Abstract
The internal circadian clock and sleep-wake homeostasis regulate and organize human brain function, physiology and behavior so that wakefulness and its associated functions are optimal during the solar day and that sleep and its related functions are optimal at night. The maintenance of a normal phase relationship between the internal circadian clock, sleep-wake homeostasis and the light-dark cycle is crucial for nominal neurobehavioral and physiological function in humans. Here we show that the phase relationship between these factors —the phase angle of entrainment (ψ)—is strongly determined by the intrinsic period (τ) of the master circadian clock located in the suprachiasmatic nucleus of the hypothalamus and the strength of the circadian synchronizer. Melatonin was used as a marker of internal biological time and circadian period was estimated during a forced desynchrony protocol. We observed relationships between the phase angle of entrainment and intrinsic period after exposure to scheduled habitual wakefulness-sleep light-dark cycle conditions inside and outside of the laboratory. Individuals with shorter circadian periods initiated sleep and awakened at a later biological time than individuals with longer circadian periods. We also observed that light exposure history influenced the phase angle of entrainment such that phase angle was later following exposure to a moderate bright light (~450 lux)-dark wakefulness-sleep schedule for 5 days than exposure to a normal indoor daytime level of light (~150 lux)-dark wakefulness-sleep schedule for 2 days. These findings demonstrate that neurobiological and environmental factors interact to regulate the phase angle of entrainment in humans. This finding has important implications for understanding physiological organization by the brain’s master circadian clock and may have implications for understanding mechanisms underlying circadian sleep disorders.
Keywords: Adult, Female, Humans, Light, Male, Melatonin, physiology, Photoperiod, Sleep, physiology, Wakefulness
Keywords: Phase angle of entrainment , Circadian Timing , Circadian Rhythms , Light Exposure , Tau, Psi
Introduction
The daily pattern of wakefulness during the day and sleep at night in humans is dependent upon an appropriate and stable phase relationship between the internal circadian clock, sleep-wake homeostasis and environmental time. In general, sleep need increases with the duration of prior wakefulness in a classic homeostatic manner (Åkerstedt and Folkard, 1995; Borbély and Achermann, 1999). However, the circadian clock produces a wakefulnesspromoting signal that increases across the day to counteract the homeostatic drive for sleep (Edgar et al., 1993; Dijk and Czeisler, 1995; Wright Jr. et al., 2002; Hull et al., 2003). The result of the interaction between these two fundamental properties emanating from the central nervous system is that humans are able to maintain alert wakefulness for approximately 15 to 17 hours (Czeisler et al., 1994). Likewise, sleep-wake homeostasis promotes sleep at the beginning of the sleep episode (Dijk and Czeisler, 1995; Wyatt et al., 1999) and the circadian clock maintains sleep near the end of the sleep episode as the homeostatic drive for sleep is dissipated (Dijk and Czeisler, 1995). In humans, these processes normally interact to maintain consolidated sleep throughout the night (Åkerstedt and Folkard 1995; Dijk et al., 1997; Borbély and Acherman 1999; Wyatt et al., 1999).
The hormone melatonin is commonly used as a marker of internal biological time (Arendt 1978; Lewy et al., 1999; Wright Jr. et al., 2001; Aeschbach et al., 2003) representing the phase of the master circadian clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus in mammals (Moore and Eichler 1972; Lee et al., 2003; Herzog et al., 2004). The SCN regulates the daily rhythm of melatonin secretion via gabaergic inhibition and glutamatergic stimulation of the paraventricular nucleus (PVN) (Perreau-Lenz et al., 2003; Perreau-Lenz et al., 2004) and noradrenergic stimulation of the pineal gland (Klein and Moore, 1979; Reppert et al., 1981). High circulating melatonin levels represent the biological night in both diurnal and nocturnal species. Humans typically initiate sleep shortly after the circadian rise in plasma melatonin levels and awaken shortly after the circadian fall in plasma melatonin levels. In humans, sleep efficiency is best during the biological night when melatonin levels are high. Wakefulness during scheduled sleep episodes is greatest during the biological day when melatonin levels are low (Dijk and Czeisler, 1995; Wyatt et al., 1999). Melatonin receptors are present on the human SCN (Weaver et al., 1993) and administration of exogenous melatonin in humans during the biological day produces drowsiness and sleep (Dollins et al., 1994; Hughes and Badia, 1997). This has lead to the hypothesis that the onset of melatonin secretion quiets the alerting signal from the SCN (Sack et al., 1997).
Maintenance of an appropriate phase relationship between the internal circadian clock, sleep-wake homeostasis and the environmental light-dark cycle is crucial for maintaining optimal physiological and behavioral function to promote survival (Pittendrigh, 1993; Daily and Ehrlich, 1996; DeCoursey et al., 2000). In fact, the internal biological timekeeping and the sleep-wake systems are important regulators of neuroendocrine, metabolic, renal, cardiovascular and neurobehavioral function (Smolensky et al., 1976; Gronfier et al., 1996; Czeisler and Klerman, 1999; Spiegel et al., 1999; Wyatt et al., 1999; Wurts and Edgar, 2000; Hull et al., 2001; Wright Jr. et al., 2002; Hull et al., 2003). Under normal conditions the internal biological timekeeping and the sleep-wakefulness systems interact to organize human physiology and behavior such that sleep and its associated functions occur during the solar night and wakefulness and its associated functions occur during the solar day. Circadian entrainment is the process that occurs when the phase relationship between biological and environmental time is stable and when the biological night occurs at the appropriate time in the solar day for that species (Pittendrigh and Daan, 1976; Wright Jr. et al., 2001; Honma et al., 2003). Humans, unlike other species, have exquisite control the light-dark cycle and are able to produce artificial light equivalent to daytime levels during solar darkness. The influence of this light exposure on the phase angle of entrainment has received little attention. In non-human species, the maintenance of an appropriate phase relationship between biological and environmental time has been reported to be dependent upon the period of the internal circadian clock as well as the timing and the strength of environmental time cues (Hoffmann, 1963; Pittendrigh and Daan, 1976). Whether the intrinsic period of the circadian clock (τ) and the strength of the environmental time cue affect the phase angle of entrainment (ψ) when sleep is scheduled to occur at the habitual time in humans is addressed in the current study.
