Abstract
Animal models of retinitis pigmentosa include the rd mouse, in which a mutation of a rod-specific phosphodiesterase leads to the rapid loss of photoreceptors during the early postnatal life. Very little is known about changes occurring in inner retinal neurons after photoreceptor loss. These changes are important in view of the possibility of restoring vision in retinas with photoreceptor degeneration by means of cell transplantation or direct stimulation of inner layers. In this paper, we show that bipolar and horizontal cells of the rd mouse retina undergo dramatic morphological modifications accompanying photoreceptor loss, demonstrating a dependence of second order neurons on these cells. While describing modifications of the rd retina, we also provide quantitative information about neurons of the wild-type mouse retina, useful for future studies on genetically altered animals.
Keywords: rd mouse, bipolar cell, horizontal cell, morphology
Retinitis pigmentosa (RP) comprises a large group of inherited retinal disorders and represents one of the major causes of blindness in the world, with an incidence of approximately 1 in 4,000 (1). There is currently no treatment for RP but there are proposed strategies. The availability of experimental animals with similar genetic defects, either occurring naturally or obtained through transgenic manipulations, has made it possible to attempt a variety of therapeutical approaches. The most promising of these include (i) the artificial stimulation of the retina by means of implanted electronic prostheses (2, 3), and (ii) the transplant of healthy retinal sheets that would develop new photoreceptors restoring appropriate connections with inner retinal neurons (4–6). Both strategies are based on the assumption that inner retinal neurons are preserved in both functional properties and connectivity after photoreceptor degeneration and can be either electrically stimulated or induced to form meaningful synaptic connections. However, there is little experimental evidence that this might be the case. Indeed, the few papers dealing with this issue noted variations (in number, immunoreactivity, and functional properties) of inner retinal neurons in both RP patients and animals models of RP (7–13). A systematic approach aimed at clarifying this important issue is still lacking.
A well established animal model of RP is represented by the rd mouse, in which a mutation of the rod-specific phosphodiesterase (14) leads to the rapid and massive death of rod photoreceptors in the first few weeks of postnatal life (15). At a lower rate, cones degenerate as well, leading to blindness in less than 2 months (16). A number of papers describe the fine morphological, physiological, and biochemical changes associated with photoreceptor degeneration (reviewed in ref. 15). However, very little is known about the possible changes of second- and higher-order neurons. We have undertaken a systematic study of the inner retina of the rd mouse. We show hereby that second-order neurons of the rd retina undergo a series of impressive alterations that occur early in the animal life and that reveal cellular interactions that govern the formation of functional synapses in normal mice.
Methods
Animals.
Mice were C57BL/6J and C3H/HeJ (The Jackson Laboratory). Animals were maintained in a 12/12 h light/dark cycle. Fifty retinas from adult (2–3 months old) mice were used for immunocytochemistry (25 retinas from rd and an equal number from control mice). Three additional retinas from each strain were used for rod bipolar cell counting; three more were used for horizontal cell counting. Immature retinas were from 42 animals (21 from each strain) killed at postnatal day 9 (P9)–P10 or P20. Animals were anesthetized by i.p. injection of Avertin (1.2% tribromoethanol and 2.4% amylene hydrate in distilled water, 0.02 ml/gm body weight) and perfused transcardially with 4% paraformaldehyde/0.1 M phosphate buffer. All procedures conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Immunocytochemistry.
