 3.6 | 130.4 | 7.2 | <0.001* | 0.467 |
| Attention | 36.6 | 0.6 | 36.4 | 0.7 | 35.5 | 1.5 | 0.002* | 0.206 |
| Initiation/perseveration | 36.8 | 0.7 | 36.5 | 1.0 | 34.1 | 3.0 | <0.001* | 0.308 |
| Construction | 5.9 | 0.2 | 5.7 | 0.2 | 5.7 | 0.5 | NS | 0.091 |
| Conceptualization | 36.5 | 3.7 | 37.6 | 1.6 | 35.8 | 2.4 | NS | 0.069 |
| Memory | 24.0 | 1.1 | 24.1 | 1.4 | 19.2 | 4.3 | <0.001* | 0.438 |
| Total MOANS | 11.9 | 2.2 | 11.8 | 2.7 | 7.3 | 3.7 | <0.001* | 0.362 |
| Mini-Mental State Exam | 29.1 | 2.5 | 28.9 | 1.2 | 27.4 | 2.2 | 0.002* | 0.205 |
| Verbal learning (RAVLT) | ||||||||
| Trials 1–5 | 48.0 | 5.2 | 50.6 | 8.5 | 28.7 | 7.9 | <0.001* | 0.651 |
| Post-interference recall | 9.3 | 2.1 | 9.4 | 3.1 | 3.8 | 2.5 | <0.001* | 0.520 |
| Delayed recall | 9.7 | 1.8 | 9.6 | 3.2 | 3.0 | 2.0 | <0.001* | 0.640 |
| Long-term per cent retentiona | 87.0 | 15.4 | 78.3 | 19.7 | 43.6 | 25.7 | <0.001* | 0.463 |
| Learning over trialsb | 16.7 | 5.6 | 17.5 | 6.7 | 7.6 | 5.4 | <0.001* | 0.372 |
| Depression | ||||||||
| GDS | 4.3 | 4.6 | 1.4 | 1.9 | 7.1 | 5.0 | <0.001** | 0.251 |
| Activities of daily living | ||||||||
| Lawton | 4.8 | 0.4 | 4.8 | 0.4 | 5.0 | 0.2 | NS | 0.042 |
| fMRI task performance | ||||||||
| Per cent correct—famous | 90.9 | 7.1 | 92.5 | 8.4 | 87.4 | 14.6 | NS | 0.041 |
| Per cent correct—unfamiliar | 96.7 | 5.9 | 97.5 | 4.8 | 90.4 | 16.5 | NS | 0.090 |
| Discriminability index (A') | 5.5 | 1.2 | 6.0 | 1.1 | 4.8 | 1.8 | 0.027** | 0.125 |
| Bias Index (B'') | 0.6 | 0.5 | 0.6 | 0.7 | 0.4 | 0.8 | NS | 0.070 |
| Reaction time—famous (ms) | 1293 | 229 | 1224 | 177 | 1334 | 242 | NS | 0.044 |
| Reaction time—unfamiliar (ms) | 1575 | 277 | 1573 | 311 | 1691 | 354 | NS | 0.031 |
a Long-term per cent retention (delayed recall/trial 5) × 100.
b Learning over trials: sum of words recalled on trials 1-5 − (trial 1 × 5).
*MCI < Control; MCI < At Risk.
**MCI < At Risk.
Functional MRI
fMRI task
The selection of famous name stimuli for this study is described in greater detail in Douville et al. (2005). Briefly, the task stimuli consisted of 30 names of famous persons who achieved their fame either between 1990 and 2000 or between 1950 and 1965 and 30 names of unfamiliar individuals selected from an original pool of 784 names because of a high rate of correct identification (<10% error rate) (Douville et al., 2005). A trial consisted of the visual presentation of a single name for 4 s. Participants were instructed to make a right index finger key press if the name was famous and a right middle finger key press if the name was unfamiliar. Both accuracy (% correct) and reaction time (in milliseconds) were recorded; signal detection indices, A' and B'', were calculated to examine discriminability and response bias (Grier, 1971). The 60 name trials were randomly interspersed with 30 4-s trials in which the participant was instructed to fixate a single centrally placed crosshair. This procedure was done to introduce ‘jitter’ into the fMRI time course. The imaging run began and ended with 12 s of fixation. Total time for the single imaging run was 5 min and 24 s.