While many stimuli have been reported to influence the phase of the internal circadian clock (Wever, 1979; Skene et al., 1996; Lockley et al., 2000; Sack et al., 2000; Wright Jr. et al., 2001; Crowley et al., 2003; Barger et al., 2004), exposure to light is the strongest naturally occurring environmental synchronizer for humans (Czeisler and Wright Jr., 1999). In humans, the duration, intensity and the biological time of exposure to light have been reported to influence the phase and amplitude of the internal circadian clock (reviewed in Czeisler and Wright Jr., 1999). However, how the strength of the light stimulus affects the phase angle of entrainment in humans, while maintaining the same day length and light-dark ratio), has not been reported. Here we show that the onset of melatonin secretion and its phase angle relationship to habitual sleep time/darkness onset in humans is strongly determined by the period of the internal circadian clock and the strength of the environmental synchronizer.
Materials and Methods
A total of 34 healthy adults (11 females, 23 males; aged 30.5 ± 8.2) participated in one of two inpatient protocols (Wright Jr. et al., 2001; Wright Jr. and Czeisler, 2002). The current phase angle assessments were performed on data from the baseline days of these protocols. Data used in the current analyses includes available data from days 1 and 3–5 of the first study (Wright Jr. and Czeisler, 2002) and days 1–2, days 5–8 and days 35–49 of the second study (Wright Jr. et al., 2001). General procedures were identical for the two protocols and are provided next. This is then followed by descriptions of study specific procedures and then the specific aims of the current analyses.
General Procedures
The Brigham and Women’s Hospital/Partners Health Care Human Research Committee approved the procedures for the protocols and participants gave written informed consent. The investigations were conducted according to the principles expressed in the Declaration of Helsinki. Participants were healthy based upon medical history, physical and psychological exams, blood and urine chemistries and electrocardiogram. Toxicology screens for drug use, including but not limited to alcohol, nicotine and caffeine, verified that participants were drug free upon admission to the laboratory. Three weeks prior to the laboratory protocol, participants were instructed to maintain a regular eight hour sleep schedule, which was verified by times called in to a time stamped voice recorder and by wrist actigraphy for at least one week. Napping was proscribed. Reported time spent in bed was on average 8-h 5 min ± 16 min (mean ± SD) the week prior to entering the laboratory.
Ceiling-mounted fluorescent T8 and T80 lamps (Phillips, Eindhoven, The Netherlands) with a 4100 K color temperature produced a spectrum of white light. Lux was measured with an IL-1400 photometer (International Light, Inc. Newburyport, MA, USA) and microwatts were determined with a PR-650 SpectraScan Colorimeter (CR-650, PhotoResearch Inc, Chatsworth, CA). Microwatts levels are provided when available.
Participants arrived at the laboratory ~12 h prior to habitual bedtime and shortly thereafter they were maintained in very dim light of ~3.0 lux in the angle of gaze (< 5 lux ambient at ~76 cm with the light sensor pointed in the direction of the ceiling fixtures, < 15 lux maximum at ~183 cm with the light sensor pointed in the direction of the ceiling fixtures). Participants were scheduled to sleep in dark, sound attenuated, temperature-controlled rooms for 8 h at their habitual sleep time. Habitual sleep time was calculated by subtracting four hours from the average midpoint of the participants’ self-selected sleep-wakefulness schedule during the week prior to laboratory admission. The average habitual sleep onset time was 2244 hr ± 1 hr 14 min (± SD).
Constant routines (CR) were used to estimate melatonin phase under very dim light conditions of ~1.5 lux angle of gaze (< 3 lux ambient at ~76 cm, < 8 lux and < 0.8 μW/cm2 maximum at ~183 cm). Wakefulness, activity, and ambient temperature were constant and food and fluid intake were evenly distributed across the CR protocol (Czeisler and Wright Jr., 1999).
Study Specific methods
Study 1
Nineteen participants, 8 females and 11 males (age 29.3 ± 9.7; mean ± SD) were studied in a 10 daylong inpatient protocol (Wright Jr. and Czeisler, 2002). These subjects included all those with available melatonin data during baseline. On days 2 through 3 of the 10 day protocol these 19 participants were exposed to light levels equivalent to normal indoor daytime levels (~150 lux in the angle of gaze, ~190 lux maximum) during 16 h of scheduled wakefulness. This was followed by a 40-h CR (Figure 1A).
Figure 1.