The following antibodies were used: protein kinase C (PKC; Amersham) to stain rod bipolar cells; calbindin D (Sigma) to stain horizontal cell bodies and dendrites; neurofilament 200 kDa (Sigma) to stain horizontal cell axonal endings; mGluR6, kindly donated by S. Nakanishi (Kyoto University, Kyoto), to stain metabotropic glutamate receptors; and L7, kindly provided by C. Cepko (Harvard Medical School, Boston), to stain inner nuclear layer cells, including rod bipolars. For whole-mount immunocytochemistry, the eyes were removed and the retinas were isolated and placed in primary antibodies, ranging in concentration from 1:50 to 1:1,000. After 3–4 days, the retinas were rinsed in PBS and immersed in secondary antibodies. They were anti-mouse Oregon Green 488 and anti-rabbit Alexa 568 (Molecular Probes). After 2 additional days, retinas were rinsed, mounted in glycerol-based medium, and viewed with a Leica true confocal scanner-NT confocal microscope equipped with an argon-kripton laser. For vertical section immunocytochemistry, whole eyes were infiltrated with 25% sucrose, frozen at −20°C, and cut serially at 12 μm with a cryostat. Sections were treated as above, with concentrations of primary antibodies ranging from 1:200 to 1:7,000.
Cell Counting.
Serial optical sections were obtained at 1-μm intervals across the inner nuclear layer (INL) to acquire the profiles of rod bipolars and horizontal cells. Scanning areas were 20 fields, 125 × 125 μm for rod bipolar cells and 250 × 250 μm in the case of horizontal cells. Fields were regularly spaced along the dorso-ventral and naso-temporal retinal meridians. Cell counts were performed on prints corresponding to each single focal plane for rod bipolars, and on extended-focus prints for horizontal cells. Retinal profiles were acquired with an image analyzer (MC4; Imaging, Inc., ON, Canada) that allowed the calculation of retinal areas. Total number of cells was obtained by multiplying average cellular densities for corresponding retinal areas.
Results
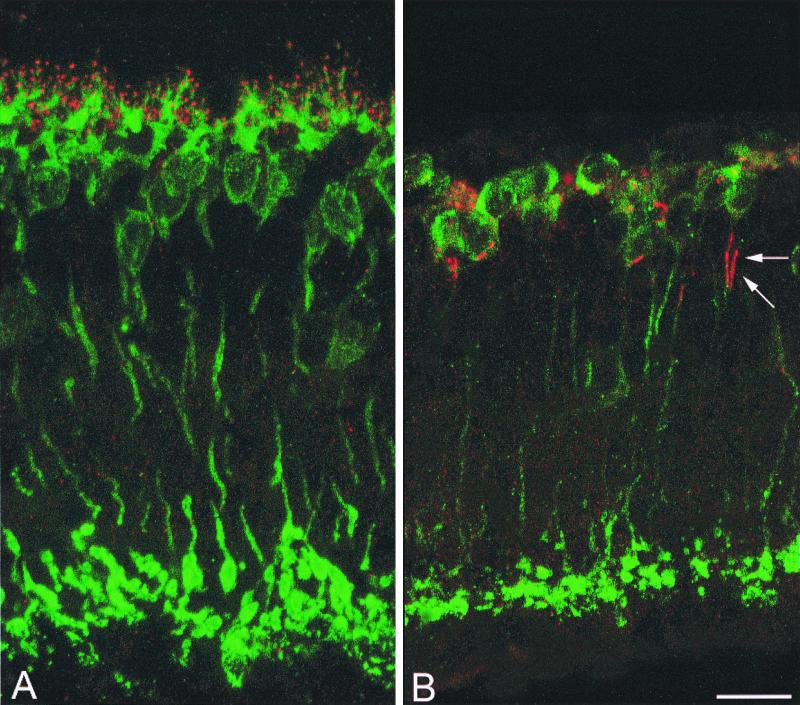
Rod bipolar cells were labeled with PKC antibodies (17) in both vertical sections and retinal whole mounts. To determine their number, retinal whole mounts were examined with a confocal microscope, where serial optical sections were obtained of the entire INL at various eccentricities along the dorso-ventral and naso-temporal retinal meridians. Rod bipolars cells are dramatically affected by photoreceptor degeneration: In retinas of adult rd mice they completely lack dendrites. The outer aspect of their cell bodies is totally devoid of processes (Fig. 1). This finding is confirmed by immunostaining with the L7 antiserum, recognizing a protein abundant in Purkinje and rod bipolar cells (18) (Fig. 2). In whole mounts of rd retinas, it is impossible to identify a focal plane corresponding to the level of stratification of bipolar cell dendrites. Only at very peripheral retinal locations, rod bipolars occasionally retain few processes in the outer plexiform layer (OPL). A topographical change in the pattern for L7 immunoreactivity in the rd retina has been previously described by Ogilvie et al. (8).