Image acquisition
Whole brain, event-related fMRI was conducted on a General Electric (Waukesha, WI, USA) Signa Excite 3.0 Tesla short bore scanner equipped with a quad split quadrature transmit/receive head coil. Echoplanar images were collected using an echoplanar pulse sequence [TE = 25 ms; flip angle = 77°; field of view (FOV) = 24 mm; matrix size = 64 × 64]. Thirty-six contiguous axial 4-mm thick slices were selected to provide coverage of the entire brain (voxel size = 3.75 × 3.75 × 4 mm). The interscan interval (TR) was 2 s. High-resolution, three-dimensional spoiled gradient-recalled at steady-state (SPGR) anatomic images were acquired [TE = 3.9 ms; TR = 9.5 ms; inversion recovery (IR) preparation time = 450 ms; flip angle = 12°; number of excitations (NEX) = 2; slice thickness = 1.0 mm; FOV = 24 cm; resolution = 256 × 224]. Foam padding was used to reduce head movement within the coil.
Image analysis
Functional images were generated with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Each image time series was time shifted to the beginning of the interscan interval and then spatially registered to reduce the effects of head motion using a rigid body iterative linear least squares method. A deconvolution analysis was used to extract a HRF for famous and unfamiliar names from the time-series. HRFs were modeled for the 0–16 s period post-stimulus onset. Motion parameters were incorporated into the model as nuisance regressors. The HRFs were also transposed so that the value of the HRF at trial onset was zero. Despite the high task accuracy rate (see below), estimation of the HRFs for identification of famous names and rejection of unfamiliar names was restricted to correct trials. Area under the curve (AUC) was calculated by summing the hemodynamic responses at time points 4, 6 and 8 s post-trial onset. We selected these time points because they yield optimal contrast to noise in calculating the AUC. Individual anatomical and functional scans were transformed into standard stereotaxic space (Talairach and Tournoux, 1988). To compensate for normal variation in anatomy across subjects, functional images were blurred using a 6 mm Gaussian full-width half-maximum filter.
Spatial extent analysis
This analysis was performed to examine within-group differences in the spatial extent of activation comparing the Famous and Unfamiliar name conditions. For each group, statistical parametric maps were generated to identify voxels where the AUC for famous names differed significantly from the AUC for unfamiliar names. An individual voxel probability threshold (P = 0.001) was coupled with a minimum cluster volume threshold of 0.28 ml. This combination of individual voxel probability and minimum cluster size thresholds is equivalent to a whole-brain family-wise error threshold of P < 0.05 based on 3000 Monte Carlo simulations (Forman et al., 1995).
Functional region of interest analysis
As a follow-up to the voxel-wise analyses, a functional region of interest (fROI) analysis was conducted to evaluate potential group differences in the magnitude of the AUC in functionally active regions. A functional region of interest map was generated by conjoining activated regions identified in the spatial extent analysis (see above) across the three groups. Any voxel deemed ‘activated’ by the Famous-Unfamiliar name subtraction in at least one of the three groups contributed to the final functional region of interest map. For each participant, an ‘averaged HRF’ was calculated for all voxels within a functional region of interest. AUC (4, 6 and 8 s post-stimulus onset) served as the dependent variable in a one-way analysis of variance (ANOVA) to examine group differences in each functional region of interest.
Structural MRI analyses
Voxel-based morphometry
Voxel-based morphometry (VBM) was conducted using SPGR anatomical images segmented with SPM 5 (Ashburner et al., 2008). A cut-off grey matter probability (P = 0.01) was used to remove spurious signals at grey–white matter boundaries. Modulated, normalized grey matter images were blurred using a 12 mm Gaussian filter to compensate for normal variation in anatomy across subjects. A voxelwise, one-way ANOVA (unpooled variance across subjects) was used to examine differences in cortical atrophy across the three participant groups, using a family-wise error threshold of P < 0.05.
Hippocampal volume
Left and right hippocampal volumes were manually traced on T1-weighted SPGR images by two raters blinded to participant group membership. Starting with a segmented mask of the medial temporal region created using SPM 5 and overlaid on the anatomical image, raters erased non-hippocampal regions on sagittal views. Using coronal views, the mask is further refined by excluding the fimbria and alveus and retaining the hippocampus (uncal apex, cornu ammonis, subiculum, gyrus of retzius and fasciola cinerea). Hippocampal volumes were normalized by dividing by the total intracranial volume. Intraclass correlation for the two raters was 0.88. Hippocampal volumes were also used as masks to calculate functional brain activation (see Results section).