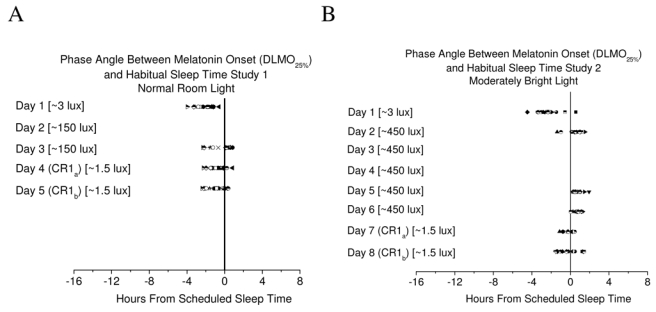
A) Phase angle of entrainment for subjects in study 1 (n=19). Following two days of exposure to a light-dark schedule with light levels equivalent to normal daytime indoor light, melatonin onset was delayed but the range of phase angles was similar when comparing day 1 to the CR. B) Phase angle of entrainment for subjects in study 2 (n=15). Following five days of exposure to a moderately bright light-dark schedule, melatonin onset was delayed and inter-individual differences in phase angle were reduced when comparing day 1 to the CR. Symbols represent individual subjects. Solid line represents habitual sleep time. Values in square brackets represent light level exposure in the angle of gaze.
Study 2
Fifteen participants, 3 females and 12 males (age 31.8 ± 6.2; mean ± SD) were studied in a 55 daylong inpatient protocol where intrinsic circadian period was assessed (Wright Jr. et al., 2001). On days 2 through 6 of the 55 day protocol these 15 participants were exposed to moderately bright indoor light of ~450 lux in the angle of gaze (< 1,100 lux ambient at ~76 cm, < 1,500 lux maximum at ~183 cm) during 16 h of scheduled wakefulness. A 40-h CR followed baseline days (Figure 1B). Subjects were then scheduled to a 24.0 or 24.6 hr day, in ~1.5 or 25 lux in the angle of gaze during scheduled wakefulness and sleep in darkness, for 25 days. Then, participants were scheduled to a 28-h day (18.66 h wakefulness and 9.33 h scheduled sleep) forced desynchrony (FD) protocol for 12 consecutive days (equivalent to fourteen 24-h days) to estimate the intrinsic circadian period of the melatonin rhythm(τm) (Czeisler et al., 1999; Wright Jr. et al., 2001). The FD days were conducted in ~1.5 lux in the angle of gaze during scheduled wakefulness. However, light levels during scheduled wakefulness of the CR and of FD protocols were ~3.0 lux in the angle of gaze for the first two subjects in study 2. Intrinsic circadian period of the melatonin rhythm was estimated using non orthogonal spectral analysis techniques described in detail elsewhere (Czeisler et al., 1999).
Specific Aims
The primary aims of the current analyses were 1) to assess whether the strength of the environmental synchronizer influenced the phase angle of entrainment in humans; 2) to determine whether there is a significant relationship between the phase angle of entrainment and intrinsic circadian period in humans after exposure to scheduled wakefulness-sleep light-dark cycle conditions inside and outside of the laboratory; 3) to determine whether the drift in circadian phase across two circadian cycles under constant conditions of the CR is related to intrinsic circadian period and whether this drift can be used to accurately assess circadian period.
In addition, since the current sample size is relatively large, we also used the available data to determine if we could replicate previous research findings. Specifically, we assessed 1) the phase angle of entrainment (the average time of melatonin onset relative to habitual sleep time/darkness onset) following scheduled light-dark sleep-wake cycles outside of the laboratory (Lewy et al., 1999; Martin and Eastman, 2002; Burgess et al., 2003; Mongrain et al., 2004). Data from the first baseline day of both studies presented in this manuscript were combined for this analysis; 2) the relationship between the clock hour of habitual sleep time and circadian period (Duffy et al., 2001). Data from study 2 were used since circadian period was not assessed in study 1; 3) the relationship between morning-eveningness and intrinsic circadian period (Duffy et al., 2001). Data from study 2 were used since circadian period was not assessed in study 1; and 4) the influence of exposure to ~150 and ~450 lux on the assessment of the phase angle of entrainment (i.e., masking of melatonin onset during exposure to these light levels; e.g., Bojkowski et al 1987; Czeisler and Wright Jr., 1999; Nathan et al 2000; Zeitzer et al. 2000). Data from study 1 and study 2 were used to demonstrate the influence of masking on assessment of melatonin onset.
Melatonin analyses
Blood samples were obtained every 30 to 60 min to assess circadian phase and period of the melatonin rhythm. Plasma melatonin levels were assayed via radioimmunoassay I125 (Diagnostic USA, Inc., Osceola, WI). The sensitivity of the assay was 2.5 pg/ml. The average inter-assay and intra-assay coefficients of variation were 13.45% and 4.99% respectively. The dim light melatonin onset (DLMO25%) was defined as the linearly interpolated point in time when melatonin levels exceeded 25% of the 3-harmonic fit peak to trough amplitude as determined on the CR on day 4 (Wright Jr. and Czeisler, 2002) or day 7 (Wright Jr. et al., 2001). The 25% criterion was applied to all data to control for individual differences in melatonin amplitude. Using this criteria, we found that the average value for the DLMO25% was 13.33 ± 7.14 pg/ml (Mean ± SD) with a range of 3.27 to 33.57 pg/ml. This average DLMO25% is slightly higher than the 10 pg/ml criteria commonly used when melatonin amplitude is not known or assessed. Melatonin data were unavailable for some individuals on different days of the study due to blood sampling difficulties (see results). Changes in melatonin onset from day 1 to the first circadian cycle on the CR (day 4 or 7), and drift in melatonin phase from one cycle to the next during constant conditions of the CR were analyzed using one-sample t-tests. The relationship between the phase angle of entrainment and the intrinsic period of the internal circadian clock was assessed using linear regression techniques. Results are presented as mean ± SD. We do not consider the results for melatonin onset during exposure to ~150 or ~450 lux to be an accurate assessment of melatonin onset since these light levels have been reported to influence melatonin levels. However, these data are presented to demonstrate masking of the phase angle of entrainment in humans under these conditions.
