Abstract
Background
Although alveolar epithelial injury is a major determinant of outcome in patients with acute lung injury, there is no reliable biological marker of alveolar epithelial injury. The primary objective was to determine whether elevated levels of the receptor for advanced glycation end products (RAGE), a marker of alveolar epithelial injury, reflect impaired alveolar fluid clearance (AFC) in an ex vivo perfused human lung preparation. A second objective was to determine whether levels of a marker of endothelial injury, von Willebrand factor antigen (vWF:Ag), are associated with impaired AFC.
Methods
Human lungs (N = 30) declined for transplantation by the California Transplant Donor Network were perfused at a constant pulmonary artery pressure of 12 mm Hg. Following rewarming to 36°C, the lungs were inflated with a continuous positive airway pressure of 10 cm H2O. RAGE and vWF:Ag levels and AFC rates were then measured.
Results
The rate of AFC was inversely correlated with RAGE levels in the alveolar fluid (p < 0.005). Similarly, the concentration of RAGE in the alveolar fluid was significantly higher in lungs with submaximal AFC, defined in a prespecified analysis as ≤ 14%/h, when compared with lungs with preserved AFC (median 0.82 vs 0.43 μg/mL; p < 0.05). In contrast, vWF:Ag levels did not correlate with the rate of AFC.
Conclusions
RAGE may be a useful biological marker of alveolar epithelial injury and impaired AFC in donor lungs prior to transplant and perhaps in patients with acute lung injury.
Keywords: acute lung injury, alveolar epithelial fluid transport, alveolar epithelial permeability, ARDS, lung transplantation, primary graft failure, receptor for advanced glycation end products, von Willebrand factor
The scarcity of available grafts and the growing number of candidates waiting for lung transplantation have led to an increase of deaths in patients on waiting lists. Moreover, clinical criteria used to screen donor lungs for transplantation may be inadequate, resulting in the under-utilization of lungs for transplantation.1 Novel methods of selecting donor lungs are therefore urgently needed, particularly given recent trends in the utilization of lungs from marginal donors.2-4
An intact alveolar epithelial barrier with preserved alveolar fluid clearance (AFC) is associated with better clinical outcomes in patients with acute lung injury (ALI)5 and patients with post-transplant reperfusion pulmonary edema.6 Biomarkers that reflect alveolar epithelial injury may therefore be useful in selecting potentially viable lungs for donation. The receptor for advanced glycation end products (RAGE) is a recently described novel marker of alveolar type I epithelial cell injury with both prognostic and pathogenetic value in patients with ALI.7,8
In our recently developed ex vivo perfused human lung preparation, using human lungs declined for transplantation, we found that the airspace and perfusate levels of RAGE were elevated in donor lungs without measurable AFC (< 3%/h).9 However, the sample size for that study was small. Therefore, the primary objective of the present study was to prospectively determine if airspace or perfusate RAGE levels in potential donor lungs are associated with AFC rate. A secondary objective was to determine if levels of circulating von Willebrand Factor antigen (vWF:Ag), a previously identified biological marker of vascular endothelial injury, are associated with alveolar epithelial fluid transport.
Materials and Methods
Ex Vivo Human Lung Preparation
Lungs from brain-dead organ donors used for this study were rejected for transplantation by the Northern California Transplant Donor Network according to international criteria for human lung donors.10 The demographic data and the reasons for rejections were collected and analyzed. The lungs were prepared as previously described.9 Briefly, after consent from family was obtained, lungs from donors were removed en bloc, inflated, and transported on ice to the laboratory. The lungs were not perfused with a preservative solution prior to transport. The left lung was used for this study. The pulmonary artery and mainstem bronchus were cannulated and the lung was then perfused with Dulbecco’s modified Eagle’s medium with low glucose containing 5% bovine serum albumin using a peristaltic pump at a mean pulmonary artery pressure of 12 mm Hg. The pulmonary veins were not cannulated, and venous drainage was passive. The lung preparation was then suspended within a container surrounded by a heated (38°C) water jacket where the inner container served as a reservoir for the perfusate solution (900 mL). The temperature of the perfusate was continuously monitored and when the temperature of the venous drainage reached 36°C, the lungs were inflated with continuous positive airway pressure of 10 cm H2O with 95% O2 and 5% CO2. After a 15 min stabilization period, AFC was measured and airspace and perfusate samples were collected. Pulmonary artery pressure and airway pressure were continuously monitored using a computer-integrated data acquisition system (Biopac Inc.; Santa Barbara, CA), and the pH, Po2 and Pco2 tensions were measured in the perfusate (Bayer RapidLab 248; Bayer HealthCare LLC; Tarrytown, NY) every 30 min.
