Abstract
Background
The mechanism of human immunodeficiency virus (HIV) transmission via heterosexual intercourse is unknown. We sought to determine whether the presence of inflammatory cells in the vagina is associated with the presence of genital tract HIV type 1 (HIV-1) RNA.
Methods
Analysis of a longitudinal prospective cohort was performed. Women with HIV-1 infection were assessed with use of paired plasma and cervicovaginal lavage specimens. Viral load measurements were performed using nucleic acid sequence—based amplification. White blood cells found in the genital tract (GT WBCs) were quantified using a hemacytometer. Common lower genital tract infections assessed for association with viral shedding (i.e., genital tract viral load [GTVL]) included bacterial vaginosis, candidiasis, and trichomoniasis. Generalized estimating equations were used to estimate the prevalence and odds of detectable GTVL by GT WBC. The association was examined both in the presence and in the absence of lower genital tract infections.
Results
A total of 97 women and 642 visits were included in the analysis. Median duration of follow-up was 30.4 months. Thirty women (31%) had detectable GTVL at any visit. The median CD4 cell count at baseline was 525 cells/μL. Most women were antiretroviral therapy naive at baseline. After adjustment for plasma viral load, the odds of detectable GTVL increased as GT WBC increased, with an odds ratio of 1.36 (95% confidence interval, 1.1–1.7) per 1000-cell increase in GT WBC among women without lower genital tract infections. After adjustment for plasma viral load and lower genital tract infections by incorporating them in a regression model, GT WBC remained significantly associated with GTVL, with an adjusted odds ratio of 1.22 (95% confidence interval, 1.08–1.37).
Conclusions
The presence of GT WBC is associated with an increased risk of detectable GTVL.
Exposure via heterosexual intercourse is the predominant mode of transmission of HIV-1 infection world-wide [1]. Although heterosexual HIV transmission appears to be inefficient [2], women are twice as likely as men to contract HIV infection during vaginal intercourse [3]. However, the exact source of HIV from the infected partner and the target cell in the mucosa of the recipient are still not known. Since the isolation of HIV-1 in the female genital tract (GT) was first reported in 1986 [4], many subsequent studies have evaluated the presence, the amount, and the determinants of HIV-1 shedding in cervicovaginal secretions in the form of cell-free HIV-1 RNA, cell-associated HIV-1 RNA, proviral DNA, or culturable virus [5-7]. Some factors that increase GT HIV-1 shedding include alterations in the vaginal environment, such as certain genital infections [2, 8]; oral contraceptive use [3]; and pregnancy [4]. Virological and immunologic cofactors, such as plasma HIV-1 RNA concentration and CD4 cell count, also influence GT shedding [6, 9]. Unfortunately, there is a paucity of data assessing the relationship of female GT HIV-1 shedding to the presence of leukocytes in the absence of other GT infections.
The lower female GT is generally thought to be the primary site of female HIV-1 infection. Much of the previous research on HIV transmission was devoted to studying the relationship between lower GT infections (LGTIs) and the transmission of HIV. In women, cervical and vaginal ulcers have been associated with increased detection of HIV-1 in cervicovaginal secretions [7], and genital ulcerative disease was found to be a major determinant of HIV-1 transmission via the coital act [5]. Nonulcerative LGTIs increase HIV-1 shedding as well. Cross-sectional studies have demonstrated increased HIV-1 prevalence among women with signs of mucopurulent cervicitis caused by Neisseria gonorrhoeae and Chlamydia trachomatis [10], vaginal candidiasis [3], trichomoniasis [11], and bacterial vaginosis [1].