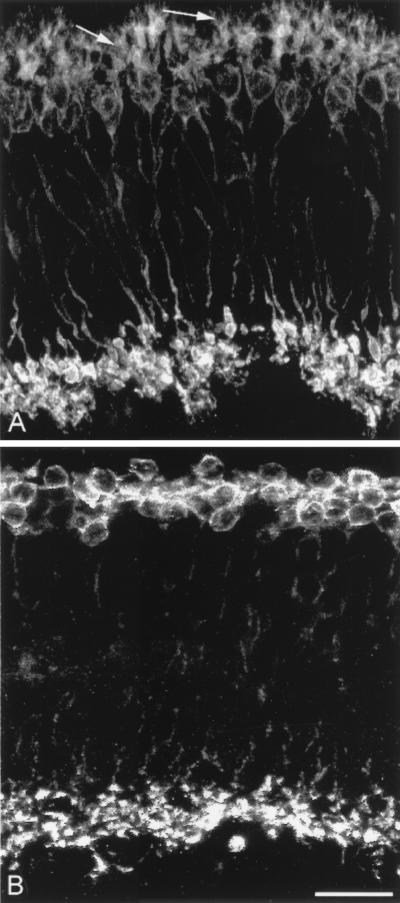
Figure 1.
Rod bipolar cells. PKC staining in wt (A) and rd (B) retinas in vertical sections. Dendrites, pointed out by arrows in A, are totally absent in B. Unless specified, illustrations have been obtained from retinas of animals ranging between 2 and 3 months of age. (Bar is 20 μm in this and subsequent figures.)
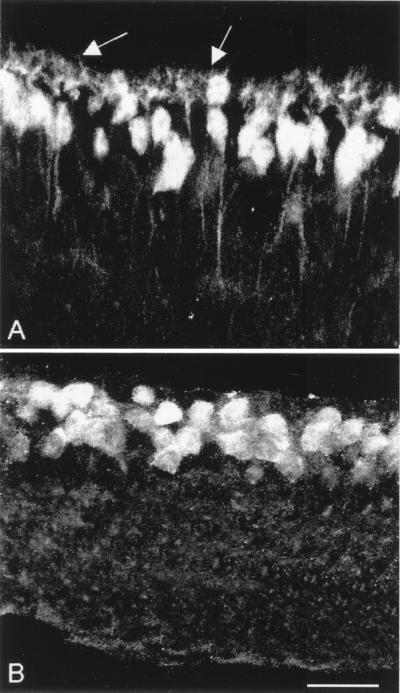
Figure 2.
Lack of dendrites in rod bipolar cells. Immunostaining with a different antiserum (L7) confirms that rod bipolars have no processes in the OPL. (A) wt retina; (B) rd retina. Arrows in A indicate dendrites.
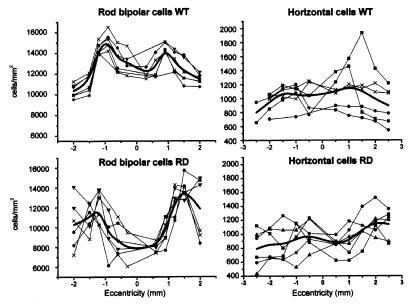
Rod bipolar densities were estimated at various retinal locations in 2-month-old rd mice. Local densities were compared with corresponding measures obtained for wild-type (wt) retinas; total numbers of rod bipolar cells also were determined. Results are reported in Fig. 3 Left. There is a marked decrease (>30%) in the density of rod bipolar cells in the rd central retina. The overall decrease in cell number is in the range of 10% but is not statistically significant (there are 187,170 ± 9,022 rod bipolar cells in the rd retina versus 207,995 ± 3,368 cells in the wt retina; values are from three retinas for each strain and are expressed as mean ± SD). It has to be noticed that the total number of cells is affected by retinal areas (in this case, rd retinas were slightly larger) and by the average cellular density. The number of samples is also relevant. What it is important to notice here is that some rod bipolar cells die off in the central retina, thus following the topography of rod degeneration (14). Dendrites of remaining rod bipolars are scant or absent.