Measurement of Distal AFC
AFC was measured as previously described.9 Briefly, following the stabilization period, a catheter (PE 240 tubing; BD; Franklin Lakes, NJ) was passed through a side port in the endobronchial tube into the lung and advanced until gentle resistance was encountered. Then, 150 mL of warmed (36°C) normal saline containing 5% bovine serum albumin was instilled through the catheter into the airspaces of the lung. After 5 min (T = 0) and 35 min (T = 30 min), samples were removed through the catheter by gentle aspiration. The change in protein concentration at T = 30 min was used to determine the volume of fluid cleared from the airspaces by the following equation:
Where Ci is the protein concentration at T = 0 min and Cf is the protein concentration at T = 30 min.
Measurement of RAGE and vWF:Ag
Airspace and perfusate levels of RAGE were measured by an ELISA as previously described.7 vWF:Ag was measured in the alveolar fluid and in the perfusate medium using a commercially available ELISA (Asserachrom® vWF:Ag; Diagnostica Stago; Asnieres, France). vWF:Ag levels are expressed as the percent increase over a pooled plasma normal reference assayed against a secondary standard of the 02/150 International Standard of vWF.
Statistical Analysis
Statistical analysis was performed with software (StatView; SAS Institute; Cary, NC). Because biomarker levels were abnormally distributed, nonparametric methods were used. We used Spearman correlation coefficients to test the correlations between biomarker levels and other continuous variables, Mann-Whitney rank-sum tests for two-group comparisons, and the Kruskal-Wallis test for multi-group comparisons. We used linear regression models to determine the relation between RAGE levels and other continuous variables; for these analyses, RAGE levels were log-transformed in order to apply linear methods. Normally distributed data are presented as mean ± SE.
Results
Demographic Data and Procurement Times
The demographic data and procurement times for the 30 lungs used in this study are summarized in Table 1. All patients died from catastrophic CNS events, including intracranial hemorrhage and anoxic brain injury. Lungs were rejected for transplantation for a variety of reasons, most commonly due to concern for suspicion of pulmonary edema, infection, or airway inflammation. The mean rewarming time (4° to 36°C) with perfusion was 53 ± 2 min.
Table 1. Donor Clinical Demographic Data and Procurement Times*.
| Variables | Data |
|---|---|
| Age, yr | 38.4 ± 2.9 |
| Female gender, % | 50 |
| Cause of death, No./total | |
| Head trauma | 4/30 |
| Intracranial hemorrhage | 16/30 |
| Anoxic brain injury | 10/30 |
| Cause of graft rejection (several reasons may be associated), No./total | |
| Suspicion of infection (airway inflammation; bronchial secretions) | 10/30 |
| Infection history (hepatitis, HIV) | 8/30 |
| Suspicion of pulmonary edema (severe hypoxemia, chest radiograph abnormalities) | 18/30 |
| Cigarette smoking | 9/30 |
| Time from death to procurement, h | 46 ± 4 |
Data are presented as mean ± SE unless otherwise indicated.
AFC Rates, Hemodynamic Data, and Gas Tensions
Consistent with our previous studies, the mean AFC rate for all of the lungs studied (N = 30) was 17 ± 2%/h. According to the previously defined cutoff value of ≤ 14%/h to divide lungs into submaximal or preserved AFC,11 14 lungs had submaximal and 16 lungs had normal AFC. The mean AFC rate was 7 ± 1%/h in the submaximal group and 27 ± 2%/h in the preserved AFC group. There were no significant differences in gas exchange, pH, or pulmonary vascular resistance between the preserved and submaximal AFC groups (Table 2).
