Abstract
Stress affects the gastrointestinal tract as part of the visceral response. Various stressors induce similar profiles of gut motor function alterations, including inhibition of gastric emptying, stimulation of colonic propulsive motility, and hypersensitivity to colorectal distension. In recent years, substantial progress has been made in our understanding of the underlying mechanisms of stress’s impact on gut function. Activation of corticotropin-releasing factor (CRF) signaling pathways mediates both the inhibition of upper gastrointestinal (GI) and the stimulation of lower GI motor function through interaction with different CRF receptor subtypes. Here, we review how various stressors affect the gut, with special emphasis on the central and peripheral CRF signaling systems.
Keywords: motility, CRF antagonists, colon, stomach, urocortin
INTRODUCTION
More than 70 years ago, Hans Selye (1) identified the gut, along with the endocrine and immune systems, as the primary target altered by a variety of chemical and physical challenges and pioneered the concept of stress as the “stereotyped biological response to any demand.” Later, the term allostasis was introduced by Sterling & Eyer (2) to refer to the “maintenance of stability through change.” Subsequently, McEwens (3) applied this concept to define stress as the physiological adaptation processes that maintain stability in times of internal or external challenges. Excessive stress can result in cumulative biological changes (known as allostatic load) and can alter adaptive mechanisms, resulting in an inefficient allostatic response and a permanent change in the basal levels of stress mediators (4). A perpetual imbalance between adaptation capacity and stressors can result in allostatic overload, leading to a state of illness (4) that may affect different body systems and induce development of functional bowel diseases (5). It is now appreciated that signaling pathways involving corticotropin-releasing factor (CRF) are altered by stress and contributetofunctional bowel diseases (5).
STRESS AND THE GUT
In recent years, our understanding of the circuitries and biochemical coding involved in the stress response has increased tremendously (6). Many studies have used the immediate early gene c-fos protein (Fos) immunohistochemistry as a marker of neuronal activity, thereby identifying brain nuclei that respond to acute or chronic stress (7) and their relation to the autonomic regulation of gut function (8–11). CRF is the primary neurohormone involved in the hallmark response to stress: the activation of the hypothalamic-pituitary-adrenal (HPA) axis. CRF also acts as a neurotransmitter/neuromodulator to coordinate the behavioral, autonomic, and visceral efferent limbs of the stress response (12–14). Convergent findings support the involvement of CRF receptors in the brain and the gut as important mediators of acute or chronic stress–related alterations of gut function (14–16). Furthermore, environmental stressors seem to play a role in the development and/or exacerbation of functional bowel diseases, such as irritable bowel syndrome (IBS), which is characterized by altered bowel habits and visceral hypersensitivity (17–20). Growing preclinical and clinical reports indicate that increased central and peripheral CRF signaling may contribute to the development and maintenance of functional bowel disorders through the alteration of autonomic, enteric nervous, and immune system activity (5, 16).
THE CORTICOTROPIN-RELEASING FACTOR FAMILY AND ITS RECEPTORS
Mammalian Corticotropin-Releasing Factor and Urocortins
CRF, originally isolated by Vale and colleagues in 1981 (21), is a 41-amino-acid (aa) hypothalamic releasing peptide that stimulates the synthesis and release of adrenocorticotropic hormone and β-endorphin from the anterior pituitary. More recently, three other mammalian CRF-related peptides have been characterized: urocortin 1 (Ucn 1), a 40-aa peptide with 45% sequence identity with rat/human (r/h) CRF; urocortin 2 (Ucn 2); and urocortin 3 (Ucn 3) (22–25). Mouse Ucn 2 (mUcn 2) is a 38-aa peptide sharing 34% homology with r/h CRF and 42% with r/h Ucn 1 (24). However, the 38-aa peptide mUcn 3 shares only 26% and 21% homology to r/h CRF and r/h Ucn 1, respectively (25). Phylogenetic profiling of the CRF peptide family indicates that these four distinct genes—those encoding CRF, Ucn 1,Ucn 2, and Ucn 3—are highly conserved through evolution and can be traced back to invertebrates, indicating their important roles in survival and adaptation (26, 27).
CRF1 and CRF2 Receptors
CRF and urocortins interact with two receptors, CRF1 and CRF2, which are encoded by two distinct genes exhibiting 70% sequence homology(28). The human and rat genomic structures of the CRF1 receptor contain 14 and 13 exons, respectively. In most mammals, the active CRF1 receptor protein results from transcription of all exons. In contrast, translation of all 14 exons in humans results in a humanspecific, 444-aa protein named CRF1b that contains an extended first intracellular loop and exhibits impaired agonist binding and signaling properties (29, 30). The 415-aa protein CRF1a is the main functional CRF1 variant resulting from the excision of exon 6. In addition to CRF1a and CRF1b, the CRF1 gene gives rise to multiple additional splice variants (1c, 1d, 1e, 1f, 1g, 1j, 1k, 1m, and 1n) that have neither a ligand binding site nor a signaling domain, although some variants modulate CRF and Ucn 1 actions (29). The expression of these CRF1 isoforms is tissue specific and can vary with the tissues’ functional activity as well as with environmental factors (29). For instance, the onset of labor is associated with an increased transcription of myometrial CRF1a gene and with differential up- and downregulation of other specific variants that may be implicated in the passage of quiescent to procontractile activity of the myometrium during labor (31). The expression and regulation of CRF1 receptor variants under conditions of acute or chronic stress in the brain and the gut are still largely unexplored.
In humans, there are three functional splicing variants of the CRF2 receptor (namely 2a, 2b, and 2c), whereas in other mammals only 2a and 2b are expressed (29, 32). The CRF2 variants display a distinct expression profile in mammals (29, 32). The CRF2 isoforms result from alternative splicing of exon 1 to exon 3. This splicing leads to structurally distinct N-terminal extracellular domains (34 aa for CRF2a, 61 aa for CRF2b, and 20 aa for CRF2c), which are involved in ligand-receptor interaction (32, 33). In addition to the wild-type CRF2a-1,five additional splice variants, CRF2a-2 to CRF2a-6, have been identified in the rat upper gut (34). Interestingly, the mouse CRF2a splice variant, originally identified in the brain as a soluble binding protein (sCRF2a) for CRF and Ucn 1 (35), is also expressed in the rat upper gut (CRF2a-6) (34).
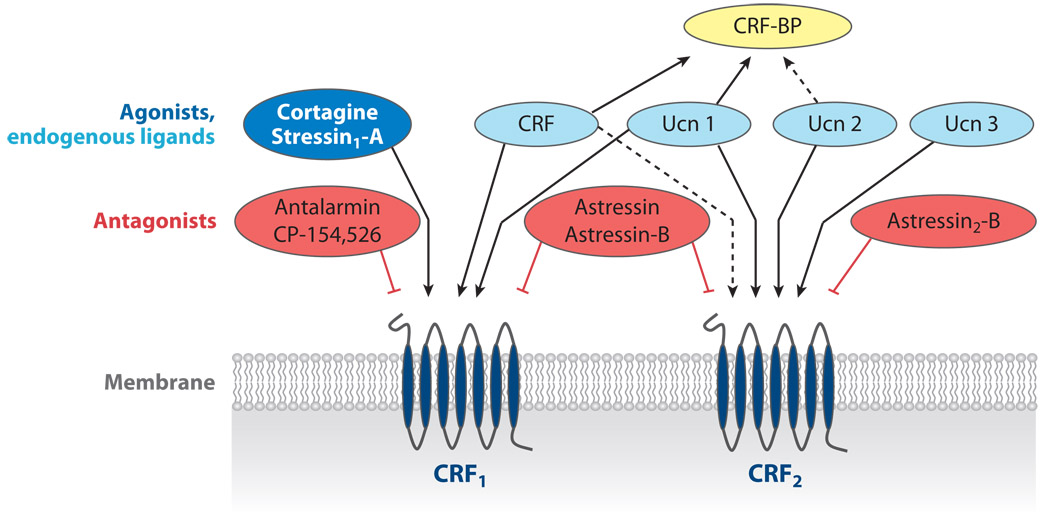
CRF1 and CRF2 receptors show distinct affinities to CRF and related peptides (29, 32). Although CRF has a 10-to40-fold higher affinity for the CRF1 receptor than for the CRF2 receptor, urocortins preferably signal through CRF2 receptors. Ucn 1 binds with equal affinity to both CRF receptors and has a 100-fold higher affinity than CRF for the CRF2 receptor (29, 36). In contrast, Ucn 2 and Ucn 3 show high selectivity for the CRF2 receptor (29, 36) (Figure 1). Because none of the endogenous CRF ligands characterized thus far exclusively activate CRF1 receptors, selective peptide CRF1 agonists (namely cortagine and stressin1-A) have recently been developed (36–38) (Figure 1). However, the molecular determinants that govern the binding of CRF-family peptides to their cognate receptors have been extensively characterized (29). The long N-terminal extracellular domain of CRF receptors primarily interacts with the C-terminal residues of CRF, whereas the N-terminal residues of CRF interact with the transmembrane region of the receptor, resulting in conformational changes that enable G protein activation (29).
Figure 1.
Schematic overview of preferential binding affinities and specificities of endogenous or synthetic corticotropin-releasing factor (CRF) receptor ligands. The CRF1 and CRF2 receptors share 70% homology and belong to the family of seven-transmembrane G protein–coupled receptors. BP, binding protein.
In most tissues, signal transduction of CRF1 and CRF2 primarily involves coupling to the Gs–adenyl cyclase system, with subsequent cAMP generation and protein kinase A activation. In addition, CRF receptors, like most heptahelical G protein–coupled receptors, can interact with multiple G protein systems including Gq, Gi, Go, Gil/2, and Gz to relay signals to diverse intracellular effectors in an agonist- and tissue-specific manner (29). Thus, CRF receptors (a) may modulate various kinases, including phosphokinases A, B, and C, (b) can phosphorylate and activate mitogenactivated protein kinase (MAPK), in particular the ERK1/2 and p38/MAPK pathways, and (c) can alter intracellular Ca2+ concentrations (29, 34).