Figure 3.
Density curves of rod bipolars cells (Left) and horizontal cells (Right). Data are from three wt and rd adult retinas. Black solid lines represent average curves. Notice the sizeable decrease in rod bipolar density in central rd retinas.
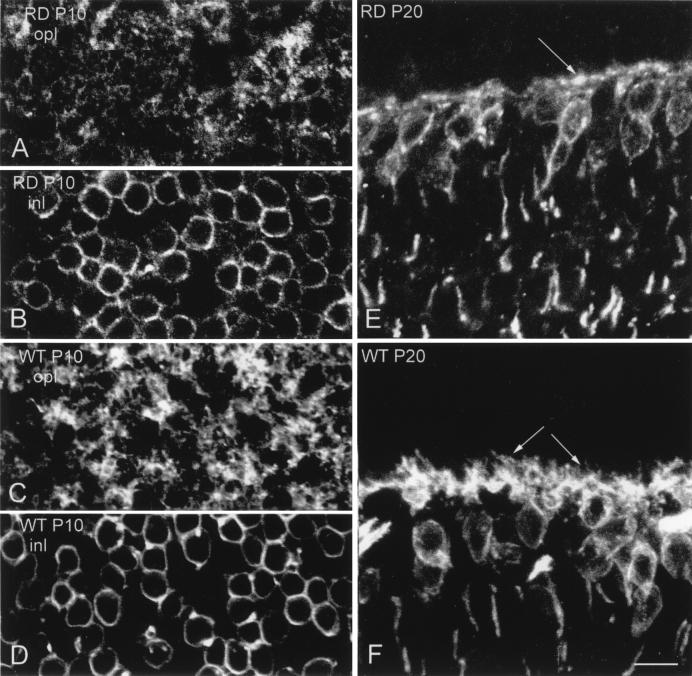
We wondered whether the absence of rod bipolar cell dendrites represented a secondary loss of processes, occurring after or strictly following the disappearance of rods, or, rather, whether bipolar cells failed to develop normal dendritic arborizations during the first weeks of postnatal life. We studied the morphology of rod bipolar cells stained with PKC antibodies at 10, 20, and 30 days of life. We never observed normally developed dendritic arborizations in the OPL (Fig. 4). At P20 there are rudimental dendrites of abnormal morphology, resembling club-shaped processes directed toward photoreceptors (Fig.4E). These abortive dendrites are absent in the adult (Fig.1B). This finding is in agreement with early observations reporting arrest of synaptogenesis in the photoreceptor terminal of the rd retina in the second postnatal week (19). We conclude that rod bipolar cells need healthy photoreceptors to develop their dendrites successfully; this requirement seems independent of the amount of glutamate effectively reaching bipolars in the OPL, because knockout mice for mGluR6 glutamate receptors do have normal bipolar cell dendrites (20).
Figure 4.
PKC staining of rd and wt young retinas (P10 and P20). (A–D) Confocal images obtained at different depths in whole-mounted retinas. Rod bipolar dendrites are scant and scarcely differentiated in the P10, rd retina (A), although they tile richly the OPL in the wt (B). There are not appreciable differences in the staining of rod bipolar cell bodies of rd and wt retinas (B and C). (E and F) Vertical sections of rd and wt retinas at the age of P20. Dendrites of rod bipolar cells in the wt (F) are well differentiated (arrows); in age-matched rd retinas they are limited to a rudimental border (arrow in E).