Results
fMRI task performance
All groups performed well on the Fame Discrimination task with mean performance levels exceeding 87% correct (chance = 50%) (Table 1). There were no statistically significant differences between groups in per cent correct or reaction times for either condition. A more sensitive signal detection measure, Discriminability Index (A'), did reveal significant differences between groups, although the effect size was relatively small. For this reason, only correct trials were incorporated into the fMRI analyses.
fMRI spatial extent analysis
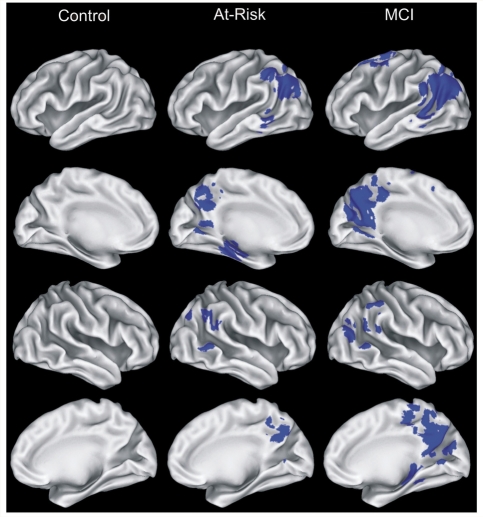
Total volume of activation (Famous > Unfamiliar comparison) was largest in the MCI group (34.7 ml) and intermediate in the At Risk group (9.7 ml); the Control group showed no significant differences in activation between the two stimulus conditions (Fig. 1, Table 2). With a few exceptions, the MCI and At Risk groups activated similar regions (bilateral posterior cingulate/precuneus, and lateral temporoparietal regions, albeit smaller in volume in the At Risk group relative to the MCI group. Small areas of activation were observed in the frontal cortex and caudate in the MCI group, which were not observed in the At Risk group. Finally, activation was observed in the left hippocampus in the At Risk group and the right hippocampus in the MCI group.
Figure 1.
Regions (shown in blue) demonstrating significant differences between the Famous and Unfamiliar Name conditions, conducted separately for each of the three groups. Brain activation projected on the lateral and medial surfaces of the left and right hemispheres. See Table 2 for additional information relating to individual activation foci.
Table 2.
Activation foci for famous versus unfamiliar name contrast for each group
| Control |
At Risk |
MCI |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Region | BA | x | y | z | Vol. | x | y | z | Vol. | x | y | z | Vol. | |
| Frontal | |||||||||||||||
| 1 | L Middle, superior frontal gyrus | 6 | −22 | 13 | 51 | 1.48 | |||||||||
| 2 | R SMA | 6, 31 | 12 | −26 | 47 | 0.59 | |||||||||
| 3 | L Middle frontal gyrus | 6 | −37 | −1 | 54 | 0.44 | |||||||||
| 4 | L Middle frontal gyrus | 6 | −26 | −6 | 60 | 0.31 | |||||||||
| Parietal | |||||||||||||||
| 5 | B Precuneus, posterior cingulate | 7, 23, 30, 31 | 0 | −54 | 35 | 2.37 | 1 | −57 | 26 | 16.04 | |||||
| 6 | L Angular gyrus | 19, 22, 39 | −42 | −63 | 25 | 11.19 | |||||||||
| 7 | R Angular gyrus, precuneus | 13, 40 | 51 | −47 | 30 | 0.32 | 42 | −73 | 35 | 0.41 | |||||
| 8 | L Inferior parietal lobule | 40 | −46 | −46 | 40 | 0.56 | |||||||||
| 9 | R Inferior parietal lobule | 40 | 53 | −42 | 47 | 0.35 | |||||||||
| 10 | L Posterior cingulate | 29, 30 | −4 | −54 | 6 | 0.53 | |||||||||
| Temporal | |||||||||||||||
| 11 | L Middle temporal, angular gyrus | 19, 39 | −41 | −64 | 29 | 3.61 | |||||||||
| 12 | R Middle temporal gyrus | 21, 22, 39 | 61 | −55 | 6 | 0.50 | 56 | −57 | 18 | 1.57 | |||||
| 13 | L Middle temporal gyrus | 37 | −57 | −47 | −6 | 0.34 | |||||||||
| 14 | L Hippocampus | − | −23 | −25 | −11 | 1.44 | |||||||||
| 15 | R Hippocampus | − | 27 | −32 | −2 | 0.65 | |||||||||
| Basal Ganglia | |||||||||||||||
| 16 | L Caudate | − | −10 | 9 | 11 | 1.65 | |||||||||
| Total Activation Volume | 0 | 9.66 | 34.67 | ||||||||||||
L = left, R = right.