Brain Distribution of Corticotropin-Releasing Factor Ligands and Receptors
The distribution of CRF-immunoreactive (ir) cells and fibers in the rat brain has been extensively described (39–41). The major brain areas expressing CRF messenger RNA (mRNA) and CRF-ir cells include the paraventricular nucleus (PVN) of the hypothalamus, the cerebral cortex, the amygdalar-hippocampal complex, and Barrington’s nucleus in the dorsolateral pons. The PVN is the major site of CRF-containing cell bodies projecting to the median eminence. CRF neurons in the central amygdala project to the PVN, the locus coeruleus (LC), and the parabrachial nucleus, and neurons in the bed nucleus of stria terminalis project to the dorsal vagal complex (DVC). CRF-containing neurons in Barrington’s nucleus contribute to the CRF innervation of the LC and the sacral spinal cord (40, 42).
CRF, Ucn 1, Ucn 2, and Ucn 3 distribution in the rat brain shows limited neuroanatomical overlap (43). In contrast to Ucn 1’s widespread peripheral distribution, peptide expression in the brain is limited (22, 44, 45). The most prominent brain site of Ucn 1 expression and immunoreactivity is the Edinger-Westphal nucleus (44, 46). Moreover, Ucn 1–ir can be detected in the lateral superior olive; the olfactory bulb; the supraoptic nucleus (SON); the ventromedial hypothalamus (VMH); and magnocellular parts of the PVN, the lateral hypothalamic area, and the ambiguous nucleus; as well as the cranial nerve motor nuclei (facial and hypoglossal) (46, 47). The majority of Ucn 1–ir projections provide descending input to the brainstem, whereas ascending projections are restricted. In particular, Ucn 1–ir fibers project to CRF2 receptor–containing nuclei including the lateral septum, the dorsal raphé,the interpeduncular nucleus, the nucleus of the solitary tract, and the area postrema (46). Ucn 2 gene expression is localized in the parvo- and magnocellular PVN, the SON, the arcuate nucleus of the hypothalamus, and the LC, as well as several cranial nerve motor nuclei (trigeminal, facial, and hypoglossal nuclei) and the ventral horn of the spinal cord (24, 48). Ucn 3 mRNA has been detected in the PVN, the amygdala (basomedial nucleus), and the basomedial nucleus of the stria terminalis (43, 48). Assessment of brain distribution of Ucn 2–ir fibers has been hampered by the lack of specific Ucn 2 antibodies. Ucn 3–ir fibers innervate the lateral septum, the amygdala (except for the central nucleus), the dorsal aspect of the VMH, and the dorsal raphé, as well as the area postrema (25, 48, 49).
In rat brain, the CRF1 receptorisdensely expressed in the forebrain and subcortical limbic structures in the septal region and amygdala. In the hypothalamus, expressionis low under basal conditions but can be significantly upregulated during stress or following CRF application (50–52). Dense CRF1 receptor representation also appears in the anterior and intermediate lobes of the pituitary, which supports its role in the activation of the HPA axis by CRF (53). In contrast, CRF2 receptor expression in rodents is confined to subfornical structures, with high expression in the lateral septum, amygdala (with the exception of central nuclei), and hypothalamus (including high levels in the VMH and SON) (54). In the hindbrain, the dorsal raphé, area postrema, nucleus of the solitary tract, and chorionic plexus express the CRF2 receptor (54). A close association has been found between Ucn 3–ir–terminal fields and expression ofCRF2 receptors in specific hypothalamic nuclei (49).
Corticotropin-Releasing Factor Receptor Antagonists and Binding Protein
Key to the understanding of the physiological role of the CRF signaling pathways is the early development of CRF receptor antagonists by Rivier et al. (55). The first of these antagonists to be developed are the nonselective CRF1/CRF2 receptor antagonists α-helical CRF9–41, D-Phe12CRF12–41, astressin, and the long-acting astressin-B. Recently, two groups developed the peptide CRF2 receptor antagonists antisauvagine-30, K41498, [D-Phe11, His12, Nle17] sauvagine11–40, and the more potent and long-acting analog astressin2-B, which are competitive antagonists that bind equally to the a, b, and c variants of CRF2 receptor (35, 56, 57) (Figure 1). A common feature of these peptide antagonists is that they display poor penetrance into the brain when administered peripherally. In an early study, our group showed that an intravenous injection of astressin did not influence the inhibition of gastric transit in response to CRF injected into the cisterna magna, although the same dose blocked peripheral CRF–induced delay of gastric emptying (58). With regard to selective CRF1 antagonists, pharmaceutical firms have developed a large number of high-affinity small hydrophobic molecules that can cross the blood-brain barrier. The impetus to create these molecules arose from their potential therapeutic application to curtail dysregulation of CRF-signaling pathways that may be relevant to the pathogenesis of human illnesses such as anxiety and depression, eating disorders, inflammatory diseases, substance abuse, preterm parturition, and functional bowel disorders (59–62). Among the CRF1 antagonists most commonly used in experimental studies are CP-154,526, antalarmin, NBI-34041, NBI-30545, and NBI-35965 (60, 63).
In addition to the synthetic antagonists that can block CRF receptors, a 332-aa endogenous CRF binding protein (CRF-BP) has been isolated across different species (64, 65). The CRF-BP functions as an endogenous antagonist by sequestering CRF ligands and therefore modulating the access of CRF and related peptides to CRF receptors (64). Rat and human CRF and Ucn 1 display high (picomolarrange) affinity for the CRF-BP, whereas Ucn 2 shows a moderate (nanomolar-range) affinity and Ucn 3 displays no affinity (65) (Figure 1). The decrease in food intake and body weight, the anxiogenic-like behavior occurring in CRF-BP-deficient mice, and, conversely, the body weight gain in CRF-BP-overexpressing mice support the contention that CRF-BP can sequester CRF/Ucn 1 and modulate endogenous CRF/Ucn 1 biological actions (66, 67). Recent studies have identified distinct regions and residues of the CRF-BPthat are responsible for r/h CRF and r/h Ucn 1 binding to CRF-BP. In particular, a single alanine mutation (R56A) in CRF-BP was effective in creating an Ucn 1–specific antagonist, thereby opening new venues for the design of selective CRF versus Ucn 1 antagonists to dissect their respective role in the stress response (68). In rat brain, CRF-BP immunoreactivity is prominently expressed in hypothalamic regions involved in the neuroendocrine and autonomic responses to stress, including the subdivision of the dorsal cap of the PVN projecting to the spinal cord (64, 69).
The isolation of CRF and endogenous selective CRF2 receptor agonists, along with the development of selective CRF1 agonists and antagonists and CRF2 antagonists, provided essential tools to establish the pleiotropic actions of CRF and urocortins in the brain by acting at one or both CRF receptors. In particular, this pharmacological approach has allowed investigators to dissect the primary involvement of CRF1 signaling pathways in the stress-related stimulation of the HPA axis, anxiogenic behavior, alterations in the autonomic nervous system activity, and visceral responses (5, 13, 70).
STRESS-RELATED ALTERATIONS OF GASTROINTESTINAL MOTILITY MEDIATED BY BRAIN CORTICOTROPIN-RELEASING FACTOR RECEPTORS
Various acute stressors most commonly delay gastric emptying in experimental animals as well as in healthy humans (12). In contrast, a range of stressors (e.g., anxiety, dichotomous listening, fear, intermittent hand immersion in cold water, and stressful interviews) increase colonic motility in healthy volunteers (12). Activation of propulsive colonic motor function has also been shown in rodents following exposure to diverse stressors such as open field tests, conditioned fear, loud sounds, restraint, cold exposure, water avoidance, inescapable foot or tail shocks, and central injection of interleukin-1 (12, 71–73). Consistent experimental evidence highlights the role of central CRF recep tors in stress-related inhibition of gastric motor function and in stimulation of colonic propulsive motor activity, as shown by central injection of CRF and urocortins in nonstressed animals as well as by the injection of CRF antagonists under stress conditions.
Brain Corticotropin-Releasing Factor Receptors Mediate Stress-Related Inhibition of Gastric Motor function
A number of studies have established that central injection [intracerebroventricularly (icv), intracisternally (ic), or into the fourth ventricle] of CRF,Ucn1,Ucn2,or nonmammalian CRFrelated peptides such as sauvagine and urotensin I inhibits gastric emptying of acaloric liquid, caloric liquid, or solid meal and alters gastric motility. These changes include the suppression of propagative contractions, cyclic activity front, high-amplitude contractions, and the disruption of fasted pattern in species including rats, mice, and dogs (74). Central injection of α-helical CRF9–41, D-Phe12CRF12–41, astressin, astressin-B, and astressin2-B blocks the icv or ic CRF-, Ucn 1–, and Ucn 2–induced delay of gastric emptying and inhibition of motility in rats, mice, and dogs. However, selective CRF1 antagonists have no effect, indicating that CRF’s and urocortins’ actions are primarily mediated by interaction with CRF2 receptors (11, 73, 75–88). The PVN and DVC, which influence autonomic outflow to the stomach, have been identified as responsive brain nuclei for CRF-induced inhibition of gastric emptying and motility, whereas the LC seems not to be involved (89–93). The expression of CRF2a receptor mRNA has been found in hypothalamic and brainstem nuclei, such as the PVN and DVC, as well as in limbic structures; this is consistent with the locations of the responsive sites (54).
The central action of CRF—inhibition of gastric transit—is independent of the activation of the HPA axis and is mediated by the autonomic nervous system, as shown by the persistence of the gastric response in hypophysectomized or adrenalectomized rats (75, 94). A number of reports have shown that (a) delay of gastric transit induced by icv or ic injection of CRF and Ucn 1 and (b) alteration of motility require the integrity of the vagus nerve in rats and dogs (75, 82, 88, 90, 92, 95–97). Only two studies reported different results that showed a primary involvement of the sympathetic nervous system (86, 94). However, the delay of gastric emptying induced by the ic injection of Ucn 2 is not altered by vagotomy but is mediated by sympathetic pathways and peripheral α-adrenergic receptors; this indicates that CRF ligands act through both vagal and sympathetic pathways to influence gastric function (88).