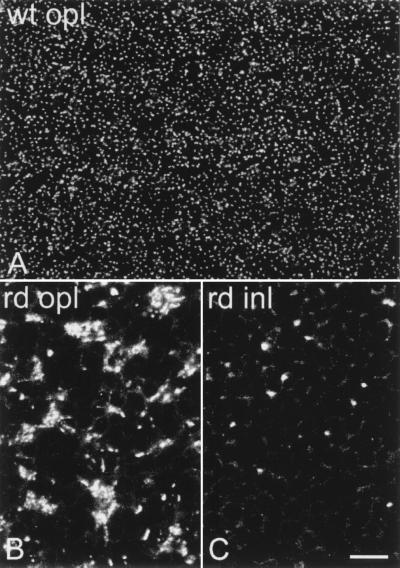
Synaptic transmission between rods and rod bipolar cells occurs through glutamate and a specific type of metabotropic glutamate receptor, namely mGluR6. As a consequence of the absence of dendrites in rod bipolar cells, mGluR6 loses its normal distribution. Immunoreactivity for mGluR6 in the OPL appears decreased, for it is restricted to large patches on the outer surface of the cell bodies and residual dendrites of bipolar cells (Fig. 5). The precise localization of the staining (21) that is typical of the normal OPL is completely lost (Fig. 6). In addition, immunoreactivity is present over the surface of the cell body and along axons as they course through the inner nuclear layer (Figs. 5 and 6). These axons are stained by anti-PKC antibodies and thus belong to rod bipolar cells. Such a pattern of staining, occurring rarely in the normal retina, demonstrates a rerouting of the receptor molecules. As for dendrites of rod bipolar cells, changes in the pattern of mGluR6 immunoreactivity occur early in development and are closely associated to photoreceptor degeneration.
Figure 5.
Glutamate metabotropic receptor. Double labeling for PKC (green) and mGluR6 (red) in wt (A) and rd (B) retinas in vertical sections. Immunoreactivity for mGluR6 in the OPL of the rd retina appears decreased; rod bipolar axons also are stained (arrows in B).
Figure 6.
Whole-mount staining for mGluR6. Compare the OPL of wt retina (A) to the OPL of rd retina (B). In B, immunoreactivity appears clustered in dense bodies. The fine punctate labeling visible in A has been lost. In the rd retina, individual bipolar axons are also stained in the INL (C).
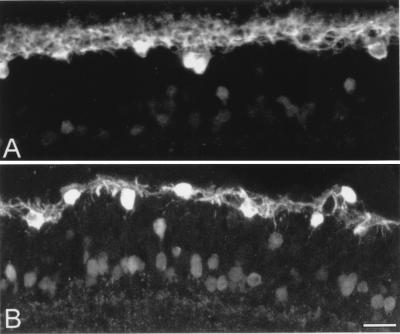
Finally, we studied the morphology and distribution of horizontal cells in the rd retinas. Cell bodies of horizontal cells are postsynaptic to cone photoreceptors; they can be labeled selectively with anti-calbindin antibodies (22) (Fig. 7). In young rd retinas (up to 3 weeks postnatally), they exhibit a normal morphology. However, in the adult retina (3.5 months of age), they have changed visibly: Cell bodies as well as first-order dendrites have become hypertrophic and small, and terminal dendrites have completely disappeared. Dendritic loss is not too surprising in neurons that have lost most of their afferent synapses. Similar to what is observed in rod bipolar cells, horizontal cell bodies have slightly but not significantly decreased in number (14,600 ± 1,360 cells in the rd retina versus 17,860 ± 548 cells in the wt; data are mean values ± SD; n = 3 for each strain). Results are summarized in Fig. 3 Right.
Figure 7.
Calbindin staining of horizontal cell bodies. Vertical sections of wt (A) and rd retinas (B). In this view it is easy to appreciate the loss of processes emerging from horizontal cell bodies. Several amacrines and ganglion cells are stained for calbindin in both A and B.