Table 2. Alveolar Fluid Clearance, Weight Gain, Hemodynamic, and Gas Exchange Variables During ex Vivo Lung Perfusion*.
| Measurements | All Lungs (n = 30) | Maximal AFC (> 14%/h) [n = 16] | Submaximal AFC (≤ 14%/h) [n = 14] |
|---|---|---|---|
| AFC, %/h | 17 ± 2 | 26 ± 2 | 7 ± 1 |
| Pao2/fraction of inspired oxygen | |||
| Initial | 214 ± 19 | 192 ± 15 | 239 ± 24 |
| Final | 188 ± 20† | 170 ± 16† | 209 ± 25† |
| pH | |||
| Initial | 7.51 ± 0.03 | 7.55 ± 0.05 | 7.47 ± 0.04 |
| Final | 7.47 ± 0.04† | 7.52 ± 0.05† | 7.42 ± 0.05† |
| Perfusion flow rate, L/min | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.26 ± 0.03 |
| Total pulmonary vascular resistance, mm Hg/L/min/100 g | |||
| Initial | 15.6 ± 3.3 | 19.4 ± 5.6 | 11.4 ± 2.8 |
| Final | 14.8 ± 3.3† | 18.4 ± 5.6† | 10.6 ± 2.8† |
Data are presented as mean ± SE.
Beginning of experiment vs end of experiment, p < 0.05.
RAGE Levels
RAGE concentrations globally differed according to the reasons of rejection, both in alveolar fluid (p < 0.005) [Fig 1] and in the perfusate (p < 0.02; Kruskal-Wallis test). More refined pairwise comparisons (Mann-Whitney test) failed to show which reason for rejection was statistically different from the others. Lungs with abnormal AFC had higher airspace RAGE levels than lungs with intact AFC (Fig 2). Specifically, lungs with an AFC rate below 14%/h had a median (interquartile range) RAGE level of 0.82 μg/mL (0.62 to 1.40 μg/mL) in the airspace fluid, while lungs with an AFC rate > 14%/h had a RAGE level of 0.43 μg/mL (0.30 to 0.66 μg/mL; p < 0.05). Similarly, when analyzed as a continuous variable, airspace RAGE levels were significantly and inversely correlated with AFC (Spearman r = - 0.55; p < 0.05; Fig 3, left, A).
Figure 1.

Alveolar fluid levels of RAGE according to the reason for lung rejection: The median is shown by the horizontal line within the box. The values between the lower and upper quartiles (25th to 75th centiles) are within the box. The whiskers represent the limits of the 90th and 10th centile values. Outliers are shown as filled circles (p < 0.005; Kruskal-Wallis test).
Figure 2.

RAGE levels and AFC. RAGE levels in alveolar fluid were significantly higher in lungs with submaximal AFC (≤ 14%/h, n = 14) than in lungs with preserved fluid clearance (> 14%/h, n = 16). The distinction between groups in terms of AFC rates was previously described in patients with hydrostatic pulmonary edema11 and in patients with ALI.5 RAGE levels in the alveolar fluid are expressed as median and centiles. The median is shown by the horizontal line within the box. The values between the lower and upper quartiles (25th to 75th centiles) are within the box. The whiskers represent the limits of the 90th and 10th centile values. Outliers are shown as filled circles (p < 0.05; Mann-Whitney U test).
Figure 3.

AFC and RAGE levels. The rate of AFC was inversely correlated with the level of RAGE in alveolar fluid (left, A) [p < 0.05; R = -0.55] but not with perfusate RAGE levels (right, B).
In contrast, perfusate RAGE was not significantly associated with AFC when analyzed as either a continuous (Fig 3, right, B) or dichotomous variable. Of note, levels of RAGE in the distal airspaces were significantly correlated with levels in the perfusate (r = 0.54; p < 0.005; Fig 4).
Figure 4.
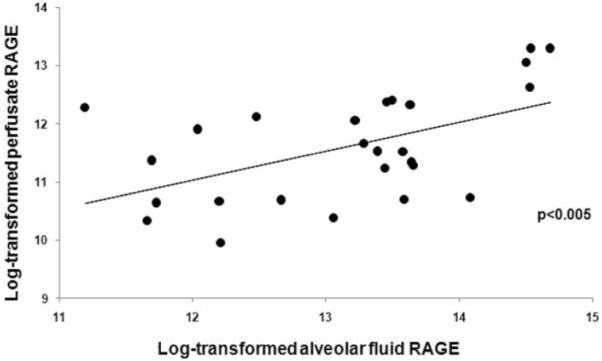
Correlation of alveolar fluid and perfusate levels of RAGE. Data have been log-transformed (natural logarithm). For every one-log increase in alveolar fluid RAGE, the perfusate level of RAGE increased by 0.5 log (p < 0.005); expressed differently, for every doubling in alveolar fluid RAGE, the concentration of RAGE in perfusate increased by 40%.
vWF:Ag Levels
AFC was not correlated with vWF:Ag levels in the airspace fluid or in the perfusate. There was also no significant difference in airspace or perfusate levels of vWF:Ag when analyzing AFC as a dichotomous variable (AFC ≤ 14%/h or > 14%/h).