Pretreatment with CRF receptor antagonists has provided pharmacological evidence that brain CRF receptors are involved in stress-induced inhibition of gastric motor function. The ic, icv, or PVN injection of α-helical CRF9–41, D-Phe12CRF12–41, astressin, or astressin-B blocks acute stress–induced delayed gastric emptying (12, 74). Stressors used in these studies fall under one of the following categories: (a) psychological/physical (swim stress, restraint), (b) visceral (abdominal surgery, trepanation, peritoneal irritation with intraperitoneal 0.6% acetic acid), immunological (intravenous or central injection of interleukin-1β), and (c) chemical (ether) (12, 74). Of interest is the demonstration that electroacupuncture normalizes both restraint-and ic-CRF-induced delay of gastric emptying, suggesting that the beneficial action of elec-troacupuncture under conditions of stress may be related to interference with brain CRF pathways (98). Also supportive of a role of brain CRF pathways is the demonstration that a variety of physical, immune, and psychological stressors (e.g., abdominal surgery, immobilization, forced swimming, and interleukins) activate CRF neurons and lead to a rapid increase in CRF gene transcription and upregulation of CRF mRNA in the PVN, which is the primary site of CRF synthesis (11, 99–102). The parvocellular division of the PVN contains neurons in the dorsal and ventral parvocellular caps that regulate autonomic outflow to the viscera (103), which is consistent with the role of CRF signaling in the PVN to influence gastric motor function. Ucn 1 and 2 are also expressed in the PVN and are upregulated by various stressors (104, 105). However, their involvement in stress-related inhibition of gastric motor function remains to be clarified. So far, investigations in Ucn 1–deficient mice suggest that Ucn 1 does not play a primary role in heart rate increase and sympathetic activation in response to acute restraint, as monitored by epinephrine and norepinephrine levels (106).
The CRF receptor subtype(s) involved in mediating stress-related inhibition of gastric motor function has yet to be fully characterized. Because CRF and urocortins inhibit gastric emptying via a CRF2-mediated pathway (73, 85, 88), it was expected that CRF2 receptors would be primarily involved in stress-induced delay of gastric emptying. This has been demonstrated in one study, where ic injection of astressin2-B blocked the restraint stress–induced delay of gastric emptying in rats (86). However, it is surprising that under conditions of surgical stress (abdominal surgery and cecal palpation), central CRF1 receptors play a predominant role: CRF1-knockout mice and wildtype animals injected centrally with a CRF1 antagonist no longer develop the inhibition of gastric emptying following abdominal surgery and cecal palpation (107). Experimental data in which the central injection of CRF1/CRF2 antagonists or selective CRF receptor subtype antagonists blocked stress-related alterations of gastric propulsive motor function provide new insights into the role of brain CRF signaling pathways as underlying mechanisms involved in both acute postoperative gastric ileus (108) and alterations of gastric digestive function during disease states associated with cytokine release (109, 110). Furthermore, pharmacological evidence indicates that several centrally administered brain-gut peptides ultimately converge on brain CRF signaling as the downstream effector to induce an autonomic-mediated suppression of gastric propulsive motor function. For instance, the delayed gastric emptying induced by icv, fourth ventricle, or ic injection of glucagon-like peptide–1 (GLP-1), cocaine and amphetamine-regulated transcript (CART) peptide, or des-acyl ghrelin is blocked by pretreatment with CRF antagonists injected via the same route (111–113). However, central application of CRF receptor antagonists does not alter basal gastric emptying of a liquid nonnutrient or solid nutrient meal in rats, mice, and dogs, indicating that central CRF pathways do not regulate fasted and postprandial gastric propulsive motor function under basal conditions, but that they do gain importance when recruited under stress conditions (12, 76, 82, 114).
Are Brain Corticotropin-Releasing Factor Receptors Involved in the Mediation of Stress-Related Alterations of Small Intestinal Motor Function?
Compared with our understanding of gastric motor function, much less is known about the effects of stress on small intestinal propulsive activity and the role of brain CRF receptors (74). As in the stomach, acute psychological stress, as well as central injection of CRF or Ucn 1, inhibits duodenal and small intestinal transit and motility (suppression of the occurrence of myoelectric migrating complex, disruption of the fasted pattern of duodenal motor activity to fed pattern) in rats and dogs (71, 95, 96, 115–118). Likewise, the central action of CRF is mediated via vagal pathways and is independent of the HPA axis activation (71, 94, 116, 119). However, the slowing of small intestinal transit induced by icv injection of CRF is not as prominent as it is in the stomach; this is probably due to the lesser vagal innervation of the small intestine compared with the stomach (120). The involvement of central CRF receptors in mediating stress-induced inhibition of small intestinal transit has been little studied, and results are conflicting. The icv injection of α-helical CRF9–41 blocks restraint stress–induced slowing of small intestinal transit in male rats, whereas there is no such effect in female rats at a dose effective to block restraintinduced stimulation of colonic transit (71, 116). Whether these divergences are sex-related or specific differences in experimental conditions needs to be clarified. Furthermore, the central CRF receptor subtype involved in the mediation of the stress-induced inhibition of small intestinal motor function has not been investigated.
Brain CRF1 Receptors Mediate Stress-Related Stimulation of Colonic Motor Function
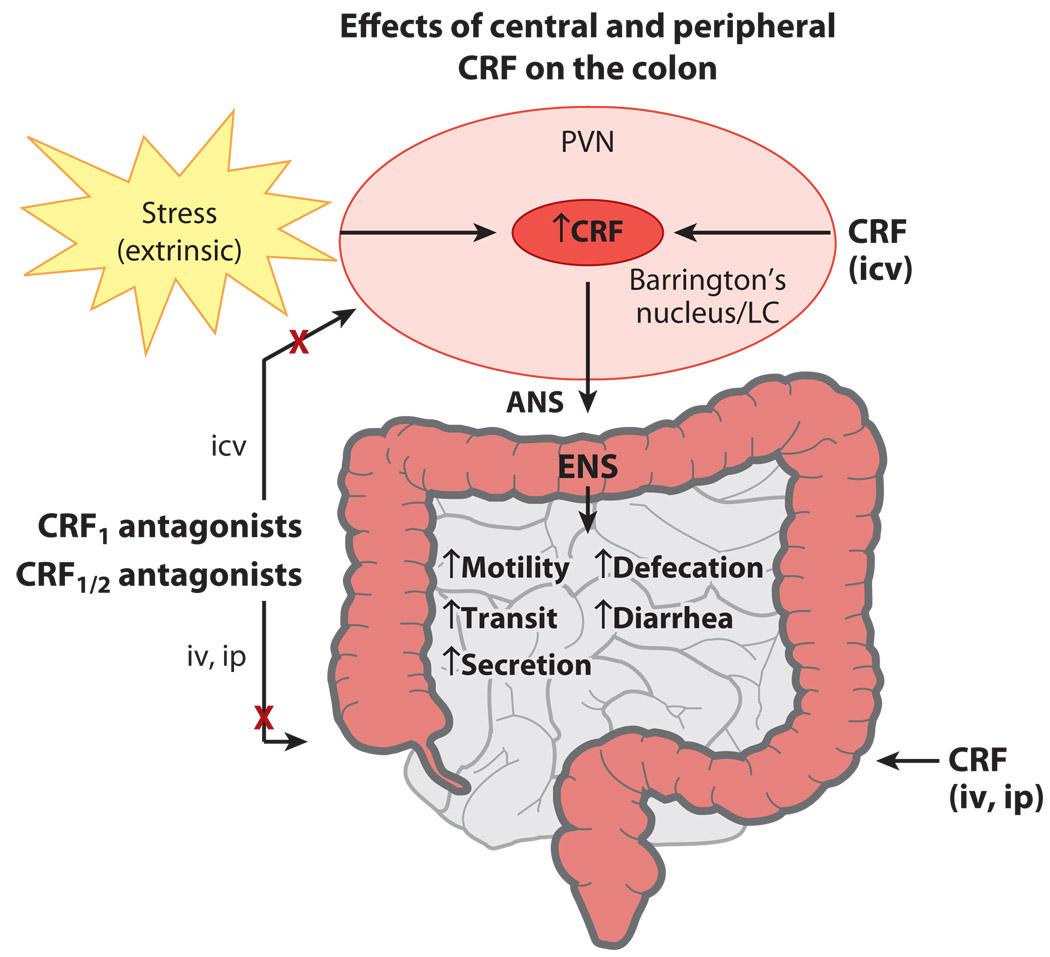
In contrast to its inhibitory effect on gastric and small intestinal transit, centrally injected CRF or Ucn 1 stimulates colonic transit and defecation and induces a pattern of cecocolonic my-oelectrical activity characterized by clustered spike-bursts of long duration in freely moving female and male rats, mice, and gerbils (9, 71–73, 81, 91, 94, 121–123) (Figure 2). Convergent evidence has established that the stimulation of colonic motor function in response to central CRF and Ucn 1, as well as various stressors, is primarily mediated via central CRF1 receptors (5, 74) (Figure 2). First, colonic propulsive motor activity in response to stress is mimicked by centrally administered CRF1 receptor–preferential agonists such as ovine CRF, r/h CRF (56), and Ucn 1, whereas Ucn 2 and Ucn 3 are inactive in mice when centrally injected at a dose similar to that of CRF (73). Second, the central injection of peptide CRF1/CRF2 receptor antagonists and selective CRF1 antagonists blocks the colonic motor stimulation induced by central injection of CRF or Ucn 1 or by various stressors (5, 124) (Figure 2). For instance, central injection of astressin, α-helical CRF9–41, and D-Phe12CRF12–41 blocks the effects of central injection of CRF, Ucn 1, or interleukin-1β; wrap or partial restraint; water avoidance; morphine withdrawal; and colorectal distention–induced stimulation of colonic transit and defecation; as well as the increased frequency of colonic spike-bursts induced by conditioned fear stress in rodents (9, 71–73, 81, 84, 116, 123, 125–128). Furthermore, central or peripheral application of the selective CRF1 antagonists CP-154,526, CRA 1000, NBI-27914, NBI-35965, JTC-017, and antalarmin reduces the acceleration of colonic transit time caused by restraint, fecal pellet output induced by water avoidance, social stress, painful stimuli, and diarrhea resulting from morphine withdrawal in rodents. CRF1-knockout mice show significantly less defecation in an open field test than do their wild-type littermates (5, 12, 129) (Table 1). Lastly, central injection of the selective CRF2 antagonist astressin2-B at a dose effective to block CRF2-mediated action on gastric emptying does not prevent the colonic response to central injection of CRF in rodents (12). As described above for gastric motor function, central CRF1 receptors are not involved in the basal and postprandial regulation of colonic motor function under nonstress conditions (5, 12, 73). Of interest, however, is the growing pharmacological evidence that a number of brain peptides influencing food intake, such as neuropeptide Y, GLP-1, CART, and ghrelin, act in the brain to stimulate colonic motor function by recruiting brain CRF signaling pathways (130–133).
Figure 2.