However, the presence of HIV-1 shedding in the female GT is not completely explained by plasma viral load (PVL) and commonly diagnosed LGTIs. Although PVL is a significant driver of genital viral shedding, there is strong evidence supporting the idea of compartmentalization, which might account for differences between GT and PVL [12]. The epithelium of the uterine cervix and underlying stromal constituents of the endo- and ectocervix change throughout a woman’s lifetime as a result of hormonal, physical, and infectious influences [13]. HIV first infects the Langherhans cells located in the epithelia of the vagina, as well as T cells, macrophages, and dendritic cells in the subepethial tissues [14]. Once in the genital mucosa, HIV leads to a decrease in CD4 cells and an increase in CD8 cells [15]. Proinflammatory cytokines, such as TNF-α, IL-1, and IL-10, are also produced by these infected leukocytes [16]. The cytokines can both attract more target cells for HIV and stimulate HIV expression in these cells through toll-like receptors [17]. In addition, IL-10 has been demonstrated to enhance the induction of HIV-1 replication in macrophages, and IL-10—stimulated monocytes may be more efficiently infected by HIV [18].
Our previous work has determined the pattern of HIV-1 RNA in paired plasma and cervicovaginal lavage (CVL) measurements during 36 months of observation and that PVL was the strongest predictor of HIV-1 RNA detection in CVL fluid [19]. In the present analysis, we assessed the relationship between leukocyte count in CVL fluid and HIV-1 shedding in the female GT among women with and women without diagnosed common LGTIs.
METHODS
A secondary analysis of a longitudinal prospective cohort was performed. The original cohort study was an observational study designed to evaluate the effect of antiretroviral therapy and factors associated with genital HIV shedding [19]. Women with HIV-1 were observed over a 36-month period by a process of serial paired plasma and CVL specimens. Women >18 years of age who had a uterus and cervix and who were receiving care for HIV infection at The Miriam Hospital (Providence, RI) or The Memorial Hospital of Rhode Island (Pawtucket) were offered inclusion in the study. Participants signed written, informed consent forms, and the study protocol was approved by the institutional review boards at both facilities. Paired plasma and CVL specimens were collected at each visit. Visits occurred at baseline, 2 weeks later, 1 month after baseline, and every 6 months thereafter. Participants were advised to abstain from sexual intercourse, use of vaginal douches, and use of other intravaginal products for ≥48 hours before examinations.
At the baseline interview, demographic, medical, sexual, and reproductive histories were collected. At each visit, CVL and plasma samples were collected. Examinations were scheduled to occur at the midpoint of the menstrual cycle, to decrease the variability that can be seen with hormonal changes. Subjects with symptomatic vaginal discharge were not included and were referred for clinical assessment. For collection of the CVL fluid, a speculum was placed in the vagina while the subject was in the dorsal lithotomy position, and the cervix was visualized. Ten cubic centimeters of normal saline was instilled into the vaginal vault, and the stream of fluid was directed at the cervical os. The fluid was allowed to pool in the posterior fornix for ∼30 s and then was aspirated. With use of this technique, 9–10 cc of fluid was typically recovered.
HIV-1 load measurements were performed using nucleic acid sequence—based amplification (bioMérieux). The lower limit of detection for this test was 400 copies/mL. Cell counts were performed by the hemacytometer method. Cells were counted in a standard fashion, using five 1-mm squares, and counts were averaged. Bacterial vaginosis was diagnosed using Amsel’s criteria [20]. Gonococcal and chlamydial infections were diagnosed using culture. The presence of semen was detected using a commercially available test (Abacus Diagnostics). Trichomoniasis was determined with wet mount and visualization of motile trichomonads. Candidal infection was determined by the presence of vaginal symptoms and the presence of hyphae and/or budding yeast on a potassium hydroxide slide. All slides were read by experienced microscopists within 15 min of collection. Syphilis was diagnosed using rapid plasma reagin. Gonococcal, chlamydial, and syphilitic screening was performed only annually because of a low prevalence of such infections. All other tests, including pregnancy tests, were performed at each visit.