Results
The results section will first present some of the analyses that were performed to determine if we could replicate previous research findings. These results are then followed by analyses that address the specific aims of the current study and additional analyses to replicate previous research.
Phase angle of entrainment following exposure to scheduled light-dark sleep-wake cycles outside of the laboratory (subject data combined from studies 1 and 2) and relationship to habitual sleep time. On the first day upon entry to the laboratory, melatonin onset occurred prior to habitual sleep time for all but one of the 34 participants, with a range from −4.46 h prior to sleep to 0.55 h after habitual sleep time. The average time of the DLMO25% was 2-h 9 min ± 1-h 1 min (± SD) prior to habitual sleep time/lights out and the median time was 2 h 14 min prior to habitual sleep time/lights out. We also assessed the relationship between the clock hour of habitual sleep time and the clock hour of the DLMO25% and found a significant correlation (r=0.67; P < 0.0001), such that for every 1 hr change in sleep time there was a 0.87 hr difference in DLMO25%.
Masking of melatonin onset during exposure to ~150 and ~450 lux in the angle of gaze. During exposure to 16 h of ~150 lux, a light intensity equivalent to normal daytime levels of indoor light, on day 3 of study 1, melatonin onset occurred very near to but after scheduled sleep time/lights out for 11 of 17 subjects whereas during the very dim light of the CR on the following day (day 4), melatonin onset occurred after scheduled sleep time/lights out in only 3 of 19 subjects (Figure 1A).
During exposure to 16 h of ~450 lux, a light intensity equivalent to moderately bright indoor light, melatonin onset occurred after scheduled sleep time/lights out for 12 of 14 subjects on day 2 and for all subjects on days 5–6 in study 2 (Figure 1B). During the very dim light of the CR, following five days of exposure to the moderately bright indoor light-dark schedule, melatonin onset occurred after scheduled sleep time/lights out for 7 of 14 subjects (Figure 1B).
Specific aim 1
Does the strength of the environmental synchronizer influenced the phase angle of entrainment in humans? Comparison of the phase angle of entrainment following exposure to a 16:8 hr ~150:0 lux light-dark wakefulness-sleep schedule (Study 1) and following exposure to a 16:8 hr ~450:0 lux light-dark wakefulness-sleep schedule (study 2). Following 2 days of exposure to scheduled 16:8 hr ~150 lux:0 lux wakefulness-sleep cycles, the DLMO25% on the CR was significantly delayed by 1 h 12 min ± 28 min compared to day 1 of entry into the laboratory (Figure 1A; t= 11.35, p<0.000001; one-sample t-test). Following 5 days of exposure to scheduled 16:8 hr ~450 lux:0 lux wakefulness-sleep cycles, the DLMO25% on the CR was significantly delayed by 2-h 5 min ± 55 min (Figure 1B; t= 8.54, p<0.000001; one-sample t-test). This phase delay of melatonin onset from day 1 to the CR (day 7) was greater in the moderately bright light conditions of study 2 than the normal daytime indoor light conditions of study 1 (t= 3.68, p<0.001). In addition to the greater phase delay in response to moderately bright light, we observed that the inter-individual variance in the phase angle of entrainment was reduced after exposure to the moderately bright light-dark circadian synchronizer (Figure 1B).
Specific aim 2
Is there a relationship between the phase angle of entrainment and intrinsic period following scheduled wakefulness-sleep light-dark conditions outside and inside of the laboratory (Study 2). Regression analysis showed positive relationships between the phase angle of entrainment and intrinsic period such that longer phase angles were associated with shorter intrinsic periods of the internal circadian clock (Figures 2). This relationship was observed to be robust upon arrival to the laboratory (Figure 2A) and during the CR following controlled laboratory conditions of 5 days 16:8 exposure to ~450:0 lux wakefulness-sleep cycles (Figure 2B–C). In general, subjects with a circadian period shorter than 24.0 h showed longer phase angles of entrainment than subjects with a period longer than 24.0 h regardless of previous light exposure history outside or inside of the laboratory. Upon entry to the laboratory on day 1, the association between intrinsic period and phase angle was such that for every 6 min change in circadian period (0.1 h) there was a 24 min difference in phase angle. Even though exposure to a moderately bright light-dark/wakefulness-sleep schedule delayed melatonin onset and reduced inter-individual differences in phase angle, the association between intrinsic period and phase angle during the first cycle of the CR (CRa) was such that for every 6 min change in circadian period there was a 14 min difference in phase angle. On the second cycle of the CR (CRb) the association between intrinsic period and phase angle was such that for every 6 min change in circadian period there was a 32 min difference in phase angle.
Figure 2.
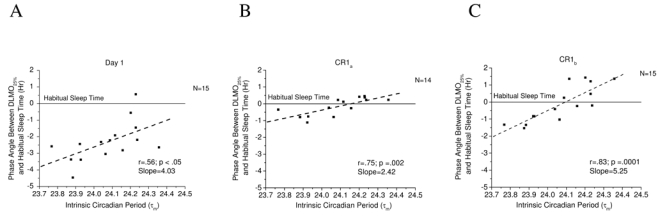
Association between intrinsic circadian period during forced desynchrony (T-Cycle= 28.0) and the phase angle of entrainment on A) day 1 of entry to the laboratory, B) day 7, the first cycle of the constant routine (CRa), and C) day 8, the second cycle of the constant routine (CRb) for subjects in study 2. Symbols represent individual subjects for whom melatonin onset and intrinsic period data were available. The dashed line represents a linear fit of the data. The phase angle of entrainment, as determined by the relationship between melatonin onset and habitual sleep time, is positively and robustly related to intrinsic circadian period (τm) with slopes ranging from 2.42 to 5.25.