Summary of corticotropin-releasing factor (CRF) actions on colonic function. Central and peripheral CRF stimulates various colonic functions, which recapitulate the effects of stress and are blocked by nonselective and CRF1-selective CRF receptor antagonists. Abbreviations: ANS, autonomic nervous system; ENS, enteric nervous system; icv, intracerebroventricularly injected; ip, intraperitoneally injected; iv, intravenously injected; LC, locus coeruleus; PVN, paraventricular nucleus of the hypothalamus.
Table 1.
Blockade of acute stress–induced stimulation of colonic motor functions by selective CRF1 antagonistsa
| Antagonist | Dose | Route | Species | Stress | Inhibition | Reference |
|---|---|---|---|---|---|---|
| Central administration | ||||||
| NBI-27914 | 100 µg | Icv | Rats | Water avoidance | Defecation (67%) | 84 |
| NBI-35965 | 50 µg | Icv | Mice | Restraint | Defecation (100%) | 73 |
| Peripheral administration | ||||||
| CP-154,526 | 20 mg kg−1 | Sc | Rats | Water avoidance | Defecation (55%) | 153 |
| CP-154,526 | 30 mg kg−1 | Sc | Rats | Morphine withdrawal | Diarrhea (50%) | 127 |
| CRA 1000 | 20 mg kg−1 | Sc | Mice | Morphine withdrawal | Diarrhea (50%) | 171 |
| CP-154,526 | 20 mg kg−1 | Sc | Rats | Partial restraint | Transit (55%) | 157 |
| NBI-35965 | 20 mg kg−1 | Sc | Rats | Water avoidance | Defecation (53%) | 172 |
| Antalarmin | 20 mg kg−1 | Ip | Rats | Restraint | Defecation (49%) | 173 |
| JTC-017 | 10 mg kg−1 | Ip | Rats | Colon distention | Defecation (100%) | 128 |
| Antalarmin | 20 mg kg−1 | Po | Monkeys | Social intruder | Defecation (40%) | 174 |
Abbreviations: CRF, corticotropin-releasing factor; Icv, intracerebroventricular; Ip, intraperitoneal; Po, per pos; Sc, subcutaneous.
The central CRF- and stress-induced CRF1-dependent stimulation of colonic motor function (transit and increased frequency of spike-burst activity) is not altered by blocking the activation of the HPA axis (72, 94). Rather, it is mediated by an increased parasympathetic outflow to the colon via vagal celiac branches innervating the proximal colon and via sacral parasympathetic fibers innervating the distal colon and rectum (72, 90, 94, 125, 126). Effector mechanisms within the colon involve parasympathetic-mediated activation of myenteric cholinergic and nitrergic neurons regulating the peristaltic reflex as well as serotonin (5-HT) acting on 5-HT3 and 5-HT4 receptors. This is supported by the demonstration that icv CRF-induced defecation is blocked by the peripheral administration of atropine and the 5-HT3 antagonists ramosetron, ondansetron, azasetron, alosetron, and cilansetron, as well as the 5-HT4 antagonist SB-204070, whereas the icv injection of these antagonists has no effects (123, 134–136). In addition, restraint stress or icv injection of CRF increases the 5-HT content in the feces of rat proximal colon (134). Taken together, these data suggest the cholinergic recruitment of peripheral serotonin pools from either enterochromaffin cells or enteric neurons by restraint stress and central CRF.
Consistent with the pharmacological evidence engaging the CRF1 signaling pathways in the colonic response to stress, functional mapping and gene regulation studies support the PVN and LC/Barrington’s complex in stressrelated, CRF-mediated activation of colonic propulsive motor function (Figure 2). Tracing studies have shown that CRF neurons in the dorsal cap of the parvocellular part of the PVN have trans-synaptic connections to the colon (42). Also, CRF-synthesizing neurons in Barrington’s nucleus project to the noradrenergic LC, as well as to the intermediolateral column of the sacral spinal cord, which contains the sacral parasympathetic nucleus innervating the descending colon (42, 120). There are CRF-efferent fibers projecting directly from the PVN and Barrington’s nucleus to the LC, as shown by anterograde tracing (137, 138). Neuroanatomical and functional studies have demonstrated that water avoidance stress activates the PVN and LC/Barrington’s nuclei and CRF gene transcription in the PVN (9, 139), whereas icv injection of α-helical CRF9–41 reduces Fos expression selectively in these hypothalamic and pontine nuclei in correlation with defecation score (9). Likewise, Lewis rats known to have a blunted hypothalamic CRF response to stress exposure (140) displayed a reduced activation of neurons in the PVN and sacral parasympathetic nucleus and showed a lower defecation response than did Fisher rats (126). Consistent with a role of CRF/CRF1 signaling in the PVN, α-helical CRF9–41 injected directly into the PVN blocks partial restraint–and water avoidance–induced stimulation of colonic transit and defecation, and various neurogenic and systemic stressors activate the transcription of the CRF1 receptor gene in the PVN (50, 90, 125). CRF increases the firing rate of noradrenergic neurons in the LC and releases noradrenalin into the brain cortex, which results in arousal and anxiogenic behavior (137, 141). Therefore, CRF/CRF1 signaling pathways in the PVN and LC may physiologically regulate the behavioral and autonomic responses to stress that influence colonic function as part of the brain-gut axis (91, 137). These pathways may play a role in diarrhea-predominant IBS patients with psychic comor-bidities such as anxiety and depression (5).
STRESS-RELATED ALTERATIONS OF GASTROINTESTINAL MOTILITY MEDIATED BY PERIPHERAL CORTICOTROPIN-RELEASING FACTOR SIGNALING
Like many peptides once thought to be restricted to the brain and pituitary that were later detected in peripheral tissues, CRF ligands and receptors are widely expressed outside the brain in spinal cord and peripheral organs, including the gastrointestinal tract in animals and humans (12, 25, 142–146). This overlapping expression pattern of CRF ligands and receptors in the gastrointestinal tract gives rise to a local CRF signaling pathway that can act directly on the gut in either a paracrine or an autocrine manner (143, 144, 146–48).The potent actions of CRF ligands upon peripheral injection and CRF receptor blockade are consistent with the notion that peripheral activation of CRF1 and CRF2 signaling pathways may be part of the local effectors involved in gastric and colonic motor alterations induced by stress.
Peripheral Corticotropin-Releasing Factor Signaling Alters Gut Motor Function
When injected peripherally, CRF strongly alters gut motility and transit in several mammalian species including rodents, dogs, and humans (71, 75, 149–152). The iv or intraperitoneal (ip) injection of CRF or Ucn 1 in rats inhibits gastric emptying, delays small intestinal transit, and stimulates colonic transit and defecation with a potency similar to that of centrally injected (icv or ic) CRF (71, 152, 153). However, although the patterns of gut motor response are similar, distinct sites and mechanismsofaction are involved in mediating the effects of centrally and peripherally injected CRF (58, 94, 96, 154). Ganglion blockade, which inhibits the icv CRF-induced delay of gastric emptying and acceleration of colonic transit, does not influence the ip CRF-induced alteration of gut transit (94). Likewise, sympathetic blockade, which prevents ic Ucn 2–induced delayed gastric emptying, does not influence iv Ucn 2–inhibitory action on gastric emptying in rats tested under otherwise-similar conditions (88). More importantly, peptide action can be reproduced in vitro in antral and colonic preparations. Studies performed on muscle strips of rat gastric antrum showed that perfusion of CRF, Ucn 1, or Ucn 2 decreases the amplitude of circular and longitudinal muscle contractions (148, 155). Moreover, in an isolated colonic rat preparation, CRF increased basal myoelectrical peristaltic activity (153, 156).
Convergent studies to characterize the CRF receptors involved in these processes have established that the delayed gastric emptying following peripheral injection of CRF, Ucn 1, Ucn 2, and Ucn 3 is mediated by CRF2 receptors, whereas the stimulation of colonic motility after peripheral administration of CRF and Ucn 1 involves CRF1 receptors located on the myenteric neurons of rats and mice (145). Ucn2 (and, less potently, Ucn 3) injected peripherally delays gastric emptying of a solid or liquid meal without modifying distal colonic transit (152, 157). In contrast, ip injection of stressin1-A induces defecation alone, without altering gastric emptying (38). In contrast, (a) ip or iv CRF and (b) Ucn 1 interacting with both CRF receptors inhibit gastric motor function while stimulating colonic propulsion and fecal pellet output in rats and mice (96, 152, 157). The use of selective CRF antagonists has shown that the peripheral injection of astressin2-B and antisauvagine-30 blocks peripheral CRF- and Ucn 1–induced inhibition of gastric emptying without affecting the increase of distal colonic transit (152, 157). Conversely, the selective CRF1 receptor antagonists CP-154,526 and NBI-27914 block peripheral CRF- or Ucn 1–induced stimulation of colonic motor function (clustered spike-burst activity, distal transit, defecation, and diarrhea) without influencing the delay of gastric emptying (152, 153, 157, 158). Consistent with a peripheral action within the gut, CRF receptors have been localized throughout the gastrointestinal tract in guinea pigs (147). In rat colon, CRF1 receptor has been detected both at the gene level and by immunohistochemistry in goblet and stem cells of the crypts, as well as on surface epithelial cells, lamina propria, and, prominently, in the myenteric nervous plexus (144, 159). CRF2 receptors are highly expressed at the gene and protein levels in the rat upper gut, including the esophagus and stomach (34, 148). Evidence for a pivotal role of the CRF2 receptors, which are expressed on gastric myenteric neurons (148), comes from in vitro studies on gastric antral strips, in which CRF- and Ucn 2–induced reduction of spontaneous circular and longitudinal muscle contraction was tetrodotoxin dependent (148, 155).