Effect of Ischemic Time on AFC and RAGE
The duration of cold ischemia (time between lung procurement and the beginning of the experiment) was inversely correlated with the rate of AFC (R = -0.50, p < 0.05) [Fig 5, left, A] and positively correlated with the RAGE concentration in the alveolar fluid (R = 0.55, p < 0.01) [Fig 5, right, B].
Figure 5.

Effect of cold ischemia time on AFC and RAGE levels. The duration of cold ischemia (the time from procurement to experiment) was inversely correlated with the rate of AFC (R = -0.50; p < 0.05) [left, A] and positively correlated with the airspace levels of RAGE (R = 0.56; p < 0.01) [right, B].
Discussion
Clinical criteria used to evaluate donor lungs prior to transplantation may not be adequate as many lungs rejected for transplant appear to have normal alveolar epithelial function.1 Biological markers of lung endothelial and alveolar epithelial injury may have clinical value in determining the severity of alveolar barrier injury in potential transplant donor lungs or in patients with ALI. Prior studies have shown a relationship between elevated levels of vWF:Ag and poor clinical outcomes in ALI,12,13 and recent work has suggested that elevated plasma levels of RAGE may reflect the severity of alveolar epithelial injury in patients following lung transplantation.14 More importantly, intact alveolar barrier function has been associated with lower mortality in patients with ALI5 and in patients with posttransplant pulmonary edema.6
We recently evaluated the effect of perfusion and β-adrenergic agonist therapy on AFC in a novel preparation of ex vivo perfused human lungs.9 In that study, RAGE levels were measured in only nine lungs, but a marked elevation in RAGE levels was observed in three lungs without measurable AFC. Therefore, the main objective of this study was to prospectively determine if levels of RAGE correlated with AFC in a larger number of donor lungs (N = 30) declined for transplantation. Our primary finding was that elevated airspace levels of RAGE were significantly correlated with impaired AFC in lungs declined for transplantation.
RAGE is a member of the immunoglobulin super-family of cell surface molecules that has been implicated as a biological marker of cellular injury in multiple pathologic conditions such as diabetes, amyloidosis, and cancer, although the role of RAGE in the pathogenesis of these conditions is uncertain.15,16 Proteolysis of full-length RAGE during tissue injury accounts for the release of the soluble 48-kd isoform into the alveolar space. In the lung, the majority of soluble RAGE is released from the epithelium. Recent studies have demonstrated that RAGE expression in the lung is primarily limited to alveolar type I epithelial cells7,17 and RAGE is not expressed at high levels in human pulmonary vascular endothelial cell lines.7 Previous clinical studies have shown that RAGE levels are higher in pulmonary edema fluid from patients with ALI than in edema fluid from patients with hydrostatic pulmonary edema.7 In addition, plasma RAGE levels in ALI/ARDS patients are significantly higher than in healthy volunteers and patients with hydrostatic pulmonary edema. Therefore, an increase in RAGE levels may reflect injury to alveolar type I epithelial cells.
In the lungs we studied, the RAGE concentrations globally differed based on the reasons for rejecting the lungs for transplantation in patients. Because of the small number of lungs in each group we failed to show which reason for rejection is statistically different from the others. We hypothesize that there is a trend for higher RAGE levels in possibly injured lungs (suspicion of pulmonary edema, or suspicion of active infection or inflammation). The RAGE levels tend to be lower in lungs with no apparent gross injury but only a potential risk due to a previous history of infection (eg, hepatitis virus or HIV) or tobacco abuse.