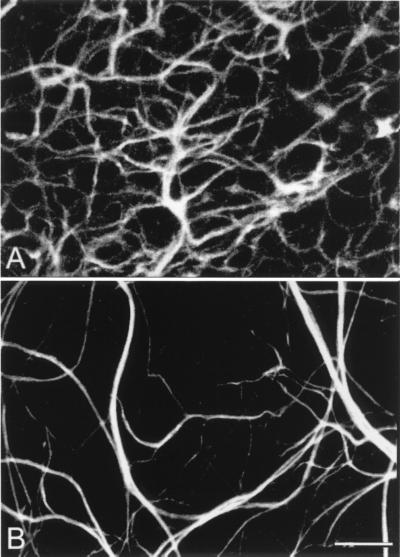
The axonal arborizations of horizontal cells are postsynaptic to rod photoreceptors. They can be visualized with antibodies against the 200-kDa neurofilament protein (22). Also, the axonal endings of horizontal cells fail to develop normally. They increase enormously in size whereas fine ramifications connected to rods are totally absent. In retinal whole mounts, they clearly form a loose meshwork with poor coverage of the retinal surface (Fig. 8). Frequently, thin processes originate from large-size endings to run a perpendicular course in the inner plexiform layer. Such processes are never encountered in the retina of normal mice.
Figure 8.
Whole-mount staining with neurofilament antibodies. Compare the tight network made by horizontal cell axonal endings in the OPL of wt retinas (A) to the loose arrangements of hypertrophic process in the rd (B).
In summary, cells or portions of cells connected to rod photoreceptors fail to develop, whereas the portions of horizontal cells connected to cones (which degenerate more slowly) develop normally, although they will show signs of degeneration thereafter.
Discussion
Many studies have been devoted to the genetics, biochemistry, and morphology of photoreceptor degeneration. Possible changes occurring in inner retinal layers have not been investigated or overlooked. Only changes in the overall number of cells in the inner nuclear layer have been systematically studied. A careful report by Santos et al. (9) describes a 20% cell loss in the INL in the macular area of RP patients, whereas in extramacular regions the loss can reach 60% (12). Previous work has been dedicated to ganglion cells of degenerating retinas (23). We found local changes occurring in the densities of both rod bipolars and horizontal cells in retinas of young adult rd mice. These changes are in the same range of the overall numerical decrease detected in central retinas of aged RP patients (9). In the future, we intend to follow in time the number of these cell types to find out whether such variations become more prominent. However, more than quantitative effects on INL cells, photoreceptor degeneration appears to trigger dramatic changes in the morphology of second-order neurons that predict even more serious functional consequences. Some of the reported alterations are confirmed by early observations by Blanks et al. (19), who described the absence of lateral processes from the synaptic complexes established by rods in the immature rd retina.
The failure of rod bipolar cells to develop dendrites as well as the absence of processes in the OPL originating from the axonal arborizations of horizontal cells demonstrate that healthy photoreceptors are required to ensure the normal development of second-order neurons. We can speculate that, even before the onset of light-dependent electrical synaptic activity at photoreceptor terminals, these cells produce and release factors guiding the normal development of bipolar and horizontal cells. Indeed, the release from photoreceptors of diffusible molecules that affect photoreceptor survival has been demonstrated recently (24). Such a type of interactions falls under the general category of non-cell-autonomous mechanisms that act during development (25). The release of toxic substances from mutant photoreceptors affecting the development of second-order neurons seems unlikely, because heterozygous rd +/− mice have a morphologically normal retina (26). The hypertrophy that we observe in horizontal cell axonal endings, including the presence of ectopic neurites extending in the inner plexiform layer, can be interpreted as events of cellular plasticity after deafferentation. Various changes also occur in the cell bodies of horizontal cells that lose fine dendrites, increase in size, and decrease in number. Alterations in the distribution of the synaptic receptor mGluR6 resembles the spread of acetylcholine receptors outside the synaptic site after muscle denervation (27). It is known for N-methyl-d-aspartate glutamate receptors that afferent activity as well as complex protein interactions are responsible for receptor clustering at the synapse (28, 29). Remarkably, the mGluR6 receptor may be displaced to the axons of rod bipolar cells. This finding suggests that sorting and targeting of the receptor proteins to the appropriate cell region have been compromised.