Underlying alterations of motility linked with changes in gut transit have been characterized. In the upper gut, the iv injection of CRF decreases gastric intraluminal pressure in rats and inhibits motilin-induced jejunal migrating motor complex in dogs (155, 160). Likewise, in healthy humans, iv CRF reduces the basal fundic tone and stimulates the nonpropulsive postprandial duodenal motor activity as well as the pyloric and duodenal pressure wave (150, 161, 162). Ucn 1 injected intravenously disrupts the fasted motor pattern of gastroduodenal motility, which in conscious rats is replaced by the fed-like motor pattern (96). In contrast, when Ucn 1 is injected intravenously in ad libitum–fed animals, the fed motor pattern remains, but there is a decrease in antral and an increase in duodenal motor index (96). However, the increase in duodenal motility index is nonpropagative, as shown by the reduction of duodenal transit (96). In the colon, peripheral injection of CRF and Ucn 1 increases clustered spike-burst propagative activity in rats (153, 163). Clinical studies show that systemic application of CRF induces a colonic motility response that includes the occurrence of clustered contractions in the descending and sigmoid colon, which is more prominent in IBS patients than in healthy controls (151). The mediation of the CRF and Ucn 1 effect on colonic motor activity may involve a direct interaction with colonic myenteric neurons (159). Peripheral injection of CRF or of stressin1-A induces robust Fos expression selectively in the myenteric ganglia of the colon; this expression can be blocked by peripheral application of astressin and CP-154,526 (159, 164). In addition, the activation takes place in cholinergic and nitrergic colonic myenteric neurons that are known to be involved in the peristaltic reflex and that bear CRF1 receptors (144, 159).
Stress-Related Alteration of Gut Motor Function: Involvement of Peripheral Corticotropin-Releasing Factor Receptors
The functionality of the CRF signaling system in the gut during stress is supported by reports that peripherally injected peptide antagonists, namely α-helical CRF9–41, D-Phe12CRF12–41, and astressin, block abdominal surgery–induced delay of gastric emptying (58, 165, 166). The inhibition of gastric emptying induced by acute wrap restraint stress is also blocked by peripheral application of a CRF2 antagonist, whereas application of CP-154,526 has no effect (157). Likewise, the stimulation of distal colonic transit and fecal pellet output induced by acute wrap restraint or water avoidance stress is blocked or blunted by peripheral injection of α-helical CRF9–41 or astressin (71, 123, 153, 167). However, peripheral CRF receptors seem not to be involved in the regulation of fasted and postprandial gut motor functions under basal conditions (71, 145, 153, 157). Researchers speculate that stress may recruit CRF ligands, which are expressed in the gut through autonomic alterations. For instance, CRF and Ucn 1 mRNA are detected in the submucosa and muscle layers, and immunoreactivity shows a cellular distribution in myenteric neurons, serotonin-containing enterochromaffin cells, and lamina propria cells of the mucosa in stomach and colon (148, 168, 169).
CONCLUSIONS
In summary, we have made major advances both in unraveling the components of CRF signaling pathways that encompass CRF, urocortins, CRF receptors, and CRF-BP and in mapping their expression in the brain and the gut. There are conclusive experimental data showing that activation of brain and colonic CRF1 pathways by exogenous CRF or Ucn 1 or stress recapitulates cardinal features encountered during stress, including the stimulation of colonic motility, defecation/watery diarrhea, and visceral hypersensitivity (5). SelectiveCRF1 antagonists abolish or reduce exogenous CRF-and stress-induced anxiogenic/depressive behavior, defective intestinal barrier, stimulation of colonic motility, myenteric neurons, mucus secretion, mast cell activation, defecation, diarrhea, and hyperalgesia (5, 170). Therefore, sustained activation of the CRF1 system at central and/or peripheral sites may represent a key underlying mechanism whereby stress alters colonic function and can lead to stress-related functional bowel disorders such as IBS (15–20). In the upper gut, the brain and gastric CRF2 signaling systems are more prominently involved in CRF ligands– and stress-related suppression of gastric motor function. Both central ligands (e.g., CRF and Ucn 1, Ucn 2, and Ucn 3) and stress inhibit propulsive gastric motor function through autonomic and enteric nervous system alterations.
Additional investigations on the stress-related regulation of CRF ligands and their receptors, including their variants in the gut and their mechanisms of action at the cellular level, may provide insight into new venues for effective therapies for patients suffering from stressrelated functional bowel disorders.
SUMMARY POINTS
In recent years, our understanding of how stress alters the function of the gastrointestinal tract via the brain-gut axis has increased dramatically.
The discovery of the CRF peptide family; Ucn 1, Ucn 2, and Ucn 3; and the cloning of CRFreceptor subtypesCRF1, CRF2, and CRF-BP;as well as the development of selective CRF1 and CRF2 antagonists, has provided relevant tools to characterize the primary implications of the brain CRF/CRF1 signaling pathways in the endocrine, anxiogenic, autonomic, and visceral responses to stress.
Among the viscera, the gut functions are highly susceptible to the effects of stress, as has been shown by alterations of gut motility such as slowing of gastric transit and stimulation of colonic propulsive motor activity, along with altered intestinal barrier function.
Components of the CRF signaling system are expressed in brain nuclei influencing autonomic outflow to the viscera such as the PVN, the Barrington’s nucleus/LC complex, and the DVC, along with myenteric, endocrine, and immune cells within the gut.
The stimulation of colonic motor function induced by various stressors is mediated by the brain (PVN, Barrington’s nucleus/LC) and gut (enteric) CRF1 signaling system, which contributes to the activation of the sacral parasympathetic and the colonic myenteric nervous systems, respectively. Such CRF1 activation also contributes to the recruitment of colonic serotonin-containing enterochromaffin and mast cells.
The inhibition of gastric motor function by CRF ligands is mediated by activation of CRF2 receptors both in the brain and in the stomach. CRF receptors are involved in the modulation of autonomic and gastric myenteric activity, which influences gastric function during stress.
Further studies on the regulation of the CRF signaling system in the gut in response to stress and specific mechanisms of action may provide the basis for effective therapeutic venues for stress-related functional bowel disorders.
ACKNOWLEDGMENTS
The authors were supported by National Institutes of Health grants R01 DK-33061 and DK 57238 (Y.T.), Center grant DK-41301 (Animal core, Y.T.), P50 AR 049550 (Y.T.), VA Career Scientist and Merit Award (Y.T.), and German Research Foundation grant STE 1765/1–1 (A.S.).
Glossary
- Irritable bowel syndrome (IBS)
a functional disease characterized by altered bowel habits and visceral pain
- Urocortins (Ucns)
mammalian CRF-related peptides
- Paraventricular nucleus (PVN)
hypothalamic brain nucleus implicated in autonomic regulation of gastrointestinal functions
- Locus coeruleus (LC)
pontine catecholaminergic nucleus involved in physiological responses to stress
- Astressin-B
long-acting CRF1/CRF2 receptor antagonist
- Astressin2-B
long-acting selective peptide CRF2 receptor antagonist
Footnotes
The Annual Review of Physiology is online at physiol.annualreviews.org/
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Selye H. A syndrome produced by diverse nocuous agents. J. Neuropsychiatr. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. [1936] [DOI] [PubMed] [Google Scholar]
- 2.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason HS, editors. Handbook of Life Stress Cognition and Health. New York: Wiley; 1981. [Google Scholar]
- 3.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 5.Martinez V, Taché Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr. Pharm. Des. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- 6.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm. Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci. Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- 8.Taché Y, Garrick T, Raybould H. Central nervous system action of peptides to influence gastrointestinal motor function. Gastroenterology. 1990;98:517–528. doi: 10.1016/0016-5085(90)90849-v. [DOI] [PubMed] [Google Scholar]
- 9.Bonaz B, Taché Y. Water-avoidance stress-induced c-Fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21–28. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- 10.Bonaz B, Taché Y. Induction of Fos immunoreactivity in the rat brain after cold-restraint-induced gastric lesions and fecal excretion. Brain Res. 1994;652:56–64. doi: 10.1016/0006-8993(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 11.Barquist E, Bonaz B, Martinez V, Rivier J, Zinner MJ, Taché Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. Am. J. Physiol. 1996;270:R888–R894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- 12.Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract. III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- 13.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 14.Caso JR, Leza JC, Menchen L. The effects of physical and psychological stress on the gastrointestinal tract: lessons from animal models. Curr. Mol. Med. 2008;8:299–312. doi: 10.2174/156652408784533751. [DOI] [PubMed] [Google Scholar]
- 15.Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J. Clin. Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J. Gastroenterol. 2007;42 Suppl. 17:48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- 17.Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 18.Mönnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, et al. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig. Dis. 2001;19:201–211. doi: 10.1159/000050681. [DOI] [PubMed] [Google Scholar]
- 19.Mulak A, Bonaz B. Irritable bowel syndrome: a model of the brain-gut interactions. Med. Sci. Monit. 2004;10:RA55–RA62. [PubMed] [Google Scholar]
- 20.Halpert A, Drossman D. Biopsychosocial issues in irritable bowel syndrome. J. Clin. Gastroenterol. 2005;39:665–669. doi: 10.1097/01.mcg.0000174024.81096.44. [DOI] [PubMed] [Google Scholar]