We also assessed the relationship between morning-eveningness and intrinsic circadian period.
In addition, we assessed the relationship between the clock hour of habitual sleep time and circadian period, but found no relationship between these measures (r = −0.0211, P = 0.94051).
Is the drift in circadian phase under constant conditions related to prior light history?. First, we assessed the degree of drift under the constant conditions of the CR following 2 days of exposure to a scheduled 16:8 hr ~150 lux:0 lux wakefulness-sleep cycle. We assessed absolute drift in phase since subjects with intrinsic periods shorter than 24.0 h would likely drift in an advance direction and subjects with periods longer than 24.0 h would drift in a delay direction. We observed an absolute change of the DLMO25% from the first to the second cycle on the CR that was significantly greater than zero (16 ± 11 min; t= 6.13, p<0.0001; one-sample t-test). Following 5 days of exposure to a scheduled 16:8 hr ~450 lux:0 lux wakefulness-sleep cycle, the absolute change of the DLMO25% from the first to the second cycle on the CR of study 2 was greater than zero [30 min ± 27 min (t= 4.18, p=0.001; one-sample t-test)]. There was also a non-significant trend for a greater absolute change in melatonin onset from the first to the second cycle on the CR after exposure to the moderately bright light of study 2 when compared with the normal daytime levels of indoor light of study 1 (t=1.96, p=0.0599).
Specific aim 3
Is the drift in circadian phase across two circadian cycles under constant conditions of the CR related to intrinsic circadian period and can the amount of drift be used to accurately assess circadian period. The drift between successive melatonin onsets on the CR was positively and robustly related to intrinsic circadian period (τm) with a slope of 2.61 (Figure 3). Subjects with a circadian period shorter than 24.0 h tended to advance and subjects with a period longer than 24.0 h tended to delay under the constant conditions of the CR. However, for every 6 min change in circadian period there was a 16 min change in melatonin onset.
Figure 3.
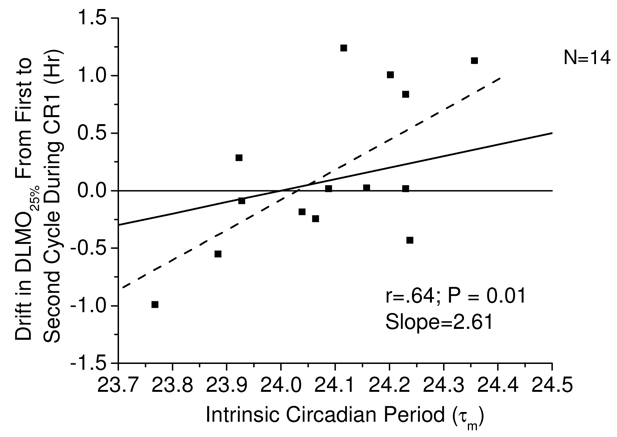
Association between the drift in melatonin onset time during CR and intrinsic circadian period during forced desynchrony (T-Cycle=28.0) of study 2. Symbols represent individual subjects for whom two melatonin onsets and intrinsic period data were available. The solid black line represents the change in melatonin onset that would have occurred had subjects drifted at a rate equal to the intrinsic period of their internal clock. The dashed line represents a linear fit of the data.
Discussion
This study demonstrates that in humans, as in other species, the phase angle of entrainment is strongly determined by the period of the internal circadian clock. Furthermore, scheduling participants to strictly controlled laboratory conditions in a light-dark cycle equivalent to normal indoor daytime levels and darkness or a light-dark cycle equivalent to moderate indoor bright light and darkness changed the phase angle of entrainment, such that under both cases it was later compared to the day of entry into the laboratory. This delay in internal biological time occurred even though the day length and the light-dark ratio were similar for the scheduled wakefulness-sleep light-dark cycle conditions inside and outside of the laboratory. Furthermore, the delay in melatonin onset time was greater following 5 days of 16 h of exposure to moderately bright light compared to the 2 days of 16 h of exposure to normal daytime indoor light, demonstrating that the strength of the laboratory synchronizer affected the phase angle of entrainment.
The current finding of a delay in melatonin onset following days of exposure to laboratory conditions is likely related to the constant light intensity exposure throughout the 16 h of scheduled wakefulness. These schedules probably exposed subjects to light of a higher intensity than normal during the evening hours in the laboratory compared to light exposure received outside of the laboratory (Guillemette et al., 1998) resulting in greater stimulation of the delay portion of the phase response curve to light (reviewed in Czeisler & Wright Jr., 1999). It is possible that exposing subjects to dim room light shortly after entry to the laboratory may have resulted in an advance of melatonin onset on day 1. However, the amount of advance is likely to be small since the average timing of melatonin onset relative to habitual sleep time that we observed upon entry to the laboratory is consistent with prior reports that exposed subjects to dim light much later in the day (Lewy et al., 1999; Burgess et al., 2003; Mongrain et al., 2004). Another study reported that the timing of melatonin onset relative to self-selected, uncontrolled sleep times was earlier in subjects who habitually went to bed later (Martin and Eastman, 2002). Consistent with previous reports, we found a relationship between habitual sleep time and the clock hour of melatonin onset (Martin and Eastman, 2002; Burgess et al., 2003). These results are consistent with the idea that the timing of melatonin onset is influenced by wakefulness-sleep and light-dark history.
