Abstract
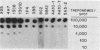
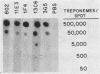
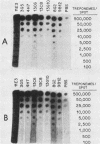
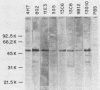
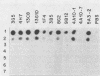
Murine anti-Treponema pallidum monoclonal antibodies were employed in studies on sensitivity and specificity of binding to examine their potential for use in the detection of low numbers of pathogenic treponemes present in various body fluids. Monoclonal antibodies were used as a primary antibody source in a solid-phase immunoblot assay system. All monoclonal antibodies assayed were capable of detecting ca. 1.0 X 10(3) to 2.5 X 10(3) treponemes. Of 13 monoclonal antibodies examined, 3 were able to detect 10(3) virulent treponemes, and 1 of these antibodies was able to reveal the presence of as few as 500 organisms. Western blot analyses showed that all anti-T. pallidum monoclonal antibodies exhibiting high sensitivities for the detection of T. pallidum cells were directed against an abundant, 47,000-dalton surface-exposed antigen of the organism (S. A. Jones, K. S. Marchitto, J. N. Miller, and M. V. Norgard, Abstr. Annu. Meet. Am. Soc. Microbiol. 1984, B173, p. 46; K. S. Marchitto, S. A. Jones, and M. V. Norgard, Abstr. Annu. Meet. Am. Soc. Microbiol. 1984, B182, p. 48). Differences in binding properties of the various monoclonal antibodies were most likely a reflection of differential binding affinities or their specificities for different epitopes on the 47,000-dalton surface antigen. With two possible exceptions, the monoclonal antibodies tested reacted specifically with T. pallidum, either purified or found within a high-contaminating tissue background, and not with Treponema phagedenis biotype Reiter, Haemophilus ducreyi, Neisseria gonorrhoeae, herpes simplex virus type 2, or normal rabbit testicular tissue. The high sensitivity and specificity exhibited by these anti-T. pallidum monoclonal antibodies make them excellent candidates for employment in new syphilis or other treponemal diagnostic tests designed to detect very low numbers of pathogenic treponemes in lesion exudates or other body fluids.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Catterall R. D. Neurosyphilis. Br J Hosp Med. 1977 Jun;17(6):585–604. [PubMed] [Google Scholar]
- Chapel T. A. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980 Oct-Dec;7(4):161–164. doi: 10.1097/00007435-198010000-00002. [DOI] [PubMed] [Google Scholar]
- Chapel T. A. The variability of syphilitic chancres. Sex Transm Dis. 1978 Apr-Jun;5(2):68–70. doi: 10.1097/00007435-197804000-00009. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Henning U., Schwarz H., Chen R. Radioimmunological screening method for specific membrane proteins. Anal Biochem. 1979 Aug;97(1):153–157. doi: 10.1016/0003-2697(79)90339-7. [DOI] [PubMed] [Google Scholar]
- Jaffe H. W., Larsen S. A., Peters M., Jove D. F., Lopez B., Schroeter A. L. Tests for treponemal antibody in CSF. Arch Intern Med. 1978 Feb;138(2):252–255. [PubMed] [Google Scholar]
- Kellogg D. S., Jr The detection of Treponema Pallidum by a rapid, direct fluorescent antibody darkfield (DFATP) procedure. Health Lab Sci. 1970 Jan;7(1):34–41. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Marchitto K. S., Jones S. A., Schell R. F., Holmans P. L., Norgard M. V. Monoclonal antibody analysis of specific antigenic similarities among pathogenic Treponema pallidum subspecies. Infect Immun. 1984 Sep;45(3):660–666. doi: 10.1128/iai.45.3.660-666.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. N. Value and limitations of nontreponemal and treponemal tests in the laboratory diagnosis of syphilis. Clin Obstet Gynecol. 1975 Mar;18(1):191–203. doi: 10.1097/00003081-197503000-00017. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Cloning and expression of Treponema pallidum (Nichols) antigen genes in Escherichia coli. Infect Immun. 1983 Nov;42(2):435–445. doi: 10.1128/iai.42.2.435-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Plasmid DNA in Treponema pallidum (Nichols): potential for antibiotic resistance by syphilis bacteria. Science. 1981 Jul 31;213(4507):553–555. doi: 10.1126/science.6264606. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Isolation of Escherichia coli mutants defective in enzymes of membrane lipid synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2274–2278. doi: 10.1073/pnas.72.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Kettman J. R., Miller J. N., Norgard M. V. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols). Infect Immun. 1982 Jun;36(3):1076–1085. doi: 10.1128/iai.36.3.1076-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., McCormack W. M., Smith R. F., Parks R. M., Bailey R., Ohlin A. C. Enzyme immunoassay for diagnosis of gonorrhea. J Clin Microbiol. 1984 Jan;19(1):57–59. doi: 10.1128/jcm.19.1.57-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Norris S. J. The biology, pathology, and immunology of syphilis. Int Rev Exp Pathol. 1983;24:203–276. [PubMed] [Google Scholar]
- Tight R. R., White A. C. Quantitative microhaemagglutination assay for Treponema pallidum antibodies in experimental syphilis. Br J Vener Dis. 1980 Oct;56(5):291–296. doi: 10.1136/sti.56.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]