- 21.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 23.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 24.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen. Comp. Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- 27.Chang CL, Hsu SY. Ancient evolution of stress-regulating peptides in vertebrates. Peptides. 2004;25:1681–1688. doi: 10.1016/j.peptides.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann. N. Y. Acad. Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 29.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr. Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 30.Teli T, Markovic D, Hewitt ME, Levine MA, Hillhouse EW, Grammatopoulos DK. Structural domains determining signaling characteristics of the CRH-receptor type 1 variant R1β and response to PKC phosphorylation. Cell. Signal. 2008;20:40–49. doi: 10.1016/j.cellsig.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Markovic D, Vatish M, Gu M, Slater D, Newton R, et al. The onset of labor alters corticotropin-releasing hormone type 1 receptor variant expression in human myometrium: putative role of interleukin-1β. Endocrinology. 2007;148:3205–3213. doi: 10.1210/en.2007-0095. [DOI] [PubMed] [Google Scholar]
- 32.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. Int. Union Pharmacol. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol. Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 33.Catalano RD, Kyriakou T, Chen J, Easton A, Hillhouse EW. Regulation ofcorticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol. Endocrinol. 2003;17:395–410. doi: 10.1210/me.2002-0302. [DOI] [PubMed] [Google Scholar]
- 34.Wu SV, Yuan PQ, Wang L, Peng YL, Chen CY, Taché Y. Identification and characterization of multiple corticotropin-releasing factor type 2 receptor isoforms in the rat esophagus. Endocrinology. 2007;148:1675–1687. doi: 10.1210/en.2006-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, et al. A soluble mouse brain splice variant of type 2α corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc. Natl. Acad. Sci. USA. 2005;102:2620–2625. doi: 10.1073/pnas.0409583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grace CR, Perrin MH, Cantle JP, Vale WW, Rivier JE, Riek R. Common and divergent structural features of a series of corticotropin releasing factor-related peptides. J. Am. Chem. Soc. 2007;129:16102–16114. doi: 10.1021/ja0760933. [DOI] [PubMed] [Google Scholar]
- 37.Farrokhi CB, Tovote P, Blanchard RJ, Blanchard DC, Litvin Y, Spiess J. Cortagine: behavioral and autonomic function of the selective CRF receptor subtype 1 agonist. CNS Drug Rev. 2007;13:423–443. doi: 10.1111/j.1527-3458.2007.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivier J, Gulyas J, Kunitake K, DiGruccio M, Cantle JP, et al. Stressin1-A, a potent corticotropin releasing factor receptor 1 (CRF1)-selective peptide agonist. J. Med. Chem. 2007;50:1668–1674. doi: 10.1021/jm0613875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swanson LW, Sawchenko PE, Lind RW. Regulation of multiple peptides in CRF parvocellular neurosecretory neurons: implications for the stress response. Prog. Brain Res. 1986;68:169–190. doi: 10.1016/s0079-6123(08)60238-1. [DOI] [PubMed] [Google Scholar]
- 40.Valentino RJ, Page ME, Luppi PH, Zhu Y, Van Bockstaele E, Aston-Jones G. Evidence for widespread afferents to Barrington’s nucleus, a brainstem region rich in corticotropin-releasing hormone neurons. Neuroscience. 1994;62:125–143. doi: 10.1016/0306-4522(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 41.De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 42.Valentino RJ, Kosboth M, Colflesh M, Miselis RR. Transneuronal labeling from the rat distal colon: anatomic evidence for regulation of distal colon function by a pontine corticotropin-releasing factor system. J. Comp. Neurol. 2000;417:399–414. [PubMed] [Google Scholar]
- 43.Venihaki M, Sakihara S, Subramanian S, Dikkes P, Weninger SC, et al. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviours. J. Neuroendocrinol. 2004;16:411–422. doi: 10.1111/j.1365-2826.2004.01170.x. [DOI] [PubMed] [Google Scholar]
- 44.Morin SM, Ling N, Liu XJ, Kahl SD, Gehlert DR. Differential distribution of urocortin-and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience. 1999;92:281–291. doi: 10.1016/s0306-4522(98)00732-5. [DOI] [PubMed] [Google Scholar]
- 45.Martinez V, Wang L, Million M, Rivier J, Taché Y. Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides. 2004;25:1733–1734. doi: 10.1016/j.peptides.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 46.Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J. Comp. Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- 47.Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J. Comp. Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Mano-Otagiri A, Shibasaki T. Distribution of urocortin 2 and urocortin 3 in rat brain. J. Nippon Med. Sch. 2004;71:358–359. doi: 10.1272/jnms.71.358. [DOI] [PubMed] [Google Scholar]
- 49.Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J. Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonaz B, Rivest S. Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. Am. J. Physiol. 1998;275:R1438–R1449. doi: 10.1152/ajpregu.1998.275.5.R1438. [DOI] [PubMed] [Google Scholar]
- 51.Imaki T, Katsumata H, Miyata M, Naruse M, Imaki J, Minami S. Expression of corticotropinreleasing hormone type 1receptor in paraventricular nucleus after acute stress. Neuroendocrinology. 2001;73:293–301. doi: 10.1159/000054646. [DOI] [PubMed] [Google Scholar]
- 52.Konishi S, Kasagi Y, Katsumata H, Minami S, Imaki T. Regulation of corticotropin-releasing factor (CRF) type-1 receptor gene expression by CRF in the hypothalamus. Endocr. J. 2003;50:21–36. doi: 10.1507/endocrj.50.21. [DOI] [PubMed] [Google Scholar]
- 53.Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc. Soc. Exp. Biol. Med. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- 54.Bittencourt JC, Sawchenko PE. Docentrally administered neuropeptides access cognate receptors? An analysis in the central corticotropin-releasing factor system. J. Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL. Constrained corticotropin releasing factor antagonists (astressin analogues) with long duration of action in the rat. J. Med. Chem. 1999;42:3175–3182. doi: 10.1021/jm9902133. [DOI] [PubMed] [Google Scholar]
- 56.Rühmann A, Bonk I, Lin CR, Rosenfeld MG, Spiess J. Structural requirements for peptidic antagonists of the corticotropin-releasing factor receptor (CRFR): development of CRFR2β-selective antisauvagine-30. Proc. Natl. Acad. Sci. USA. 1998;95:15264–15269. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, et al. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J. Med. Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 58.Martinez V, Rivier J, Taché Y. Peripheral injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks peripheral CRF-and abdominal surgery-induced delayed gastric emptying in rats. J. Pharmacol. Exp. Ther. 1999;290:629–634. [PubMed] [Google Scholar]
- 59.Lanier M, Williams JP. Small molecule corticotropin-releasing factor antagonists. Expert Opin. Ther. Patents. 2002;12:1619–1630. [Google Scholar]
- 60.Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin. Investig. Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- 61.Chen C. Recent advances in small molecule antagonists of the corticotropin-releasing factor type 1 receptor–focus on pharmacology and pharmacokinetics. Curr. Med. Chem. 2006;13:1261–1282. doi: 10.2174/092986706776873014. [DOI] [PubMed] [Google Scholar]
- 62.Ising M, Zimmermann US, Kunzel HE, Uhr M, Foster AC, et al. High-affinity CRF1 receptor antagonist NBI-34041: preclinical and clinical data suggest safety and efficacy in attenuating elevated stress response. Neuropsychopharmacology. 2007;32:1941–1949. doi: 10.1038/sj.npp.1301328. [DOI] [PubMed] [Google Scholar]
- 63.Seymour PA, Schmidt AW, Schulz DW. The pharmacology of CP-154526,a nonpeptide antagonist of the CRH1 receptor: a review. CNS Drug Rev. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front. Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- 65.Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front. Biosci. 2006;11:1878–1891. doi: 10.2741/1931. [DOI] [PubMed] [Google Scholar]
- 66.Lovejoy DA, Aubry JM, Turnbull A, Sutton S, Potter E, et al. Ectopic expression of the CRF-binding protein: minor impact on HPA axis regulation but induction of sexually dimorphic weight gain. J. Neuroendocrinol. 1998;10:483–491. doi: 10.1046/j.1365-2826.1998.00206.x. [DOI] [PubMed] [Google Scholar]
- 67.Karolyi IJ, Burrows HL, Ramesh TM, Nakajima M, Lesh JS, et al. Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proc. Natl. Acad. Sci. USA. 1999;96:11595–11600. doi: 10.1073/pnas.96.20.11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huising MO, Vaughan JM, Shah SH, Grillot KL, Donaldson CJ, et al. Residues of corticotropin releasing factor-binding protein (CRF-BP) that selectively abrogate binding to CRF but not to urocortin 1. J. Biol. Chem. 2008;283:8902–8912. doi: 10.1074/jbc.M709904200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henry BA, Lightman SL, Lowry CA. Distribution of corticotropin-releasing factor binding protein immunoreactivity in the rat hypothalamus: association with corticotropin-releasing factor-, urocortin 1-and vimentin-immunoreactive fibres. J. Neuroendocrinol. 2005;17:135–144. doi: 10.1111/j.1365-2826.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 70.Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol. Disord. Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am. J. Physiol. 1987;253:G582–G586. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- 72.Gué M, Junien JL, Buéno L. Conditioned emotional response in rats enhances colonic motility through the central release of corticotropin-releasing factor. Gastroenterology. 1991;100:964–970. doi: 10.1016/0016-5085(91)90270-u. [DOI] [PubMed] [Google Scholar]
- 73.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J. Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taché Y, Million M. Central corticotropin-releasing factor and the hypothalamic-pituitary-adrenal axis in gastrointestinal physiology. In: Johnson LR, Wood J, editors. Physiology of the Gastrointestinal Tract. Burlington, MA: Elsevier Academic; 2006. pp. 791–816. [Google Scholar]
- 75.Taché Y, Maeda-Hagiwara M, Turkelson CM. Central nervous system action of corticotropinreleasing factor to inhibit gastric emptying in rats. Am. J. Physiol. 1987;253:G241–G245. doi: 10.1152/ajpgi.1987.253.2.G241. [DOI] [PubMed] [Google Scholar]
- 76.Sheldon RJ, Qi JA, Porreca F, Fisher LA. Gastrointestinal motor effects of corticotropin-releasing factor in mice. Regul. Pept. 1990;28:137–151. doi: 10.1016/0167-0115(90)90013-m. [DOI] [PubMed] [Google Scholar]
- 77.Taché Y, Barquist E, Stephens RL, Rivier J. Abdominal surgery-and trephination-induced delay in gastric emptying is prevented by intracisternal injection of CRF antagonist in the rat. J. Gastrointest. Motil. 1991;3:19–25. [Google Scholar]