Functional ROI analysis
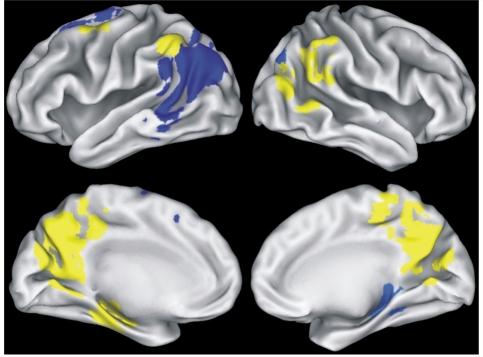
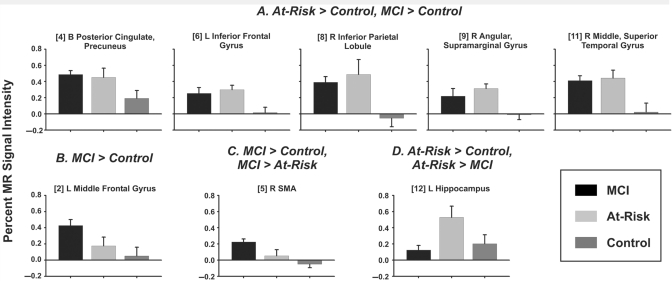
The fMRI mask (Fig. 2), formed by conjoining the activation maps in Fig. 1, consisted of 14 functional regions of interest. Eight of the 14 regions, shown in yellow in Fig. 2, showed significant group differences in the magnitude of activation, as reflected by per cent MR signal intensity (blue regions did not show group differences). Table 3 (post hoc analyses in rightmost column) and Fig. 3 illustrate that lateral temporoparietal and posterior cingulate/precuneus regions showed a similar pattern of increased activation in both the At Risk and MCI groups relative to the Control group, whereas increased activation in frontal regions (left middle frontal, right SMA) was limited to the MCI group. In contrast, activation in left hippocampus was increased only in the At Risk group. In the right hippocampal region of interest, there were no group differences in activation.
Figure 2.
Functional ROIs derived from conjoining activation maps in Fig. 1. Yellow regions indicate significant group differences in the magnitude of the MR signal intensity between groups; blue regions indicate no significant group differences. See Table 3 for additional information relating to individual fROIs.
Table 3.
Functional ROIs resulting from conjunction of famous versus unfamiliar name contrasts
| Region | BA | x | y | z | Vol. | P | Post hoc's | |
|---|---|---|---|---|---|---|---|---|
| Frontal lobe | ||||||||
| L Middle frontal gyrus | 6, 8 | −22 | 13 | 51 | 1.5 | NS | ||
| L Middle frontal gyrus | 6 | −37 | −1 | 54 | 0.4 | 0.03 | MCI > C | |
| L Middle frontal, precentral gyrus | 6 | −26 | −6 | 60 | 0.3 | NS | ||
| Parietal lobe | ||||||||
| B Posterior cingulate, precuneus | 7, 31 | 1 | −56 | 27 | 16.6 | 0.05 | AR > C, MCI > C | |
| R SMA | 5, 6, 31 | 12 | −26 | 47 | 0.6 | 0.003 | MCI > AR, MCI > C | |
| L Inferior parietal lobule | 40 | −46 | −46 | 40 | 0.6 | 0.007 | AR > C, MCI > C | |
| R Angular gyrus | 19, 39 | 42 | −73 | 35 | 0.4 | NS | ||
| R Inferior parietal lobule | 40 | 53 | −42 | 47 | 0.4 | 0.01 | AR > C, MCI > C | |
| R Angular, supramarginal gyrus | 13, 40 | 51 | −47 | 30 | 0.3 | 0.008 | AR > C, MCI > C | |
| Temporal lobe | ||||||||
| L Middle temporal, angular gyrus | 19, 39, 40 | −43 | −62 | 25 | 12.0 | NS | ||
| R Middle, superior temporal gyrus | 21, 39 | 57 | −57 | 16 | 1.9 | 0.003 | AR > C, MCI > C | |
| L Hippocampus | −23 | −25 | −11 | 1.4 | 0.03 | AR > C, AR > MCI | ||
| R Hippocampus | 27 | −32 | −2 | 0.7 | NS | |||
| Basal ganglia | ||||||||
| L Caudate | −10 | 9 | 11 | 1.6 | NS |
L = left; R = right; NS = not significant; C = Controls; AR = At Risk.
Figure 3.
Mean (SEM) per cent MR signal intensity differences between the Famous and Unfamiliar Name conditions for the three groups. HRFs for representative (A) positive and (B) negative fROIs. (A) Regions in which MCI and At Risk groups demonstrate greater activation than the Control group; (B) MCI activation greater than Control activation; (C) MCI activation greater than At Risk and Control activation; and (D) At Risk greater than MCI and Control activation.