All data related to visits at which results positive for pregnancy or semen were obtained were excluded from the analysis, to eliminate these factors as a source of GT WBCs or as a cause of inflammation. In the analysis of the relationship between genital viral shedding and GT WBCs in the absence of infection, data relative to visits with evidence of bacterial vaginosis, candidiasis, trichomoniasis, gonorrhea, chlamydia, or syphilis infections were excluded. For the analysis of the relationship between genital viral shedding and GT WBCs in the presence of LGTIs, data relative to visits with these documented LTGIs were included. Because of the low prevalence of gonococcal, chlamydial, and syphilitic infections in the cohort, we could not examine the influence of these infections on GT HIV-1 shedding. GT viral load (GTVL) and PVL measurements were dichotomized as detectable (>400 copies/mL) or undetectable.
We used a receiver operating characteristic (ROC) curve to determine the ability of GT WBC count to correctly classify visits as involving or not involving detectable GT viral shedding, and we used bootstrapping to calculate 95% CIs of the area under the ROC curve, with each patient as the basic resampling unit. In addition, regression models for longitudinal binary data were fitted using generalized estimating equations to examine the odds of GT shedding as a function of GT WBC count alone and after controlling for other potential predictors of GT shedding, which included age, ethnicity, HAART use, and detectable PVL. Models were fitted using subsets of visits with and without evidence of LGTIs, as described above. Statistical significance was determined, using robust SEs, at α = .05.
RESULTS
The data of our study comprise results of 642 visits by 97 women. The baseline demographic and clinical characteristics of the cohort are shown in table 1. Additional details about the original cohort were published elsewhere [19]. After exclusion of data relative to visits at which LGTIs or semen were detected, there were 257 visits by 89 women. The median GT WBC count was 260 cells per μL of cervicovaginal fluid (range, 0–16,600 cells/μL). At 64% of the visits at which GT WBCs were detected, neutrophils were the only leukocyte type detected in the CVL fluid. Macrophages were detected in 17% of samples, lymphocytes were detected in 20%, eosinophils were detected in 17%, basophils were detected in 0%, and other leukocytes were detected in 2.4%. At baseline, only 13.7% of subjects were receiving HAART. However, during the course of the study, 48.9% of visits involved women receiving any HAART. The regimens included nucleoside reverse-transcriptase inhibitors, such as zidovudine, lamivudine, and/or stavudine; nonnucleoside reverse-transcriptase inhibitors, such as efavirenz and nevirapine; and protease inhibitors, such as indinavir, nelfinavir, or saquinavir.
Table 1.
Baseline demographic and clinical characteristics of the longitudinal prospective cohort of women with HIV-1 infection.
| Variable | Patients (n = 97) |
|---|---|
| Age, median years (range) | 35 (20–58) |
| Ethnicity | |
| Black | 32 (33) |
| White | 35 (36) |
| Latina | 28 (29) |
| Detectable GTVL at any visit | 30 (31) |
| Baseline CD4 cell count, median cells/μL (range) |
446 (4–1517) |
| Plasma viral load, median log10 copies/mL (range) |
3.8 (<2.6 to 6.0) |
| Follow-up duration, median months (range) | 30.4 (0–36) |
| Antiretroviral naive at baseline | 67 (69) |
| STI (detected at ≥1 visit) | |
| Bacterial vaginosis | 73 (75) |
| Candidiasis | 57 (59) |
| Trichomoniasis | 21 (22) |
| Gonococcal infection | 0 (0) |
| Chlamydial infection | 0 (0) |
| Syphilis | 0 (0) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. GTVL, genital tract viral load; STI, sexually transmitted infection.