In studies that have altered the light-dark ratio during a 24 h day, it has been reported that the duration of melatonin secretion was shorter for short versus long nights (Wehr et al., 1993; Vondrasová et al., 1997). These data were interpreted as support for the hypothesis that the human circadian system, like that of other mammals (Arendt, 1979; Hastings et al. 1986) has the capacity to respond to seasonal changes in light exposure that naturally occur at latitudes away from the equator. Given that the human circadian system can be reset with very dim light (Zeitzer et al. 2000) as low as ~12 lux in the angle of gaze (Wright Jr. and Czeisler, 2002), and that intermittent exposure to light has a greater resetting capacity per minute basis compared to constant light (Rimmer et al. 2000; Gronfier et al., 2004), and that the human circadian system maybe reset by light exposure at most if not all circadian phases (Jewett et al. 1997; Khalsa et al., 2003), it is likely that both the natural and the artificial light exposure that humans are exposed to across the 24 h day is important in determining the phase angle of entrainment in modern society. Our current finding that 2 days of exposure to 16 h of light, equivalent to normal indoor daytime levels, during scheduled wakefulness and 8 h of darkness during scheduled sleep delayed the phase of melatonin onset compared to scheduled conditions outside of the laboratory supports this idea.
On average, melatonin onset time drifted in a delay direction from one cycle to the next during the constant conditions of the constant routine protocol. Although the absolute drift on the constant routine tended to be larger following exposure to 5 days of moderately bright light in study 2 than 2 days of indoor daytime levels of light in study 1, it is possible that the different number of days of exposure to the light-dark cycles influenced this result (Hebert et al. 2002). This would be consistent with the effect of light intensity history on the period of the circadian clock in diurnal mammals (Pittendrigh and Daan, 1976). Whether differences in the distribution of intrinsic periods between studies influenced this result cannot be determined because intrinsic period was not estimated in study 1.
In study 2, there was a significant relationship between the magnitude and direction of drift in melatonin onset on the constant routine and intrinsic circadian period. However, the average drift in melatonin onset time was 2.6 times greater than would be expected based on the average intrinsic circadian period observed. Melatonin onset delayed in some individuals who had shorter than 24.0 h circadian periods and advanced in some individuals who had longer that 24.0 h circadian periods. These findings suggest that the drift in melatonin onset from one circadian cycle to the next under constant routine conditions cannot be used to estimate circadian period accurately. It is possible that the drift in phase under constant conditions reflects the influence of transients on melatonin phase (Pittendrigh et al. 1958), although transients in response to light exposure have not been reported in humans (Jewett et al. 1997). As previously mentioned (Wright Jr. et al 2001), the conditions to which subjects were exposed to prior to the forced desynchrony (i.e., scheduled 24.0 and 24.6 day lengths in ~1.5 or ~25 lux) might have influenced our estimates of circadian period.
It has long been acknowledged in non-human species that both the strength of the environmental synchronizer and the period of the circadian clock determine the phase angle of entrainment. Specifically, it has been reported that the phase relationship between activity onsets and the light-dark cycle in lizards (Hoffmann, 1963); sparrows (Eskin, 1969), chaufinches (Aschoff, 1965) and hamsters (Pittendrigh and Daan, 1976) is related to intrinsic period. Previously, we reported that the phase angle of entrainment to a weak synchronizing stimulus in sighted humans was associated with intrinsic period (Wright Jr. et al., 2001) and Lewy and colleagues reported that the phase angle of entrainment to melatonin treatment in blind humans was associated with intrinsic period (Lewy et al., 2001). The phase relationship between the circadian clock and environmental time in non-human animals has also been reported to be influenced by the duration and the intensity of light exposure [reviewed in (Aschoff, 1965; Pittendrigh and Daan, 1976; Aschoff et al., 1975)]. Most non-human studies have examined the phase angle relationship between biological and environmental time during exposure to the environmental stimulus. In the present study, we also observed a change in the phase relationship between melatonin onset and the timing of light and darkness during the days of exposure to the daytime levels of indoor light on day 3 of study 1 and the moderately bright light on days 2, 5 and 6 of study 2. Using melatonin as a circadian phase marker as we did, it is impossible to determine whether this change in phase angle represents a true change in the phase relationship between biological and environmental time or whether this simply reflects the acute suppressant/masking effects of daytime levels of indoor and moderately bright light exposure on melatonin levels (Bojkowski et al 1987; Czeisler and Wright Jr., 1999; Nathan et al 2000; Zeitzer et al. 2000). Therefore, we assessed phase angle upon release from entrainment under constant routine conditions in very dim light. Based on the latter analysis more subjects were shown to have melatonin onsets prior to sleep time. These results suggest that melatonin onset was suppressed by daytime levels of indoor light and moderately bright light laboratory conditions and thus provide further evidence that melatonin levels need to be assessed in dim light (Lewy et al 1999).
We observed that if an individual’s circadian period was shorter than the scheduled day length, then the phase angle was longer relative to the onset of habitual sleep time, whereas if circadian period was longer, the phase angle was shorter. This resulted in individuals with shorter intrinsic circadian periods going to sleep and awaking at a later biological time.
It has been previously reported that circadian period was correlated with clock hour of habitual sleep time (Duffy et al., 2001). We did not replicate this finding. We did however replicate previous findings of a significant correlation between the phase angle of entrainment and circadian period, such that a longer phase angle of entrainment was associated with a shorter intrinsic period (Duffy et al. 2001). We also replicated the finding that circadian period influences morning and evening behavior preferences in humans such that those with shorter intrinsic periods tend to be early birds and those with longer circadian periods tend to be night owls (Duffy et al., 2001). In addition, morning and evening types have been reported to exhibit different phase angles of entrainment (Duffy et al., 2001; Baehr et al., 2000) and gene polymorphisms are reported to be associated with diurnal preference (Katzenberg et al. 1998; Archer et al., 2003).