Voxel-based morphometry
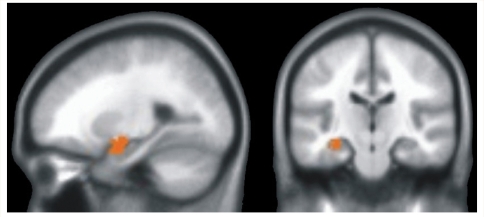
VBM was used to evaluate potential group differences in brain tissue density within the cortical grey matter. Only one significant group difference was observed, a reduction in the left hippocampus in the MCI participants compared to the At Risk and Control groups (Fig. 4). No other brain region showed significant tissue density reductions in the MCI group compared to the At Risk and Control groups.
Figure 4.
Results of VBM analysis indicating a significant difference in brain tissue density in the left hippocampus (shown in red) of the MCI group relative to the Control and At Risk groups.
Hippocampal volumes
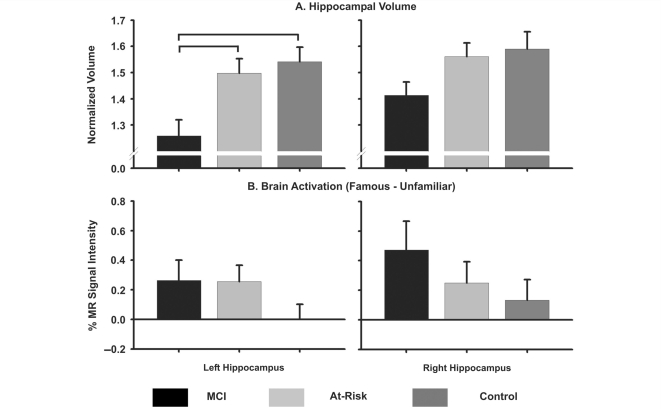
Results of hippocampal volumetric tracings are presented in Fig. 5A (upper panel) and confirm the VBM findings: left hippocampal volume was significantly reduced in MCI participants compared to the At Risk and Control groups (P = 0.002); likewise, there was a non-significant trend for the MCI group to have a smaller right hippocampal volume than the two cognitively intact groups (P = 0.069).
Figure 5.
(A) Mean (SEM) normalized volume of left and right hippocampus as a function of group. Brackets indicate significant group differences. (B) Mean (SEM) per cent MR signal intensity for the left and right hippocampi (derived from anatomical tracings) for each group.
The functional ROI analysis presented above indicated that activation in the left hippocampus was increased only in the At Risk group. Since the same functional region of interest mask was applied to all participants, it is conceivable that the reduced activation within the hippocampus of the MCI group could be due to atrophy. Figure 5B (lower panel) addresses this problem by presenting the averaged mean per cent MR signal intensity values for the left and right hippocampi based on the anatomically defined regions of interest. When this correction was applied, there were no significant differences in activation between the MCI and At Risk groups within the left and right hippocampus of the MCI participants.
Discussion
Our study demonstrated that increased activation of semantic memory circuits is observed in persons with MCI when task performance remains at levels comparable to healthy controls. In fact, the spatial extent and magnitude of the BOLD response was greater in the MCI group than that observed in cognitively intact individuals with two known Alzheimer's disease risk factors (family history and ApoE ε4 allele). Both the MCI and At Risk groups, in turn, demonstrated greater activation than a Control group consisting of cognitively intact older adults with no known risk factors. Thus, our results suggest that persons with MCI show evidence of functional compensation in brain regions subserving semantic memory systems; the degree of compensation appearing to be somewhat greater than that seen for healthy At Risk individuals. This increased activation was particularly evident in the posterior cingulate cortex, posterolateral parietal cortex, and frontal cortex [right supplementary motor area (SMA) and left middle frontal gyrus]. While functional connectivity disruptions have been noted between posterior cingulate cortex and brain regions critical to memory performance in persons with Alzheimer's disease (Greicius et al., 2004; Zhou et al., 2008) and in At Risk controls (Sorg et al., 2007), a recent study has identified increased functional connectivity between the posterior cingulate cortex and frontal-parietal cortices in persons with mild Alzheimer's disease (Zhang et al., 2008). This increase in functional connectivity could account for the pattern of increased activity seen in MCI patients and At Risk controls in our study.