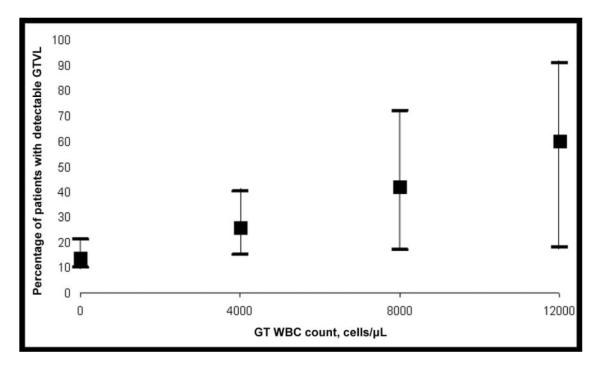
The logistic regression model that regressed GTVL on GT WBC count alone—controlling for age, ethnicity, HAART use, CD4 cell count, and PVL—fit the data well. The ROC curve indicated that GT WBC count alone is moderately able to distinguish between samples that were positive and those that were negative for GTVL; the area under the ROC curve was 0.66, with a bootstrapped 95% CI of 0.56–0.75. The unadjusted OR of detectable GTVL was estimated to be 1.20 (95% CI, 1.02–1.42) per 1000-cell/μL increase in GT WBC count. Eighty-six women with 240 observations contributed to the multivariate logistic regression analysis. Age, ethnicity, CD4 cell count, and HAART use did not statistically significantly account for variability in GTVL shedding in the multivariate model, but GT WBC count and PVL were statistically significantly associated with GTVL shedding (table 2). The predicted prevalence of GT shedding increased substantially as GT WBC count increased (figure 1).
Table 2.
Multiple logistic regression—model significant predictors of detectable genital tract (GT) viral load among women without detectable genital infections.
| Variable | Adjusted OR (95% CI) |
|---|---|
| GT WBC count, per 1000-cell/μL increase |
1.47 (1.12–1.92) |
| Detectable plasma viral load | 17.5 (2.5–121.6) |
| Receipt of HAART | 0.78 (0.27–2.29) |
| CD4 cell count, per 100-cell/μL increase | 0.87 (0.74–1.03) |
| Age, per year increase | 1.0 (0.94–1.06) |
| Ethnicity | |
| Latina versus black | 0.95 (0.37–2.42) |
| White versus black | 0.75 (0.27–2.05) |
NOTE. Adjusted ORs were calculated on the basis of 240 visits by 86 women.
Figure 1.
Predicted prevalence of genital tract viral load (GTVL) with increasing genital tract WBC (GT WBC) counts among women without detectable genital infections.
When visits involving diagnosed LGTIs were included in the analysis, there were 642 observations from 97 women. The median GT WBC count of these women was 340 cells/μL (range, 0–85,000 cells/μL). The prevalence of the infections detected in this cohort is shown in table 1. No gonococcal, syphilitic, or chlamydial infection was detected in any woman in the cohort; therefore, we could not examine the influence of these infections on GT HIV-1 shedding. Results from a multivariate regression analysis that included sexually transmitted infections and GT WBC count indicated that none of the detected LGTIs was independently associated with the presence of GTVL after controlling for the presence of GT WBCs (table 3). In an analysis that included GTVL and other covariates, the presence of GT WBCs was independently associated with the presence of GTVL, even after controlling for LGTIs and the known strongest predictor of GT viral shedding, PVL. In addition, we found that the odds of detectable GTVL were lower among women with higher CD4 cell counts. The relationship between LGTI and GTVL was examined both as single infections (table 3) and as a group (data not shown), and our analysis showed that the relationship was not statistically significant in either form. In bivariate analyses that examined the association between the presence of GT WBCs and each of the LTGIs, only trichomoniasis was statistically significantly associated with the presence of GT WBCs (P < .001).
Table 3.
Multiple logistic regression—model results among women with detectable genital infections.
| Variable | Adjusted OR (95% CI) |
|---|---|
| Genital WBCs | 1.25 (1.08–1.44)a |
| Bacterial vaginosis | 1.00 (0.57–1.75) |
| Candida infection | 1.19 (0.64–2.21) |
| Trichomoniasis | 1.09 (0.29–4.09) |
| Detectable semen | 1.34 (0.30–5.99) |
| Detectable plasma viral load | 26.1 (7.32–93.1)a |
| Receipt of HAART | 1.03 (0.54–1.96) |
| CD4 cell count, per 100-cell/μL increase | 0.85 (0.76–0.96)a |
| Age, per year increase | 0.97 (0.93–1.01) |
| Ethnicity | |
| Latina versus black | 0.96 (0.43–2.10) |
| White versus black | 0.96 (0.45–2.05) |
NOTE. Adjusted ORs were calculated on the basis of 536 visits by 94 women.