While elevated levels of RAGE in the airspaces were associated with impaired AFC in our study, perfusate RAGE levels were not associated with alveolar epithelial function. The lack of significant correlation between perfusate RAGE and AFC in our data may be explained by the dilution, in the perfusion circuit, of RAGE proteins principally released in the alveolar compartment, which must then cross the injured capillary-alveolar barrier to reach the vascular compartment. In a previous study with this model,9 we found elevated levels of RAGE in the perfusion solution of lungs with essentially no intact AFC (20% of the studied lungs). The lungs we used in the present series were in better condition, as only 3% of the studied lungs had no evidence of net AFC. This difference between the two studies may be explained by the fact that we were more reluctant to use lungs that had more macroscopic evidence of injury in the current study, thus reducing the magnitude of RAGE measurements in the perfusate.
We also found that longer cold ischemic time was associated with higher levels of RAGE and submaximal AFC (Fig 5). Although some previous studies concluded that graft ischemic time was not an independent predictor of increased adverse outcomes18,19 except with older donors,20 other studies have shown that lung dysfunction and clinical outcomes may be influenced by cold ischemic time. For example, Fischer et al21 reported an association between cold preservation times and post-transplant lung function in rat lung transplants. In addition, Ware et al6 reported a correlation between graft ischemic time and capillary-alveolar protein permeability in patients with reperfusion edema after lung transplantation. Snell et al22 studied 106 transplant patients and reported poorer outcomes with graft ischemic times beyond 5 h. Finally, Thabut et al23 found, in a cohort of 505 patients with lung transplantation, a close relationship between graft ischemic time and both early gas exchange and long-term survival.
The lungs that we received were not used for lung transplantation for a variety of clinical reasons, thus creating a natural experiment in which ischemia time varied substantially. Based on prior studies,1,9 we knew that these lungs may be normal, mildly injured, or more severely injured. Thus we designed this study of AFC in the human lung anticipating that there would be a spectrum of normal to injured lungs and taking advantage of the various degrees of injury to test for a correlation between biomarkers and alveolar epithelial function, as reflected by the rate of AFC. The correlations demonstrated in this study between RAGE levels and both AFC and ischemia time suggest that increased ischemia time negatively impacts alveolar epithelial function.
The lack of correlation between airspace and perfusate vWF:Ag levels and AFC serves as a useful negative control for our studies of RAGE. Plasma vWF:Ag levels are an early biochemical marker of endothelial injury and predict the development of ALI in patients with nonpulmonary sepsis syndrome.13,24 The degree of endothelial injury is associated with poorer outcomes in patients with ALI. In a study of 559 adult patients with ALI/ARDS, plasma levels of vWF:Ag were significantly higher in non-survivors.13 Flori et al25 found that plasma vWF:Ag levels in pediatric patients with ALI were associated with an increased risk of death and prolonged mechanical ventilation. In the present study, AFC was not associated with circulating vWF:Ag levels. Consistent with our previous study,9 these data suggest that lung endothelial injury is not a major determinant of AFC in this preparation. Separate measures of markers of endothelial and epithelial injury may provide additional information on the severity of lung injury and improve pretransplant assessment of donor lungs.
This study has some limitations. First, the number of human lungs available for study is by necessity rather small; thus, outlying data points may significantly impact the results. To address this issue, we used nonparametric statistical methods, which analyze the distribution of data based on rank rather than absolute value. In addition, the small sample size limits our ability to draw conclusions about nonsignificant associations, due to the risk of a type II error. Second, the method of lung preservation used in this study differs from methods used in studies by other groups,2-4,26 most of which were designed to keep lungs in perfect condition for possible re-transplantation. In these studies, the lungs were flushed with preservation solutions during the procurement surgery and were reperfused with a physiologic solution with RBCs. These differing methods of preservation may affect biomarker levels in either the airspaces, the perfusate, or both.
Clinical criteria inadequately predict preserved AFC and graft function in donor lungs. We found that elevated airspace RAGE levels were a marker for impaired AFC in donor lungs rejected for transplant. These data support the hypothesis that RAGE is a valuable biological marker of alveolar epithelial injury and function.