- 78.Sütö G, Király A, Taché Y. Interleukin 1 β inhibits gastric emptying in rats: mediation through prostaglandin and corticotropin-releasing factor. Gastroenterology. 1994;106:1568–1575. doi: 10.1016/0016-5085(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 79.Smedh U, Uvnas-Moberg K, Grill HJ, Kaplan JM. Fourth ventricle injection of corticotropinreleasing factor and gastric emptying of glucose during gastric fill. Am. J. Physiol. 1995;269:G1000–G1003. doi: 10.1152/ajpgi.1995.269.6.G1000. [DOI] [PubMed] [Google Scholar]
- 80.Coskun T, Bozkurt A, Alican I, Ozkutlu U, Kurtel H, Yegen BC. Pathways mediating CRF-induced inhibition of gastric emptying in rats. Regul. Pept. 1997;69:113–120. doi: 10.1016/s0167-0115(96)02066-6. [DOI] [PubMed] [Google Scholar]
- 81.Martinez V, Rivier J, Wang L, Taché Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF-and stress-related alterations of gastric and colonic motor function. J. Pharmacol. Exp. Ther. 1997;280:754–760. [PubMed] [Google Scholar]
- 82.Lee C, Sarna SK. Central regulation of gastric emptying of solid nutrient meals by corticotropin releasing factor. Neurogastroenterol. Motil. 1997;9:221–229. doi: 10.1046/j.1365-2982.1997.d01-58.x. [DOI] [PubMed] [Google Scholar]
- 83.Martinez V, Barquist E, Rivier J, Taché Y. Central CRF inhibits gastric emptying of a nutrient solid meal in rats: the role of CRF2 receptors. Am. J. Physiol. 1998;274:G965–G970. doi: 10.1152/ajpgi.1998.274.5.G965. [DOI] [PubMed] [Google Scholar]
- 84.Martinez V, Taché Y. Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res. 2001;893:29–35. doi: 10.1016/s0006-8993(00)03277-7. [DOI] [PubMed] [Google Scholar]
- 85.Chen CY, Million M, Adelson DW, Martinez V, Rivier J, Taché Y. Intracisternal urocortin inhibits vagally stimulated gastric motility in rats: role of CRF2. Br. J. Pharmacol. 2002;136:237–247. doi: 10.1038/sj.bjp.0704713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R427–R432. doi: 10.1152/ajpregu.00499.2004. [DOI] [PubMed] [Google Scholar]
- 87.Nagata T, Uemoto M, Yuzuriha H, Asakawa A, Inui A, et al. Intracerebroventricularly administered urocortin inhibits gastric emptying in mice. Int. J. Mol. Med. 2005;15:1041–1043. [PubMed] [Google Scholar]
- 88.Czimmer J, Million M, Taché Y. Urocortin 2 acts centrally to delay gastric emptying through sympathetic pathways while CRF and urocortin 1 inhibitory actions are vagal dependent in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G511–G518. doi: 10.1152/ajpgi.00289.2005. [DOI] [PubMed] [Google Scholar]
- 89.Heymann-Mönnikes I, Taché Y, Trauner M, Weiner H, Garrick T. CRF microinjected into the dorsal vagal complex inhibits TRH analog-and kainic acid-stimulated gastric contractility in rats. Brain Res. 1991;554:139–144. doi: 10.1016/0006-8993(91)90181-t. [DOI] [PubMed] [Google Scholar]
- 90.Mönnikes H, Schmidt BG, Raybould HE, Taché Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am. J. Physiol. 1992;262:G137–G143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- 91.Mönnikes H, Schmidt BG, Tebbe J, Bauer C, Taché Y. Microinfusion of corticotropin releasing factor into the locus coeruleus/subcoeruleus nuclei stimulates colonic motor function in rats. Brain Res. 1994;644:101–108. doi: 10.1016/0006-8993(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 92.Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J. Physiol. 2002;543:135–146. doi: 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smedh U, Moran TH. The dorsal vagal complex as a site for cocaine-and amphetamine-regulated transcript peptide to suppress gastric emptying. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R124–R130. doi: 10.1152/ajpregu.00234.2004. [DOI] [PubMed] [Google Scholar]
- 94.Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropinreleasing factor on gastrointestinal transit in the rat. Gastroenterology. 1988;94:598–602. doi: 10.1016/0016-5085(88)90229-6. [DOI] [PubMed] [Google Scholar]
- 95.Gué M, Fioramonti J, Frexinos J, Alvinerie M, Buéno L. Influence of acoustic stress by noise on gastrointestinal motility in dogs. Dig. Dis. Sci. 1987;32:1411–1417. doi: 10.1007/BF01296668. [DOI] [PubMed] [Google Scholar]
- 96.Kihara N, Fujimura M, Yamamoto I, Itoh E, Inui A, Fujimiya M. Effects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G406–G419. doi: 10.1152/ajpgi.2001.280.3.G406. [DOI] [PubMed] [Google Scholar]
- 97.Zhang H, Han T, Sun LN, Huang BK, Chen YF, et al. Regulative effects of essential oil from Atractylodes lancea on delayed gastric emptying in stress-induced rats. Phytomedicine. 2008;15:602–611. doi: 10.1016/j.phymed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 98.Iwa M, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture elicits dual effects: Stimulation of delayed gastric emptying and inhibition of accelerated colonic transit induced by restraint stress in rats. Dig. Dis. Sci. 2006;51:1493–1500. doi: 10.1007/s10620-006-9083-7. [DOI] [PubMed] [Google Scholar]
- 99.Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J. Endocrinol. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- 100.Bonaz B, Plourde V, Taché Y. Abdominal surgery induces Fos immunoreactivity in the rat brain. J. Comp. Neurol. 1994;349:212–222. doi: 10.1002/cne.903490205. [DOI] [PubMed] [Google Scholar]
- 101.Rivest S, Rivier C. Stress and interleukin-1β-induced activation of c-Fos, NGFI-B and CRF gene expression in the hypothalamic PVN: comparison between Sprague-Dawley, Fisher-344 and Lewis rats. J. Neuroendocrinol. 1994;6:101–117. doi: 10.1111/j.1365-2826.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 102.Yao M, Denver RJ. Regulation of vertebrate corticotropin-releasing factor genes. Gen. Comp. Endocrinol. 2007;153:200–216. doi: 10.1016/j.ygcen.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 103.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka Y, Makino S, Noguchi T, Tamura K, Kaneda T, Hashimoto K. Effect of stress and adrenalectomy on urocortin II mRNA expression in the hypothalamic paraventricular nucleus of the rat. Neuroendocrinology. 2003;78:1–11. doi: 10.1159/000071700. [DOI] [PubMed] [Google Scholar]
- 105.Korosi A, Schotanus S, Olivier B, Roubos EW, Kozicz T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain Res. 2005;1046:172–179. doi: 10.1016/j.brainres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 106.Wang X, Su H, Copenhagen LD, Vaishnav S, Pieri F, et al. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol. Cell Biol. 2002;22:6605–6610. doi: 10.1128/MCB.22.18.6605-6610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luckey A, Wang L, Jamieson PM, Basa NR, Million M, et al. Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology. 2003;125:654–659. doi: 10.1016/s0016-5085(03)01069-2. [DOI] [PubMed] [Google Scholar]
- 108.Luckey A, Livingston E, Taché Y. Mechanisms and treatment of postoperative ileus. Arch. Surg. 2003;138:206–214. doi: 10.1001/archsurg.138.2.206. [DOI] [PubMed] [Google Scholar]
- 109.Taché Y, Saperas E. Central actions of interleukin 1 on gastrointestinal function. In: Part B, De Souza EB, editors. Neurobiology of Cytokines. San Diego: Academic; 1993. pp. 169–183. [Google Scholar]
- 110.Rivest S. Molecular mechanisms and neural pathways mediating the influence of interleukin-1 on the activity of neuroendocrine CRF motoneurons in the rat. Int. J. Dev. Neurosci. 1995;13:135–146. doi: 10.1016/0736-5748(94)00063-9. [DOI] [PubMed] [Google Scholar]
- 111.Smedh U, Moran TH. Peptides that regulate food intake: separable mechanisms for dorsal hindbrain CART peptide to inhibit gastric emptying and food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1418–R1426. doi: 10.1152/ajpregu.00665.2002. [DOI] [PubMed] [Google Scholar]
- 112.Chen CY, Inui A, Asakawa A, Fujino K, Kato I, et al. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 113.Nakade Y, Tsukamoto K, Pappas TN, Takahashi T. Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res. 2006;1111:117–121. doi: 10.1016/j.brainres.2006.06.090. [DOI] [PubMed] [Google Scholar]
- 114.Taché Y, Martinez V, Million M, Rivier J. Corticotropin-releasing factor and the brain-gut motor response to stress. Can. J. Gastroenterol. 1999;13 doi: 10.1155/1999/375916. 18–25A. [DOI] [PubMed] [Google Scholar]
- 115.Buéno L, Fioramonti J. Effects of corticotropin-releasing factor, corticotropin and cortisol on gastrointestinal motility in dogs. Peptides. 1986;7:73–77. doi: 10.1016/0196-9781(86)90064-1. [DOI] [PubMed] [Google Scholar]
- 116.Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology. 1988;95:1510–1517. doi: 10.1016/s0016-5085(88)80070-2. [DOI] [PubMed] [Google Scholar]
- 117.Wittmann T, Crenner F, Angel F, Hanusz L, Ringwald C, Grenier JF. Long-duration stress. Immediate and late effects on small and large bowel motility in rat. Dig. Dis. Sci. 1990;35:495–500. doi: 10.1007/BF01536925. [DOI] [PubMed] [Google Scholar]
- 118.Kellow JE, Langeluddecke PM, Eckersley GM, Jones MP, Tennant CC. Effects of acute psychologic stress on small-intestinal motility in health and the irritable bowel syndrome. Scand. J. Gastroenterol. 1992;27:53–58. doi: 10.3109/00365529209011167. [DOI] [PubMed] [Google Scholar]
- 119.Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611–621. doi: 10.1016/0016-5085(88)90231-4. [DOI] [PubMed] [Google Scholar]
- 120.Taché Y. The parasympathetic nervous system in the pathophysiology of the gastrointestinal tract. In: Bolis CL, Licinio J, Govoni S, editors. Handbook of Autonomic Nervous System in Health and Disease. New York: Dekker; 2002. pp. 463–503. [Google Scholar]
- 121.Jimenez M, Buéno L. Inhibitory effects of neuropeptide Y (NPY) on CRF and stress-induced cecal motor response in rats. Life Sci. 1990;47:205–211. doi: 10.1016/0024-3205(90)90321-h. [DOI] [PubMed] [Google Scholar]
- 122.Gué M, Tekamp A, Tabis N, Junien JL, Buéno L. Cholecystokinin blockade of emotional stress-and CRF-induced colonic motor alterations in rats: role of the amygdala. Brain Res. 1994;658:232–238. doi: 10.1016/s0006-8993(09)90030-0. [DOI] [PubMed] [Google Scholar]
- 123.Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induced defecation in rats. Am. J. Physiol. 1998;274:G827–G831. doi: 10.1152/ajpgi.1998.274.5.G827. [DOI] [PubMed] [Google Scholar]
- 124.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involvedin stressrelated alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br. J. Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716–723. doi: 10.1016/0016-5085(93)91006-4. [DOI] [PubMed] [Google Scholar]
- 126.Million M, Wang L, Martinez V, Taché Y. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res. 2000;877:345–353. doi: 10.1016/s0006-8993(00)02719-0. [DOI] [PubMed] [Google Scholar]