P<.05.
DISCUSSION
This study examined the association between GT WBC count as a marker of inflammation and the genital shedding of HIV-1 among HIV-infected women, in both the presence and the absence of diagnosed common LTGIs. We found that the presence of GT WBCs was an important predictor of viral shedding, independent of the presence of infections. In addition, we did not find that the individual infections were independently associated with increased viral shedding, after controlling for GT WBC count. This finding was unanticipated; because of the epidemiologic associations between the presence of sexually transmitted infections and HIV infection that are discussed in the literature [8, 21], we suspected that LGTIs would cause increased viral shedding. Our data suggest that it may not be the infections themselves but the inflammation caused by infections that drives the viral shedding. This concept is further supported by the fact that we found that there was increased viral shedding with increased GT WBC count, even in the absence of LTGIs.
The source of such elevated GT WBC counts in the absence of infections is unclear. One possible explanation may be associated with the fact that GT WBCs are known to be present throughout the female GT. In our study, the WBCs were mostly neutrophils. It is known that the distribution of these cells differs in various tissues of the GT. In general, there are higher numbers of neutrophils present in more-proximal tissues, and the number of neutophils decreases in the more distal GT [22]. They tend to increase in number in response to epithelial injury and chemokines. Neutrophils release collagen-degrading enzymes that can injure tissue. It is possible that the tissue injury allows for more expression of HIV RNA. Alternatively, IL-8, a potent chemokine produced by neutrophils, is known to increase HIV-replication in vitro [23]. The increase in HIV shedding could be attributable to cytokines and chemokines produced by neutrophils of certain patients. One could examine this possibility with a large cohort study that included women who experienced HIV seroconversion. Our study was not designed to determine causality.
Cummins et al. [24] examined women with HIV-1 infection and also found that high leukocyte counts in the fluid were associated with increased shedding of HIV-1. They reported certain factors that were associated and correlated with high leukocyte levels in the vaginal fluid. They found that the level of lactoferrin, an endogenous antimicrobial peptide that functions as part of innate immunity and is released by leukocytes, was found at higher levels in women with both high vaginal leukocyte counts and high viral loads. Interestingly, they also found that levels of gp340, a glycoprotein that functions in preventing HIV infectivity of the host, were also increased in the same setting. They did not, however, find that cytokine or chemokine concentrations were increased in this group of women [24]. A study examining factors associated with HIV shedding in men found that advanced disease, low CD4 cell counts, and high seminal leukocyte counts were independently associated with viral shedding [25].
Our study examined the association between types of infection that have previously been epidemiologically linked to HIV seroconversion [21] and HIV shedding [8] (specifically, trichomoniasis and bacterial vaginosis). We did not find an independent association between these infections and GTVL shedding; rather, we found that GT WBCs appear to be driving the association, at least in the case of trichomoniasis. We were unable to identify a source of the WBCs, despite testing for several common infections that have been linked to HIV transmission and acquisition. Laga et al. [21] found that other non-ulcerative LTGIs were associated with an increased risk of HIV seroconversion, particularly gonococcal and chlamydial infections. Although it has been shown that treatment of cervicitis decreases shedding of HIV in the GT [26, 27], there were no women with symptomatic cervicitis or gonococcal or chlamydial infections in our population; therefore, we could not draw conclusions about the relationship between these infections and GTVL. Both infections are typically associated with high GT WBC counts as a result of the cervicitis that they cause.