Several articles have been recently published about successful transplantation of lungs from marginal donors (ie, non-heart-beating donors or lungs previously rejected for transplantation).2-4 In these publications, evaluation criteria are based on hemodynamic values and gas exchange capacities of the lungs. In this context, clinically relevant biomarkers of lung injury may be useful in the assessment of lungs prior to transplantation, as well as in selecting patients for clinical trials with ALI.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute HL51856 and HL088263 (Dr. Matthay), HL88440 (Dr. Frank), and Egide-Programme Lavoisier (Ministère Français des Affaires Etrangères) [Dr. Briot]. Dr. Calfee is supported by a National Heart, Lung, and Blood Institute K23 award (HL090833).
Abbreviations
- AFC
alveolar fluid clearance
- ALI
acute lung injury
- RAGE
receptor for advanced glycation end products
- T
time
- vWF:Ag
von Willebrand factor antigen
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Ware LB, Wang Y, Fang X, et al. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet. 2002;360:619–620. doi: 10.1016/s0140-6736(02)09774-x. [DOI] [PubMed] [Google Scholar]
- 2.Steen S, Sjoberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825–829. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- 3.de Antonio DG, Marcos R, Laporta R, et al. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant. 2007;26:529–534. doi: 10.1016/j.healun.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Steen S, Ingemansson R, Eriksson L, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83:2191–2194. doi: 10.1016/j.athoracsur.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 6.Ware LB, Golden JA, Finkbeiner WE, et al. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. Am J Respir Crit Care Med. 1999;159:980–988. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]
- 7.Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end-products and clinical outcomes in acute lung injury. Thorax. 2008 Jun 19; doi: 10.1136/thx.2008.095588. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank JA, Briot R, Lee JW, et al. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L52–L59. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orens JB, Boehler A, de Perrot M, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22:1183–1200. doi: 10.1016/s1053-2498(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 11.Verghese GM, Ware LB, Matthay BA, et al. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol. 1999;87:1301–1312. doi: 10.1152/jappl.1999.87.4.1301. [DOI] [PubMed] [Google Scholar]
- 12.Ware LB, Conner ER, Matthay MA. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med. 2001;29:2325–2331. doi: 10.1097/00003246-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Ware LB, Eisner MD, Thompson BT, et al. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 14.Calfee CS, Budev MM, Matthay MA, et al. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant. 2007;26:675–680. doi: 10.1016/j.healun.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt AM, Yan SD, Yan SF, et al. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 17.Shirasawa M, Fujiwara N, Hirabayashi S, et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells. 2004;9:165–174. doi: 10.1111/j.1356-9597.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- 18.Gammie JS, Stukus DR, Pham SM, et al. Effect of ischemic time on survival in clinical lung transplantation. Ann Thorac Surg. 1999;68:2015–2019. doi: 10.1016/s0003-4975(99)00903-0. discussion 2019-2020. [DOI] [PubMed] [Google Scholar]
- 19.Fiser SM, Kron IL, Long SM, et al. Influence of graft ischemic time on outcomes following lung transplantation. J Heart Lung Transplant. 2001;20:1291–1296. doi: 10.1016/s1053-2498(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 20.Meyer DM, Bennett LE, Novick RJ, et al. Effect of donor age and ischemic time on intermediate survival and morbidity after lung transplantation. Chest. 2000;118:1255–1262. doi: 10.1378/chest.118.5.1255. [DOI] [PubMed] [Google Scholar]
- 21.Fischer S, Maclean AA, Liu M, et al. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia-reperfusion injury in lung transplantation. Am J Respir Crit Care Med. 2000;162:1932–1939. doi: 10.1164/ajrccm.162.5.9910064. [DOI] [PubMed] [Google Scholar]
- 22.Snell GI, Rabinov M, Griffiths A, et al. Pulmonary allograft ischemic time: an important predictor of survival after lung transplantation. J Heart Lung Transplant. 1996;15:160–168. [PubMed] [Google Scholar]
- 23.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–791. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 24.Rubin DB, Wiener-Kronish JP, Murray JF, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flori HR, Ware LB, Milet M, et al. Early elevation of plasma von Willebrand factor antigen in pediatric acute lung injury is associated with an increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med. 2007;8:96–101. doi: 10.1097/01.PCC.0000257097.42640.6F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egan TM, Haithcock JA, Nicotra WA, et al. Ex vivo evaluation of human lungs for transplant suitability. Ann Thorac Surg. 2006;81:1205–1213. doi: 10.1016/j.athoracsur.2005.09.034. [DOI] [PubMed] [Google Scholar]