Taken together, these findings demonstrate that the correct development and maintenance of second-order neurons depends on the presence of photoreceptors. If conclusions drawn from rd retinas are of general applicability, then other models of early-occurring retinal degeneration (i.e., rhodopsin mutant mice) (30, 31) should show similar modifications of inner retinal neurons. The observed morphological changes, suggesting profound functional effects on inner retinal neurons, should be carefully taken into account when attempting to restore vision in degenerating retinas by means of photoreceptor transplantation or direct electrical stimulation. In particular, strategies have to be devised to promote dendritic regrowth in bipolar and horizontal cells.
Finally, the reported data related to the distribution of rod bipolar cells and horizontal cells in the wt mouse provide additional quantitative information about retinal cell populations in this important mammalian species (32).
Acknowledgments
This work is dedicated to the memory of Piero Strettoi. The mGluR6 antibody was kindly donated by S. Nakanishy, and the L7 antibody was a gift of C. Cepko. This research was supported by the TeleThon Foundation, Project E833.
Abbreviations
- RP
retinitis pigmentosa
- Pn
postnatal day n
- INL
inner nuclear layer
- PKC
protein kinase C
- OPL
outer plexiform layer
- wt
wild type
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190291097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190291097
References
- 1.Wong F. Prog Ret Eye Res. 1997;16:353–373. [Google Scholar]
- 2.Humayun M S, de Juan E., Jr Eye. 1998;12:605–607. doi: 10.1038/eye.1998.151. [DOI] [PubMed] [Google Scholar]
- 3.Humayun M S, de Juan E, Jr, Weiland J D, Dagnelie G, Katona S, Greenberg R, Suzuki S. Vision Res. 1999;39:2569–2576. doi: 10.1016/s0042-6989(99)00052-8. [DOI] [PubMed] [Google Scholar]
- 4.Gouras P, Du J, Kjeldbye H, Yamamoto S, Zack D J. Invest Ophthalmol Visual Sci. 1992;33:2579–2586. [PubMed] [Google Scholar]
- 5.Kwan A S L, Wang S, Lund R D. Exp Neurol. 1999;159:21–33. doi: 10.1006/exnr.1999.7157. [DOI] [PubMed] [Google Scholar]
- 6.Radtke N D, Aramant R B, Seiler M, Petry H M. Am J Ophthalmol. 1999;128:384–387. doi: 10.1016/s0002-9394(99)00250-0. [DOI] [PubMed] [Google Scholar]
- 7.Duvoisin R M, Zhang C, Hamassaki-Britto D E, Britto L R. Neurosci Lett. 1995;183:83–86. doi: 10.1016/0304-3940(94)11120-8. [DOI] [PubMed] [Google Scholar]
- 8.Ogilvie J M, Tenkova T, Lett J M, Landgraf M, Silvermen M S. Curr Eye Res. 1997;16:244–251. doi: 10.1076/ceyr.16.3.244.15411. [DOI] [PubMed] [Google Scholar]
- 9.Santos A, Humayun M S, de Juan E, Jr, Greenburg R, Marsha M J, Klock I B, Milam A. Arch Ophthalmol. 1997;115:511–515. doi: 10.1001/archopht.1997.01100150513011. [DOI] [PubMed] [Google Scholar]
- 10.Yazulla S, Studholme K M, Pinto L H. Vision Res. 1997;37:3471–3482. doi: 10.1016/S0042-6989(96)00223-4. [DOI] [PubMed] [Google Scholar]
- 11.Ekstrom P, Sanyal S, Narfstrom K, Chader G J, van Veen T. Invest Ophthalmol Vis Sci. 1988;29:1363–1371. [PubMed] [Google Scholar]