Strengths of our study include the longitudinal nature of the sample collection. We carefully selected the time frame for specimen collection and data elements, to control for potential confounding variables, such as PVL and use of medications. We excluded women who had recently used vaginal products or had vaginal sexual intercourse, thereby decreasing other potential confounders of genital shedding. We were able to show the independent association between GTVL and GT WBC counts after controlling for confounders, including PVL, which is the strongest predictor of genital shedding [19]. We also used statistical analysis for repeated measures and created our model’s confidence intervals with the bootstrapping method, which uses the patient as the basic resampling unit so that statistical independence is not violated.
There were several limitations to our study. The LTGIs were included or excluded from the analysis on the basis of tests with imperfect sensitivity and specificity. The wet-mount method was used to diagnose trichomoniasis, bacterial vaginosis, and vulvovaginal candidiasis. It is possible that there were some missed cases of these infections. The use of more-sensitive tests, such as culture for trichomoniasis and Candida infection and Gram stain and Nugent scores for bacterial vaginosis, may have improved the detection of these common LGTIs. Recently, bacterial PCR quantification has been shown to correlate better with GT HIV RNA levels, but this method is not yet clinically available [28]. The use of practical clinic-based diagnosis for LGTIs, such as wet mount, however, makes the findings of this study very applicable in clinical settings.
We also did not collect information regarding asymptomatic shedding of herpes simplex virus, which has been associated with genital shedding of HIV [29]. To our knowledge, however, no data have been published that document an association between asymptomatic herpes simplex virus infection and the presence of increased numbers of genital leukocytes. There were no cases of gonorrhea, chlamydia, or syphilis in this cohort of older HIV-infected women. These findings may not be applicable to younger women with higher rates of sexually transmitted diseases. We also did not test for nonspecific causes of cervicitis, such as Mycoplasma species, but we did exclude collection from patients with symptomatic vaginal discharge.
We used CVL, a technique that has been criticized for having a dilutional effect. We also determined GTVL with the use of HIV-1 RNA; others prefer the use of proviral DNA for measurement of GTVL. We also did not examine other inflammatory markers, such as cytokine levels, to determine the extent of inflammation that was related to the presence of the WBCs. We did not control for HIV subtype, but we believe that most of our subjects had clade B disease, because of the country of origin of enrolled subjects. Consent forms were available only in English and Spanish. The majority of subjects contracted their disease in the United States.
In summary, this analysis shows that GT WBC counts are independently associated with GTVL. Implications of this data include the possible usefulness of using GT WBC counts as a surrogate marker for the presence of GT HIV. Currently, there are no commercially available tests for detection of GT HIV. Future directions include determining the relationship between asymptomatic genital herpes simplex virus infection and the presence of genital leukocytes in the context of HIV shedding, as well as elucidating the mechanism by which GT WBCs upregulate the shedding of virus.
Acknowledgments
Financial support. Brown Medical School—Women & Infants’ Hospital of Rhode Island Women’s Reproductive Health Research (Career Development Program K12 HD050108 through the National Institutes of Health), National Institutes of Health (R01 AI40350, K24 AI066884, and P30 AI42853), and Emory Center for AIDS Research (P30 AI050409).
