The notion of habituation is as old as humankind. As Ctesippus says in Plato's Lysis:
“Indeed, Socrates, he has literally deafened us and stopped our ears with the praises of Lysis; and if he is a little intoxicated, there is every likelihood that we may have our sleep murdered with a cry of Lysis.”
To take an even older example:
“A fox who had never yet seen a lion, when he fell in with him for the first time in the forest was so frightened that he was near dying with fear. On his meeting with him for the second time, he was still much alarmed, but not to the same extent as at first. On seeing him the third time, he so increased in boldness that he went up to him and commenced a familiar conversation with him.” (Æsop's Fables.)
Experimental studies, or at least observations of phenomena of habituation for a variety of responses in a wide range of organisms from amoebas to humans literally exploded at the end of the nineteenth century and early twentieth century. See Harris (1943) and Jennings (1906). I was unable to determine who first used the term habituation in this context, but it was in widespread use early in the twentieth century. In his classic text on learning, Humphrey (1933) notes that a range of terms, “acclimatization,” “accommodation,” “negative adaptation,” “fatigue” have been used to describe the phenomenon. Harris (1943) in his classic review adds the terms “extinction” and “stimulatory inactivation” to the list. As he notes,
“While none of the terms cited is especially appropriate to this type of response decrement, we shall use the term “habituation” throughout. Little can be said in favor of this term except that all the others imply an explanation which is unjustified by the facts, or have some more valid use in another connection. Perhaps the most commonly applied term, negative adaptation, seems to deny that response decrement may be an active process. Habituation on the other hand, has had in its favor that it is not ordinarily applied to other types of behavior, implies the knowledge of no specific or general mechanism underlying the phenomenon (of which we are as yet in almost total ignorance), and in addition has been freely used in referring to exactly the type of behavior modification of which we speak.” (Harris, 1943, pp. 385-386).”
The companion phenomenon of “dishabituation” or “dehabituation,” the restoration of an habituated response by extraneous stimulation, was early studied by Holmes (1912) in the sea urchin. Humphrey (1933) provides an example with human infants:
“The phenomenon may easily and prettily be demonstrated on a young baby. The hands are clapped behind the child's back every two seconds; blinking occurs several times, but has generally died down by the sixth or seventh stimulation. Habituation has set in. The cradle is then given a sharp blow, and the hands are once more clapped, keeping the proper interval by counting. The child will be observed to blink again. The explanation seems to be that the blow on the cradle requires a new adjustment on the part of the organism which is inconsistent with that involved in effecting the habituation.” (p.142.)
Humphrey provides the following explanation:
“The peculiar process involved in the establishment of equilibrium are thus nullified and habituation has to be re-established. Dehabituation by lapse of time and by another stimulus are thus fundamentally the same, for they involve each of them the derangement of an established state of equilibrium by altered conditions, the alteration being one of increase of environmental energy in the one case, of decrease in the other.” (p.142.)
Humphrey thus argues that dehabituation is an actual removal or elimination of the process of habituation, a restoration to the original unhabituated condition, a view that persisted at least into the 1960s.
In his classic text, “The Integrative Action of the Nervous System” Sherrington (1906) analyzed fatigue of the scratch and flexion reflexes in the spinal dog. He was able to rule out sensory receptor adaptation as a mechanism of reflex fatigue by showing that stimulation of skin areas adjacent to the repeatedly stimulated area also exhibited fatigue, indeed he described the phenomena of stimulus generalization, the further separate the skin area was, the less the fatigue. Similarly he ruled out muscle fatigue by showing that the fatigued muscle response was in fact normal when activated by a different reflex. In short, fatigue was a central phenomenon. He also described several parametric features, i.e., weak stimulation led to more rapid fatigue (he notes this to be paradoxical). Reflex fatigue is in fact an example of habituation.
In an elegant series of experiments Proser and Hunter (1936) compared habituation (they used the term extinction) of the startle response in the intact rat and of spinal reflexes in the spinal rat, showing that they indeed exhibited common properties. By the time of Humphrey (1933) and Harris (1943) it was generally agreed that habituation was a central phenomenon, at least in organisms with nervous systems, and that it was an instance of elementary learning.