Our current finding that light-dark history and intrinsic period influenced the phase angle of entrainment has implications for circadian sleep disorders. The timing of melatonin onset has been reported to be earlier in a familial form of advanced sleep phase syndrome (Jones et al., 1999) and the phase angle of entrainment has been reported to be altered in circadian sleep disorders (Shibui et al., 1999; Uchiyama et al., 2000). In addition, the circadian period of one individual with advanced sleep phase syndrome was reported to be shorter than 24-h, consistent with the desire to go to bed early at night (Jones et al., 1999). Whether the responses to light exposure and/or circadian period are altered in other circadian sleep disorders remains to be determined.
In summary, the current findings demonstrate that the intrinsic period of the master circadian clock is important for determining the phase relationship between biological and environmental time and suggest that, as in other species, intrinsic circadian period determines key elements of circadian entrainment in humans. Furthermore, the current findings show that the strength of the light-dark cycle also has important influence on the relationship between biological and environmental time in humans.
Acknowledgments
We thank the participating volunteers; research staff; subject recruiters, S. Ma, M. Hines, C. O’Brien; and J.M. Ronda and B. Cade for technical support. Supported by NASA Cooperative Agreement NCC 9-58 with the National Space Biomedical Research Institute, by NIH R01-MH45130 and by General Clinical Research Center Grant GCRC-M01-RR02635 from the National Center for Research Resources. KPW was supported by fellowships from the NIH (T32-DK07529), the Medical Foundation and Harold Whitworth Pierce Charitable Trust and Start-up funds from the University of Colorado.
References
- Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- Arendt J. Melatonin assays in body fluids. J Neural Transm. 1978;(13):265–78. [PubMed] [Google Scholar]
- Aschoff J. The phase-angle difference in circadian periodicity. In: Aschoff J, editor. Circadian Clocks. Amsterdam: North-Holland Publishing Company; 1965. pp. 262–276. [Google Scholar]
- Aschoff J, Hoffmann K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase-shifts of the zeitgeber. Chronobiologia. 1975;2:23–78. [PubMed] [Google Scholar]
- Åkerstedt T, Folkard S. Validation of the S and C components of the three-process model of alertness regulation. Sleep. 1995;18:1–6. doi: 10.1093/sleep/18.1.1. [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. J Clin Endocrinol Metab. 2003;88:26–30. doi: 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: With an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Barger LK, Wright KP, Jr, Hughes RJ, Czeisler CA. Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. Am J Physiol. 2004;286:R1077–1084. doi: 10.1152/ajpregu.00397.2003. [DOI] [PubMed] [Google Scholar]
- Bojkowski CJ, Aldhous ME, English J, Franey C, Poulton AL, Skene DJ, Arendt J. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm Metab Res. 1987;19:437–440. doi: 10.1055/s-2007-1011846. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D. The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav Sleep Med. 2003;1:102–114. doi: 10.1207/S15402010BSM0102_3. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–23. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Dijk D-J, Duffy JF. Entrained phase of the circadian pacemaker serves to stabilize alertness and performance throughout the habitual waking day. In: Ogilvie RD, Harsh JR, editors. Sleep Onset: Normal and Abnormal Processes. Washington, D.C: American Psychological Association; 1994. pp. 89–110. [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk D-J, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–132. [PubMed] [Google Scholar]
- Czeisler CA, Wright JrKP. Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. Regulation of Sleep and Circadian Rhythms. New York: Marcel Dekker, Inc; 1999. pp. 149–180. [Google Scholar]
- Daily GC, Ehrlich PR. Nocturnality and species survival. Proc Natl Acad Sci USA. 1996;93:11709–11712. doi: 10.1073/pnas.93.21.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey PJ, Walker JK, Smith SA. A circadian pacemaker in free-living chipmunks: essential for survival? J Comp Physiol. 2000;186:169–180. doi: 10.1007/s003590050017. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk D-J, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol. 1997;505:851–858. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci USA. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: Evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin A. Dissertation. The University of Texas at Austin; 1969. The sparrow clock: Behavior of the free running rhythm and entrainment analysis; pp. 1–224. [Google Scholar]
- Gronfier C, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A, Brandenberger G. A quantitative evaluation of the relationships between growth hormone secretion and delta wave electroencephalographic activity during normal sleep and after enrichment in delta waves. Sleep. 1996;19:817–824. doi: 10.1093/sleep/19.10.817. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Jr, Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol. 2004;287:E174–181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette J, Hebert M, Paquet J, Dumont M. Natural bright light exposure in the summer and winter in subjects with and without complaints of seasonal mood variations. Biol Psychiatry. 1998;44:622–628. doi: 10.1016/s0006-3223(97)00543-x. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Walker AP, Herbert J. Effect of asymmetrical reductions of photoperiod on pineal melatonin locomotor activity and gonadal condition on male Syrian hamsters. J Endocrinol. 1986;114:221–229. doi: 10.1677/joe.0.1140221. [DOI] [PubMed] [Google Scholar]
- Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: A reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. Zur beziehung zwischen phasenlage und spontanfrequenz bei der endogenen tagesperiodik. Z Naturforsch. 1963;18:154–157. [Google Scholar]