Footnotes
Presented in part: 34th Annual Meeting of the Infectious Diseases Society for Obstetrics and Gynecology, Boston, Massachusetts, August 2007; and 45th Annual Meeting of the Infectious Diseases Society of America, San Diego, California, October 2007 (abstract 1452).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Cu-Uvin S, Hogan JW, Caliendo AM, Harwell J, Mayer KH, Carpenter CCJ. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis. 2001;33:894–6. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 2.Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001;183:1017–22. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- 3.Mostad SB, Overbaugh J, DeVange DM, et al. Hormonal contraception, vitamin A deficiency, and other risk factors for shedding of HIV-1 infected cells from the cervix and vagina. Lancet. 1997;350:922–7. doi: 10.1016/S0140-6736(97)04240-2. [DOI] [PubMed] [Google Scholar]
- 4.Clemetson DB, Moss GB, Willerford DM, et al. Detection of HIV DNA in cervical and vaginal secretions: prevalence and correlates among women in Nairobi, Kenya. JAMA. 1993;269:2860–4. [PubMed] [Google Scholar]
- 5.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs A, Wasserman SS, Burns D, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358:1593–601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 7.Rottingen J-A, Cameron D William, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis. 2001;28:579–97. doi: 10.1097/00007435-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Cu-Uvin S, Hogan JW, Caliendo AM, Harwell J, Mayer KH, Carpenter CC. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis. 2001;33:894–6. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs A, Chan LS, Chen ZC, et al. HIV-1 RNA in plasma and genital tract secretions in women infected with HIV-1. J Acquir Immune Defic Syndr. 1999;22:124–31. doi: 10.1097/00126334-199910010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Ghys PD, Fransen K, Diallo MO, et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote d’Ivoire. AIDS. 1997;11:F85–93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 11.McClelland RS, Sangare L, Hassan W, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 12.Neely MN, Benning L, Xu J, et al. Cervical shedding of HIV-1 RNA among women with low levels of viremia while receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:38–42. doi: 10.1097/01.qai.0000248352.18007.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17:455–80. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- 14.Bergmeier LA, Lehner T. Innate and adaptive mucosal immunity in protection against HIV infection. Adv Dent Res. 2006;19:21–8. doi: 10.1177/154407370601900106. [DOI] [PubMed] [Google Scholar]
- 15.Miller CJ, Kang DW, Marthas M, et al. Genital secretory immune response to chronic simian immunodeficiency virus (SIV) infection: a comparison between intravenously and genitally inoculated rhesus macaques. Clin Exp Immunol. 1992;88:520–6. doi: 10.1111/j.1365-2249.1992.tb06481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sha BE, D’Amico RD, Landay AL, et al. Evaluation of immunologic markers in cervicovaginal fluid of HIV-infected and uninfected women: implications for the immunologic response to HIV in the female genital tract. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:161–8. doi: 10.1097/00042560-199711010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Alfano M, Poli G. The cytokine network in HIV infection. Curr Mol Med. 2002;2:677–89. doi: 10.2174/1566524023361925. [DOI] [PubMed] [Google Scholar]
- 18.Cohen CR, Plummer FA, Mugo N, et al. Increased interleukin-10 in the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS. 1999;13:327–32. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 19.Cu-Uvin S, Snyder B, Harwell JI, et al. Association between paired plasma and cervicovaginal lavage fluid HIV-1 RNA levels during 36 months. J Acquir Immune Defic Syndr. 2006;42:584–7. doi: 10.1097/01.qai.0000229997.52246.95. [DOI] [PubMed] [Google Scholar]
- 20.Amsel R, Totten PA. Non-specific vaginitis: diagnostic criteria and microbiologic and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 21.Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Givan AL, White HD, Stern JE, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–9. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 23.Narimatsu R, Wolday D, Patterson BK. IL-8 increases transmission of HIV type 1 in cervical explant tissue. AIDS Res Hum Retroviruses. 2005;21:228–33. doi: 10.1089/aid.2005.21.228. [DOI] [PubMed] [Google Scholar]
- 24.Cummins JE, Christensen L, Lennox JL, et al. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses. 2006;22:788–95. doi: 10.1089/aid.2006.22.788. [DOI] [PubMed] [Google Scholar]
- 25.Anderson DJ, O’Brien TR, Politch JA, et al. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. JAMA. 1992;267:2769–74. [PubMed] [Google Scholar]
- 26.Marrazzo JM, Martin DH. Management of women with cervicitis. Clin Infect Dis. 2007;44(Suppl 3):S102–10. doi: 10.1086/511423. [DOI] [PubMed] [Google Scholar]
- 27.McClelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–10. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 28.Sha BE, Zariffard MR, Wang QJ, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 29.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]