The absence of within-group activation differences between famous and unfamiliar names for the Control participants contrasts with the intermediate activation in the At Risk group and greater activation in the MCI group. Although the process of famous name identification is typically done automatically and without much conscious effort, cognitive models include distinct stages of recognition and identification, which can be dissociable at both the behavioural and neural levels. We previously reported (Seidenberg et al., 2009) that MCI patients are unimpaired in the recognition of famous names but show significant impairment in the ability to access semantic knowledge about famous names that they correctly recognized, compared to age matched controls. It is possible that the healthy controls are able to proceed through the stages of recognition and identification of famous and unfamiliar names without disruption and, therefore, produce similar levels of activation for both types of stimuli. In contrast, the At Risk and MCI groups may have less automatic access to the semantic stores of knowledge about the famous names. Subtle neuropathological burden associated with increased disease risk may begin to interfere with the neural circuitry associated with semantic and episodic memory processing networks. Thus, the neural circuitry associated with access to and retrieval of their fine-grained semantic knowledge of famous individuals is more challenged. As a result, the task may be more effortful for these individuals and may produce increased activation relative to controls.
On the basis of a functional ROI analysis, compensatory activation was observed in the left hippocampus in the At Risk group, but apparently not in the MCI group. This finding may be an artifact of the analysis approach, which defined the hippocampal volume based on a conjunction mask of functional activity derived from each of the three groups and applied to all study participants. A subsequent volumetric analysis revealed that the MCI group had a significantly smaller left hippocampus than the two cognitively intact groups, suggesting that the decreased activation in the MCI group may have been the result of partial volume effects due to atrophy rather than a true decrease in hippocampal activity. When anatomical regions of interest were applied to define the hippocampus on an individual participant basis, MCI patients showed levels of activation comparable to the At Risk group. Our results underscore the importance of evaluating functional activation within the hippocampus using anatomical regions of interest because of the propensity for this structure to become atrophic in MCI.
The brain regions activated by our semantic task are similar to the so-called default network (Greicius et al., 2004), which reflects the state of the brain during rest. Binder and colleagues (Binder et al., 1999; McKiernan et al., 2003) have suggested that the resting state is not a passive state, but rather is characterized by rich cognitive activity, including semantic memory processing. Our event-related design permits a more systematic assessment of semantic memory processes during our fame discrimination task because we are comparing two active states: discrimination of famous names (high semantic memory) relative to unfamiliar names (low semantic memory). Because rest is, by definition, an uncontrolled state, blocked trial fMRI designs employing rest as a control condition cannot unambiguously separate the semantic memory processes from the active states. In addition, carriers of the ApoE ɛ4 allele show diminished task-induced deactivation relative to non-carriers in brain regions associated with the default network during a semantic categorization task (Persson et al., 2008). These reductions may suggest a propensity for the increased activation that may underlie compensatory recruitment.
This cross-sectional study implies that regional brain activity during a semantic memory task increases as the disease progresses. Longitudinal studies would be helpful in confirming whether task-induced activation does indeed increase in proportion to disease progression in an individual. If semantic memory tasks do track progression, they could be used as a biomarker to monitor disease progression or in treatment studies where reduced activation may signal a decreased need for compensatory recruitment. An obvious clinical application of our task would be its use in assessing efficacy of novel interventions or for identifying changes in the pattern of activation that may correspond to worsening of the disease. Future research studies might investigate whether our results using a semantic memory task involving person identity might generalize to other approaches for assessing semantic memory. That is, it will be important to determine whether the effect found in our study is dependent on person recognition specifically or semantic memory in general. In addition, the extent to which our approach can differentiate persons with single versus multiple-domain amnestic MCI would be of interest.
As noted earlier, prior fMRI studies of MCI and healthy controls revealed discrepant findings. We assert that one possible explanation for these divergent findings is that the majority of studies used an episodic memory task that may have been overly challenging for MCI participants. This type of difficulty would clearly limit the ability of such a task to be useful in tracking disease progression or response to pharmacological intervention in patients with Alzheimer's disease, who have even more severe deficits in episodic memory. A second limitation of prior studies may have been the use of blocked trial design fMRI paradigms, which do not have the ability to separate correct trials from errors in the imaging results. As a result, patients with memory disorders may demonstrate a different pattern of activation simply because their behaviour included more incorrect responses than controls. That is, the neural activity likely reflects more ‘dysfunction’ than ‘function’ compared to controls. Discrepancies in the direction of group activation differences among Alzheimer's disease risk groups may be a result of differences in the cognitive task used at baseline. Cognitive tasks in which performance would be expected to be more accurate and less variable over time may provide a more consistent index of disease progression involving brain networks subserving these cognitive skills.
Although our sample was intended to include persons with amnestic MCI, the limited neuropsychological testing performed with them makes a clear distinction between single domain and multiple-domain amnestic MCI difficult. Therefore, it is important to recognize that our sample may represent a combination of persons with single and multiple-domain amnestic MCI, which may influence the risk of progression to Alzheimer's disease and the nature of the dementia itself.