- Honma K, Hashimoto S, Nakao M, Honma S. Period and phase adjustments of human circadian rhythms in the real world. J Biol Rhythms. 2003;18:261–270. doi: 10.1177/0748730403018003008. [DOI] [PubMed] [Google Scholar]
- Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20:124–131. [PubMed] [Google Scholar]
- Hull JT, Wright KP, Jr, Czeisler CA. Circadian and sleep-wake dependent control of urine volume output on a 28-h forced desynchrony. Sleep. 2001;24:a90. [Google Scholar]
- Hull JT, Wright KP, Jr, Czeisler CA. The Influence of Subjective Alertness and Motivation on Human Performance Independent of Circadian and Homeostatic Regulation. J Biol Rhythms. 2003;18:329–338. doi: 10.1177/0748730403253584. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol. 1997;273:R1800–R1809. doi: 10.1152/ajpregu.1997.273.5.r1800. [DOI] [PubMed] [Google Scholar]
- Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ, Jones B, Czajkowski L, Ptacek LJ. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- Katzenberg D, Young T, Finn L, Lin L, King DP, Takahashi JS, Mignot E. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–76. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Moore RY. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: Control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979;174:245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- Lee HS, Billings HJ, Lehman MN. The Suprachiasmatic Nucleus: A Clock of Multiple Components. J Biol Rhythms. 2003;18:435–449. doi: 10.1177/0748730403259106. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Hasler BP, Emens JS, Sack RL. Pretreatment circadian period in free-running blind people may predict the phase angle of entrainment to melatonin. Neurosci Lett. 2001;313:158–160. doi: 10.1016/s0304-3940(01)02261-3. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19:695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase Relationships between Sleep-Wake Cycle and Underlying Circadian Rhythms in Morningness-Eveningness. J Biol Rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Wyndham EL, Burrows GD, Norman TR. The effect of gender on the melatonin suppression by light: a dose response relationship. J Neural Transm. 2000;107:271–279. doi: 10.1007/s007020050022. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, van der Vliet J, van Heijningen C, Simonneaux V, Pevet P, Buijs RM. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur J Neurosci. 2003;17:221–228. doi: 10.1046/j.1460-9568.2003.02442.x. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Kalsbeek A, Pevet P, Buijs RM. Glutamatergic clock output stimulates melatonin synthesis at night. Eur J Neurosci. 2004;19:318–324. doi: 10.1111/j.0953-816x.2003.03132.x. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Temporal organization: Reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:17–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Bruce V, Kaus P. On the significance of transients in daily rhythms. Proc Natl Acad Sci USA. 1958;44:965–973. doi: 10.1073/pnas.44.9.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- Reppert SM, Perlow MJ, Ungerleider LG, Mishkin M, Tamarkin L, Orloff DG, Hoffman HJ, Klein DC. Effects of damage to the suprachiasmatic area of the anterior hypothalamus on the daily melatonin and cortisol rhythms in the rhesus monkey. J Neurosci. 1981;1:1414–1425. doi: 10.1523/JNEUROSCI.01-12-01414.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmer DW, Boivin DB, Shanahan TL, Kronauer RE, Duffy JF, Czeisler CA. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am J Physiol. 2000;279:R1574–1579. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep-promoting effects of melatonin: At what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20:908–915. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]
- Shibui K, Uchiyama M, Okawa M. Melatonin rhythms in delayed sleep phase syndrome. J Biol Rhythms. 1999;14:72–76. doi: 10.1177/074873049901400110. [DOI] [PubMed] [Google Scholar]
- Skene D, Deacon S, Arendt J. Use of melatonin in circadian rhythm disorders and following phase shift. Acta Neurobiol Exp (Warsz) 1996;56:359–362. doi: 10.55782/ane-1996-1139. [DOI] [PubMed] [Google Scholar]
- Smolensky MH, Tatar SE, Bergman SA, Losman JG, Barnard CN, Dacso CC, Kraft IA. Circadian rhythmic aspects of human cardiovascular function: a review by chronobiolgic statistic methods. Chronobiologia. 1976;3:337–371. [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Okawa M, Shibui K, Kim K, Tagaya H, Kudo Y, Kamei Y, Hayakawa T, Urata J, Takahashi K. Altered phase relation between sleep timing and core body temperature rhythm in delayed sleep phase syndrome and non-24-hour sleep-wake syndrome in humans. Neurosci Lett. 2000;294:101–104. doi: 10.1016/s0304-3940(00)01551-2. [DOI] [PubMed] [Google Scholar]
- Vondrasová D, Hájek I, Illnerová H. Exposure to long summer days affects the human melatonin and cortisol rhythms. Brain Res. 1997;759:166–170. doi: 10.1016/s0006-8993(97)00358-2. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Stehle JH, Stopa EG, Reppert SM. Melatonin receptors in human hypothalamus and pituitary: Implications for circadian and reproductive responses to melatonin. J Clin Endocrinol Metab. 1993;76:295–301. doi: 10.1210/jcem.76.2.8381796. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- Wever RA. The circadian system of man: Results of experiments under temporal isolation. New York: Springer-Verlag; 1979. [Google Scholar]
- Wright KP, Jr, Czeisler CA. Absence of circadian phase resetting in response to bright light behind the knees. Science. 2002;297:571. doi: 10.1126/science.1071697. Supplementary online material. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, Hughes RJ, Kronauer RE, Dijk D-J, Czeisler CA. Intrinsic near-24-hour pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci USA. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol. 2002;283:R1370–R1377. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- Wurts SW, Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci. 2000;20:4300–4310. doi: 10.1523/JNEUROSCI.20-11-04300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk D-J. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