- 127.Lu L, Liu D, Ceng X, Ma L. Differential roles of corticotropin-releasing factor receptor subtypes 1 and 2 in opiate withdrawal and in relapse to opiate dependence. Eur. J. Neurosci. 2000;12:4398–4404. [PubMed] [Google Scholar]
- 128.Saito K, Kasai T, Nagura Y, Ito H, Kanazawa M, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks brain-gut activation induced by colonic distention in rats. Gastroenterology. 2005;129:1533–1543. doi: 10.1053/j.gastro.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 129.Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J. Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gulpinar MA, Bozkurt A, Coskun T, Ulusoy NB, Yegen BC. Glucagon-like peptide (GLP-1) is involved in the central modulation of fecal output in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G924–G929. doi: 10.1152/ajpgi.2000.278.6.G924. [DOI] [PubMed] [Google Scholar]
- 131.Tebbe JJ, Ortmann E, Schumacher K, Mönnikes H, Kobelt P, et al. Cocaine-and amphetamineregulated transcript stimulates colonic motility via central CRF receptor activation and peripheral cholinergic pathways in fed, conscious rats. Neurogastroenterol. Motil. 2004;16:489–496. doi: 10.1111/j.1365-2982.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 132.Tebbe JJ, Mronga S, Tebbe CG, Ortmann E, Arnold R, Schäfer MK. Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1-and corticotrophin-releasing factor 1 receptor activation. J. Neuroendocrinol. 2005;17:570–576. doi: 10.1111/j.1365-2826.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 133.Nakade Y, Tsukamoto K, Iwa M, Pappas TN, Takahashi T. Glucagon-like peptide-1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Auton Neurosci. 2007;131:50–56. doi: 10.1016/j.autneu.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 134.Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, et al. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1037–G1044. doi: 10.1152/ajpgi.00419.2006. [DOI] [PubMed] [Google Scholar]
- 135.Ataka K, Kuge T, Fujino K, Takahashi T, Fujimiya M. Wood creosote prevents CRF-induced motility via 5-HT3 receptors in proximal and 5-HT4 receptors in distal colon in rats. Auton. Neurosci. 2007;133:136–145. doi: 10.1016/j.autneu.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 136.Hirata T, Keto Y, Nakata M, Takeuchi A, Funatsu T, et al. Effects of serotonin 5-HT3 receptor antagonists on CRF-induced abnormal colonic water transport and defecation in rats. Eur. J. Pharmacol. 2008;587:281–284. doi: 10.1016/j.ejphar.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 137.Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: pharmacological target for pelvic visceral dysfunctions. Trends Pharmacol. Sci. 1999;20:253–260. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- 138.Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur. J. Neurosci. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- 139.Kresse AE, Million M, Saperas E, Taché Y. Colitis induces CRF expression in hypothalamic magnocellular neurons and blunts CRF gene response to stress in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G1203–G1213. doi: 10.1152/ajpgi.2001.281.5.G1203. [DOI] [PubMed] [Google Scholar]
- 140.Sternberg EM, Young WS, 3rd, Bernardini R, Calogero AE, Chrousos GP, et al. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc. Natl. Acad. Sci. USA. 1989;86:4771–4775. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 142.Kawahito Y, Sano H, Mukai S, Asai K, Kimura S, et al. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut. 1995;37:544–551. doi: 10.1136/gut.37.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chatzaki E, Murphy BJ, Wang L, Million M, Ohning GV, et al. Differential profile of CRF receptor distribution in the rat stomach and duodenum assessed by newly developed CRF receptor antibodies. J. Neurochem. 2004;88:1–11. doi: 10.1046/j.1471-4159.2003.02078.x. [DOI] [PubMed] [Google Scholar]
- 144.Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J. Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 145.Taché Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol. Motil. 2004;16 Suppl. 1:137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 146.Porcher C, Juhem A, Peinnequin A, Sinniger V, Bonaz B. Expression and effects of metabotropic CRF1 and CRF2 receptors in rat small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G1091–G1103. doi: 10.1152/ajpgi.00302.2004. [DOI] [PubMed] [Google Scholar]
- 147.Liu S, Gao X, Gao N, Wang X, Fang X, et al. Expression of type 1 corticotropin-releasing factor receptor in the guinea pig enteric nervous system. J. Comp. Neurol. 2005;481:284–298. doi: 10.1002/cne.20370. [DOI] [PubMed] [Google Scholar]
- 148.Porcher C, Peinnequin A, Pellissier S, Meregnani J, Sinniger V, et al. Endogenous expression and in vitro study of CRF-related peptides and CRF receptors in the rat gastric antrum. Peptides. 2006;27:1464–1475. doi: 10.1016/j.peptides.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 149.Pappas T, Debas H, Taché Y. Corticotropin-releasing factor inhibits gastric emptying in dogs. Regul. Pept. 1985;11:193–199. doi: 10.1016/0167-0115(85)90050-3. [DOI] [PubMed] [Google Scholar]
- 150.Mayer EA, Sytnik B, Reddy NS, Van Deventer G, Taché Y. Corticotropin releasing factor (CRF) increases postprandial duodenal motor activity in humans. J. Gastrointest. Motil. 1992;4:53–60. [Google Scholar]
- 151.Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropinreleasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J. Pharmacol. Exp. Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 153.Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569–1579. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]
- 154.Taché Y. Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Dig. Dis. Sci. 1999;44 79S–86S. [PubMed] [Google Scholar]
- 155.Raybould H, Koelbel CB, Mayer EA, Taché Y. Inhibition of gastric motor function by circulating corticotropin-releasing factor in anesthetized rats. J. Gastrointest. Motil. 1990;2:265–272. [Google Scholar]
- 156.Mancinelli R, Azzena GB, Diana M, Forgione A, Fratta W. In vitro excitatory actions of corticotropin-releasing factor on rat colonic motility. J. Auton. Pharmacol. 1998;18:319–324. doi: 10.1046/j.1365-2680.1998.1860319.x. [DOI] [PubMed] [Google Scholar]
- 157.Million M, Maillot C, Saunders P, Rivier J, Vale W, Taché Y. Human urocortin II, a new CRF-related peptide, displays selective CRF2-mediated action on gastric transit in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G34–G40. doi: 10.1152/ajpgi.00283.2001. [DOI] [PubMed] [Google Scholar]
- 158.Saunders PR, Maillot C, Million M, Taché Y. Peripheral corticotropin-releasing factor induces diarrhea in rats: role of CRF1 receptor in fecal watery excretion. Eur. J. Pharmacol. 2002;435:231–235. doi: 10.1016/s0014-2999(01)01574-6. [DOI] [PubMed] [Google Scholar]
- 159.Yuan PQ, Million M, Wu SV, Rivier J, Taché Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A, activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol. Motil. 2007;19:923–936. doi: 10.1111/j.1365-2982.2007.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Buéno L, Fargeas MJ, Gué M, Peeters TL, Bormans V, Fioramonti J. Effects of corticotropinreleasing factor on plasma motilin and somatostatin levels and gastrointestinal motility in dogs. Gastroen-terology. 1986;91:884–889. doi: 10.1016/0016-5085(86)90690-6. [DOI] [PubMed] [Google Scholar]
- 161.Su YC, Doran S, Wittert G, Chapman IM, Jones KL, et al. Effects of exogenous corticotropin-releasing factor on antropyloroduodenal motility and appetite in humans. Am. J. Gastroenterol. 2002;97:49–57. doi: 10.1111/j.1572-0241.2002.05422.x. [DOI] [PubMed] [Google Scholar]
- 162.Van Den Elzen BD, Van Den Wijngaard RM, Tytgat GN, Boeckxstaens GE. Influence of corticotropin-releasing hormone on gastric sensitivity and motor function in healthy volunteers. Eur. J. Gastroenterol. Hepatol. 2007;19:401–407. doi: 10.1097/01.meg.0000252635.26538.a2. [DOI] [PubMed] [Google Scholar]
- 163.Maillot C, Wang L, Million M, Taché Y. Intraperitoneal corticotropin-releasing factor and urocortin induce Fos expression in brain and spinal autonomic nuclei and long lasting stimulation of colonic motility in rats. Brain Res. 2003;974:70–81. doi: 10.1016/s0006-8993(03)02553-8. [DOI] [PubMed] [Google Scholar]
- 164.Miampamba M, Maillot C, Million M, Taché Y. Peripheral CRF activates myenteric neurons in the proximal colon through CRF1 receptor in conscious rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G857–G865. doi: 10.1152/ajpgi.00434.2001. [DOI] [PubMed] [Google Scholar]
- 165.Barquist E, Zinner M, Rivier J, Taché Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am. J. Physiol. 1992;262:G616–G620. doi: 10.1152/ajpgi.1992.262.4.G616. [DOI] [PubMed] [Google Scholar]
- 166.Nozu T, Martinez V, Rivier J, Taché Y. Peripheral urocortin delays gastric emptying: role of CRF receptor 2. Am. J. Physiol. 1999;276:G867–G874. doi: 10.1152/ajpgi.1999.276.4.G867. [DOI] [PubMed] [Google Scholar]
- 167.Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, et al. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am. J. Physiol. 1996;271:G884–G892. doi: 10.1152/ajpgi.1996.271.5.G884. [DOI] [PubMed] [Google Scholar]
- 168.Harada S, Imaki T, Naruse M, Chikada N, Nakajima K, Demura H. Urocortin mRNAisexpressed in the enteric nervous system of the rat. Neurosci. Lett. 1999;267:125–128. doi: 10.1016/s0304-3940(99)00349-3. [DOI] [PubMed] [Google Scholar]
- 169.Yuan PQ, Wu SV, Million M, Larauche MH, Wang L, Taché Y. Corticotropin releasing factor (CRF) in rat colon: expression, localization and regulation by stress. Gastroenterology. 2008;134 Suppl. 1:A291. [Google Scholar]
- 170.Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr. Mol. Med. 2008;8:274–281. doi: 10.2174/156652408784533760. [DOI] [PubMed] [Google Scholar]
- 171.Funada M, Hara C, Wada K. Involvement of corticotropin-releasing factor receptor subtype 1 in morphine withdrawal regulation of the brain noradrenergic system. Eur. J. Pharmacol. 2001;430:277–281. doi: 10.1016/s0014-2999(01)01402-9. [DOI] [PubMed] [Google Scholar]
- 172.Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JA, et al. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res. 2003;985:32–42. doi: 10.1016/s0006-8993(03)03027-0. [DOI] [PubMed] [Google Scholar]
- 173.Gabry KE, Chrousos GP, Rice KC, Mostafa RM, Sternberg E, et al. Marked suppression of gastric ulcerogenesis and intestinal responses to stress by a novel class of drugs. Mol. Psychiatry. 2002;7:474–483. doi: 10.1038/sj.mp.4001031. [DOI] [PubMed] [Google Scholar]
- 174.Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, et al. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc. Natl. Acad. Sci. USA. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]