Modern interest in habituation really began with an extraordinarily influential paper by Sharpless and Jasper (1956) on habituation of EEG arousal. Using repeated presentations of brief tones they found that cortical EEG arousal of the normally sleeping cat (recorded through implanted electrodes) becomes progressively shorter and finally disappears. After cessation of stimulation the arousal response exhibits spontaneous recovery over a period of minutes or hours. Further, a strong sudden stimulus that differs markedly from the habituating stimulus causes dishabituation of the EEG arousal to the original stimulus. One very interesting aspect of Sharpless and Jasper's experiment was the specificity of the EEG arousal habituation in terms of stimulus characteristics. If the EEG arousal response of the sleeping animal was habituated to presentations of a 500-cycle tone to the point at which no arousal occurred, a 1000-cps tone would exhibit strong EEG arousal. However, if a 600-cps tone was presented after habituation to the 500-cps tone, no EEG arousal occurred. In behavioral terminology this could be described as an auditory frequency generalization gradient for EEG arousal.
The human alpha blocking response, which resembles EEG arousal in the cat, was shown to habituate to tactile, auditory, and visual stimulation by Sokolov and his associates in the Soviet Union (Sokolov, 1960). Glickman and Feldman (1961) demonstrated that peripheral receptors are probably not involved in habituation of EEG arousal to sensory stimulation. They induced cortical EEG arousal by electrical stimulation through electrodes implanted in the midbrain reticular formation in animals. Under these conditions habituation of EEG arousal occurred just as it did in earlier experiments using tones.
Following Sharpless and Jasper's study great interest developed in habituation as a fundamental form of behavioral plasticity. As noted earlier, it occurs for virtually all behavioral responses in virtually all organisms. A number of investigators reported evoked response habituation to various types of stimuli at most levels of the CNS, from first-order sensory nuclei to the cerebral cortex. The first experiment to report evoked response habituation was that of Hernández-Peón, Scherrer, and Jouvet (1956); they recorded responses to click stimulation at several levels of the auditory system. Trains of clicks were delivered once every two seconds for long periods of time and evoked responses of the cochlear nucleus (the first relay of the auditory system) were reported to habituate. This paper was also extremely influential in reviving interest in habituation.
Careful studies by Worden and associates (see Worden and Marsh, 1963; Marsh, Worden, and Hicks, 1962) demonstrated that click evoked responses at the cochlear nucleus do not show habituation. Instead, amplitudes of responses at this first relay nucleus in the auditory system are rigidly controlled by the physical properties of the sound stimulus. Because of acoustic factors, the intensity of a sound is often weaker at the floor of a test cage. If an animal gradually became bored and rested his head on the floor, the cochlear nucleus evoked response to click would decrease because of reduced sound intensity. If sound at the ear is held constant, there is no habituation of the evoked response at the cochlear nucleus.
Some of the basic properties of habituation were described in the classic works noted above (Harris, 1943; Humphrey, 1933; Jennings, 1906; Prosser and Hunter, 1936). In 1966, Thompson and Spencer surveyed the by then very extensive behavioral literature on habituation and identified some nine basic parametric properties or characteristics exhibited by behavioral habituation.
A few of personal remarks, if I may. William Alden Spencer and I were undergraduates together at Reed College in Portland Oregon and we became close friends. He went to Medical School (University of Oregon) and I went to graduate school (University of Wisconsin). Alden then did a postdoc at NIH where he and Eric Kandel did their pioneering work on hippocampal physiology. Meanwhile I spent a several year postdoc in neurophysiology with Clinton Woolsey at the University of Wisconsin School of Medicine. Alden then did a further postdoc in Moruzzi's Laboratory at the University of Pisa. I had by that time accepted an assistant professorship at the University of Oregon Medical School in Psychiatry. Alden then accepted a position as Assistant Professor in the Physiology Department at the University of Oregon Medical School. We shared two adjoining basement laboratories.
Earlier we had planned our initial joint project: Spinal conditioning, i.e., classical conditioning of the hindlimb flexion reflex in the acute spinal cat. This was a very controversial phenomenon then. But at that time more was known about the circuitry and physiology of the mammalian spinal cord than other regions of the nervous system, due largely to the work of John Eccles and his many associates. Initially, we used hindlimb paw shock as an unconditioned stimulus. However, each time we gave a series of shocks the flexion reflex habituated dramatically. It was such a robust phenomenon that we decided to study it instead of classical conditioning, and the rest is published history. (Later, junior colleagues and I did establish spinal conditioning as a genuine phenomenon, e.g., Patterson et al., 1973.) Alden was a brilliant and creative person and a superb neurophysiologist. He died tragically at an early age.
Below I list the parameters of habituation, together with some supporting evidence from the earlier literature. Note that the focus was on short term or within session habituation (characteristics 1, 2, 4, 5, 6, 7, 8). Characteristic 3 dealt with long term or between session habituation and property 9 dealt with long-term or between session dishabituation (sensitization).
“1. Given that a particular stimulus elicits a response, repeated applications of the stimulus result in decreased response (habituation). The decrease is usually a negative exponential function of the number of stimulus presentations.