- 12.Humayun M S, Prince M, de Juan E, Jr, Barron Y, Moskowitz M, Klock I B, Milam A. Invest Ophthalmol Vis Sci. 1999;40:143–148. [PubMed] [Google Scholar]
- 13.Falsini B, Iarossi G, Porciatti V, Merendino E, Fadda A, Cermola S, Buzzonetti L. Invest Ophthalmol Vis Sci. 1994;35:4282–4290. [PubMed] [Google Scholar]
- 14.Bowes C, Li T, Dancer M, Baxter L C, Applebury M L, Farber D. Nature (London) 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 15.Farber D B, Flannery J G, Bowes-Richman C. Prog Ret Eye Res. 1994;13:31–64. [Google Scholar]
- 16.Jimenez A J, Garcia-Fernandez J M, Gonzales B, Foster R G. Cell Tissue Res. 1996;284:193–202. doi: 10.1007/s004410050579. [DOI] [PubMed] [Google Scholar]
- 17.Usuda N, Kong Y, Hagiwara M, Uchida C, Terasawa M, Nagata T, Hidaka H. J Cell Biol. 1991;112:1241–1247. doi: 10.1083/jcb.112.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berrebi A S, Oberdick J, Sangameswaran L, Christakos S, Morgan J I, Mugnaini E. J Comp Neurol. 1991;308:630–649. doi: 10.1002/cne.903080409. [DOI] [PubMed] [Google Scholar]
- 19.Blanks J C, Adinolfi A M, Lolley R N. J Comp Neurol. 1974;156:95–106. doi: 10.1002/cne.901560108. [DOI] [PubMed] [Google Scholar]
- 20.Tagawa Y, Sawai H, Ueda Y, Tauchi M, Nakanishi S. J Neurosci. 1999;19:2568–2579. doi: 10.1523/JNEUROSCI.19-07-02568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda Y, Iwakabe H, Masu M, Suzuki M, Nakanishi S. J Neurosci. 1997;17:3014–3023. doi: 10.1523/JNEUROSCI.17-09-03014.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wässle H, Peichl L, Airaksinen M S, Meyer M. Cell Tissue Res. 1998;292:211–218. doi: 10.1007/s004410051052. [DOI] [PubMed] [Google Scholar]
- 23.Stone J L, Barlow W E, Humayun M S, de Juan E, Jr, Milam A H. Arch Ophthalmol. 1992;110:1634–1639. doi: 10.1001/archopht.1992.01080230134038. [DOI] [PubMed] [Google Scholar]
- 24.Mohand-Said S, Deudon-Combe A, Hicks D, Simonutti M, Forster V, Fintz A C, Leveillard T, Dreyfus H, Sahel J A. Proc Natl Acad Sci USA. 1998;95:8357–8362. doi: 10.1073/pnas.95.14.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kedzierski W, Bork D, Travis G H. J Neurosci. 1998;18:4076–4082. doi: 10.1523/JNEUROSCI.18-11-04076.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Vail M M, Sidman R L. Arch Ophthalmol. 1974;91:394–400. doi: 10.1001/archopht.1974.03900060406015. [DOI] [PubMed] [Google Scholar]
- 27.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D. The Molecular Biology of the Cell. New York: Garland; 1983. [Google Scholar]
- 28.Kamboj S, Huganir R L. Curr Biol. 1998;8:719–721. doi: 10.1016/s0960-9822(98)70457-9. [DOI] [PubMed] [Google Scholar]
- 29.Hsueh Y-P, Sheng M. Prog Brain Res. 1998;116:123–131. doi: 10.1016/s0079-6123(08)60434-3. [DOI] [PubMed] [Google Scholar]
- 30.Olsson J E, Gordon J W, Pawlyk B S, Roof D, Hayes A, Molday R S, Mukai S, Cowley G S, Berson E L, Dryja T P. Neuron. 1992;9:815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- 31.Humphries M M, Rancourt D, Farrar G J, Kenna P, Hazel M, Bush R A, Sieving P A, Sheils D M, McNally N, Creighton P, et al. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 32.Jeon C J, Strettoi E, Masland R H. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]