Our results suggest that persons at increased risk for Alzheimer's disease, whether they have been diagnosed with MCI or are healthy controls with multiple risk factors, display increased activation in posterior parietal and temporal regions while performing a semantic memory task that involves the recognition of famous people, a finding that is consistent with several other reports using different cognitive tasks with At Risk groups, MCI patients or Alzheimer's disease patients (Woodard et al., 1998; Saykin et al., 1999; Bookheimer et al., 2000; Bondi et al., 2005; Dickerson et al., 2005; Johnson et al., 2006b; Lind et al., 2006; Wishart et al., 2006; Han et al., 2008). This increased activity associated with increasing disease severity appears to reflect compensatory recruitment of neural resources, presumably due to the neuropathological effects on brain regions that are critical to performing the task of interest (Nielson et al., 2006; Han et al., 2008). These activated regions are salient because they show considerable overlap with the default state network—brain areas that demonstrate greater activity during ‘rest’ conditions (Greicius et al., 2004; van den Heuvel et al., 2008). In addition, unlike prior studies that have made use of challenging episodic memory tasks, our approach uses a semantic memory task that even persons with impaired episodic memory impairment can perform with a high degree of accuracy. This advantage suggests that our famous name discrimination task can be used to track dementia progression even in cognitively impaired individuals. As noted earlier, a strong candidate biomarker for Alzheimer's disease would be sensitive not only to the risk factors for developing the disease, but also to the presence of cognitive impairment that may be associated with the disease. We conclude that this famous name recognition task has the potential to be used as a biomarker of Alzheimer's disease risk and progression, given the increased compensatory activity seen in persons at genetic risk for the disease and even greater compensation seen in persons with mild cognitive impairment.
Funding
National Institutes of Health (R01AG44407, M01RR00058); Advancing Healthier Wisconsin foundation.
Glossary
Abbreviations
- DRS-2
Mattis Dementia Rating Scale-2
- fMRI
functional magnetic resonance imaging
- GDS
Geriatric Depression Scale
- HRF
haemodynamic response function
- MCI
mild cognitive impairment
- RAVLT
Rey Auditory Verbal Learning Test
References
- Ashburner J, Flandin G, Henson R, Kiebel S, Kilner J, Mattout J, et al. SPM5 Manual. London: Functional Imaging Laboratory: Wellcome Trust Centre for Neuroimaging; 2008. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–8. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer's Disease. N Engl J Med. 2000;343:450–6. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren AC, Small GW, Sabb FW, Bookheimer SY. Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. Am J Geriatr Psychiatr. 2002;10:44–51. [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–31. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertkow H, Black S. Imaging biomarkers and their role in dementia clinical trials. Can J Neurol Sci. 2007;34(Suppl 1):S77–83. doi: 10.1017/s031716710000562x. [DOI] [PubMed] [Google Scholar]
- Chong MS, Lim WS, Sahadevan S. Biomarkers in preclinical Alzheimer's disease. Curr Opin Investing Drugs. 2006;7:600–7. [PubMed] [Google Scholar]
- Chong MS, Sahadevan S. Preclinical Alzheimer's disease: diagnosis and prediction of progression. Lancet Neurol. 2005;4:576–9. doi: 10.1016/S1474-4422(05)70168-X. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Diamond EL, Miller S, Dickerson BC, Atri A, DePeau K, Fenstermacher E, et al. Relationship of fMRI activation to clinical trial memory measures in Alzheimer disease. Neurology. 2007;69:1331–41. doi: 10.1212/01.wnl.0000277292.37292.69. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and Alzheimer's disease. Neurology. 2005;65:404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville K, Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, et al. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 'Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier JB. Nonparametric indexes for sensitivity and bias: computing formulas. Psychol Bull. 1971;75:424–9. doi: 10.1037/h0031246. [DOI] [PubMed] [Google Scholar]
- Han SD, Bangen KJ, Bondi MW. Functional magnetic resonance imaging of compensatory neural recruitment in aging and risk for Alzheimer's disease: review and recommendations. Dement Geriatr Cogn Disord. 2008;27:1–10. doi: 10.1159/000182420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J Neurosci. 2008;28:116–24. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, et al. Activation of brain regions vulnerable to Alzheimer's disease: the effect of mild cognitive impairment. Neurobiol Aging. 2006a;27:1604–12. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, et al. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006b;26:6069–76. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Lutz. FL: Psychological Assessment Resources; 2001. Dementia Rating Scale-2 professional manual. [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- Le TH, Patel S, Roberts TP. Functional MRI of human auditory cortex using block and event-related designs. Magn Reson Med. 2001;45:254–60. doi: 10.1002/1522-2594(200102)45:2<254::aid-mrm1034>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, et al. Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers. Brain. 2006;129:1240–8. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, et al. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998;20:536–47. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Mattis S. Odessa. Florida: Psychological Assessment Resources; 1988. Dementia rating scale professional manual. [Google Scholar]
- Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1:182–8. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Douville KL, Seidenberg M, Woodard JL, Miller SK, Franczak M, et al. Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiol Aging. 2006;27:1494–504. doi: 10.1016/j.neurobiolaging.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Sleegers K, Van Broeckhoven C, et al. Altered deactivation in individuals with genetic risk for Alzheimer's disease. Neuropsychologia. 2008;46:1679–87. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Coleman RE, Doraiswamy PM. Neuroimaging and early diagnosis of Alzheimer disease: a look to the future. Radiology. 2003;226:315–36. doi: 10.1148/radiol.2262011600. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Wang L, Krishnan S, Slavin MJ, Prince SE, Tran TT, et al. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245:224–35. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–8. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1958. [Google Scholar]
- Sandstrom CK, Krishnan S, Slavin MJ, Tran TT, Doraiswamy PM, Petrella JR. Hippocampal atrophy confounds template-based functional MR imaging measures of hippocampal activation in patients with mild cognitive impairment. Am J Neuroradiol. 2006;27:1622–7. [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Hulette O, Welsh-Bohmer KA, Schmechel DE, Crain B, Burke JR, et al. Specificity, sensitivity, and predictive value of apolipoprotein-E genotyping for sporadic Alzheimer's disease. Lancet. 1996;348:90–3. doi: 10.1016/s0140-6736(96)01251-2. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Flashman LA, Frutiger SA, Johnson SC, Mamourian AC, Moritz CH, et al. Neuroanatomic substrates of semantic memory impairment in Alzheimer's disease: patterns of functional MRI activation. J Int Neuropsychol Soc. 1999;5:377–92. doi: 10.1017/s135561779955501x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonenboom SN, Visser PJ, Mulder C, Lindeboom J, Van Elk EJ, Van Kamp GJ, et al. Biomarker profiles and their relation to clinical variables in mild cognitive impairment. Neurocase. 2005;11:8–13. doi: 10.1080/13554790490896785. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Zhang Q, et al. Semantic knowledge for famous names in mild cognitive impairment. J Int Neuropsychol Soc. 2009;15:9–18. doi: 10.1017/S1355617708090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Bookheimer SY, Thompson PM, Cole GM, Huang SC, Kepe V, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7:161–72. doi: 10.1016/S1474-4422(08)70019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, et al. Altered brain activation in cognitively intact individuals at high risk for Alzheimer's disease. Neurology. 1999;53:1391–6. doi: 10.1212/wnl.53.7.1391. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci US A. 2007;104:18760–5. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland T, Hampel H, Takeda M, Putnam KT, Cohen RM. Biomarkers in the diagnosis of Alzheimer's disease: are we ready? J Geriatr Psychiatr Neurol. 2006;19:172–9. doi: 10.1177/0891988706291088. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, et al. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer's disease: a cross-sectional study. BMC Med. 2006;4:1. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbulcke M, Peeters R, Dupont P, Van Hecke P, Vandenberghe R. Word reading and posterior temporal dysfunction in amnestic mild cognitive impairment. Cereb Cortex. 2007;17:542–51. doi: 10.1093/cercor/bhj179. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci. 2008;28:10844–51. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, et al. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am J Psychiatr. 2006;163:1603–10. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]
- Woodard JL, Grafton ST, Votaw JR, Green RC, Dobraski ME, Hoffman JM. Compensatory recruitment of neural resources during overt rehearsal of word lists in Alzheimer's disease. Neuropsychology. 1998;12:491–504. doi: 10.1037//0894-4105.12.4.491. [DOI] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Miller SK, Franczak M, Antuono P, et al. Temporally graded activation of neocortical regions in response to memories of different ages. J Cogn Neurosci. 2007;19:1113–24. doi: 10.1162/jocn.2007.19.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Xing J, Liu B, Ma ZL, Yang M, et al. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behav Brain Res. 2008;197:103–8. doi: 10.1016/j.bbr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Dougherty JH, Jr, Hubner KF, Bai B, Cannon RL, Hutson RK. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimers Dement. 2008;4:265–70. doi: 10.1016/j.jalz.2008.04.006. [DOI] [PubMed] [Google Scholar]