“Examples of response habitation can probably be found in essentially all behaviorial studies where a stimulus is regularly presented. In earlier experiments devoted to habituation, per se, parametric characteristics were studied for a variety of responses (cf. Harris, 1943) ranging from postrotatory nystagmus (Griffith, 1920; Wendt, 1951) to startle (Prosser & Hunter, 1936) and galvanic skin response (GSR—Davis, 1934). With the exception of the “knee jerk” reflex (Lombard, 1887; Prosser & Hunter, 1936) habituation was a consistent finding, usually exhibiting an exponential course.
“2. If the stimulus is withheld, the response tends to recover over time (spontaneous recovery).
“Spontaneous recovery is reported in most of the studies noted above and has come to be the most common method of demonstrating that a given response decrement is an example of habituation (Harris, 1943). The time course of spontaneous recovery is markedly influenced by many variables and is not necessarily characteristic of a given response. Thus, the habituated startle response to sound in the intact rat may recover in 10 minutes (Prosser & Hunter, 1936) or fail to recover in 24 hours (J.S. Brown, Personal communication, 1964), depending upon details of testing. Consequently any categorization of types of habituation based solely on recovery time is likely to be somewhat artificial.
“3. If repeated series of habituation training and spontaneous recovery are given, habituation becomes successively more rapid (this might be called potentiation of habituation).
“Humphrey (1933) noted this effect in his studies on turtle leg withdrawal to shell tap. Konorski (1948) describes it for the orientating response, and it has been described in many studies where repeated habituation series were given (e.g., Davis, 1934).
“4. Other things being equal, the more rapid the frequency of stimulation, the more rapid and/or more pronounced is habituation.
“Numerous examples of this were noted in the earlier reflex studies (Harris, 1943) as well as in more recent work on stimulus satiation and curiosity (Glanzer, 1953; Welker, 1961). The effect occurs in terms of real time course and occurs within certain limits in terms of number of trials as well.
“5. The weaker the stimulus, the more rapid and/or more pronounced is habituation. Strong stimuli may yield no significant habituation.
“This relationship is characteristic of most types of responses ranging from simple reflexes (Harris, 1943) to complex exploratory behavior (Welker, 1961). Postrotatory optic nystagmus may be an exception in that under some conditions the degree of habituation is directly related to velocity of rotation (G. Crampton, personal communication, 1964).
“6. The effects of habituation training may proceed beyond the zero or asymptotic response level.
“Additional habituation training given after the response has disappeared or reached a stable habituated level will result in slower recovery. Although relatively few experiments have studied “below-zero” habituation as such (Humphrey, 1933; Prosser & Hunter, 1936, Wendt, 1951), the observations may be viewed as an extension of the relationship between number of stimulus presentations and degree of habituation. Zero response level is of course to some degree dependent upon the particular response measures used.
“7. Habituation of response to a given stimulus exhibits stimulus generalization to other stimuli.
“Coombs (1938) demonstrated generalization of GSR habituation to different types of auditory stimulation, and Porter (1938) demonstrated cross-modal generalization of the habituated GSR for light and tone stimuli. Mowrer (1934) showed some generalization of postrotatory nystagmus habituation in the pigeon. In a recent study, Crampton and Schwam (1961) reported generalization of optic nystagmus habituation in the cat to different degrees of angular acceleration.
“8. Presentation of another (usually strong) stimulus results in recovery of the habituated response (dishabituation).
“This phenomenon appears to be as ubiquitous as habituation itself and is commonly used to demonstrate that habituation has occurred. Pavlov (1927) was perhaps the first to describe this process (i.e., disinhibition) in relation to an extinguished conditioned response (CR), but also applied it to the habituated orienting response. Humphrey (1933) studied dishabituation extensively in lower vertebrates. Essentially all responses of mammals that can be habituated can also be dishabituated (Harris, 1943). It is not always necessary for the dishabituatory stimulus to be strong. In fact Sokolov (1960) and Voronin and Sokolov (1960) reported that a decrease in the intensity of an auditory stimulus results in dishabituation of the habituated orienting response in humans. Dishabituation, viewed as neutralization of the process of habituation (Humphrey, 1933), has been perhaps the most important method of distinguishing between habituation and “fatigue.”
“9. Upon repeated application of the dishabitutory stimulus, the amount of dishabituation produced habituates (this might be called habituation of dishabituation).
“Most studies of dishabituation (see above) have noted its habituation. Lehner (1941) has done the most careful parametric studies, showing that habituation of dishabituation follows a negative exponential course for the startle response in the rat and the abdominal reflex in man. More recently, Hagbarth and Kugelberg (1958) and Hagbarth and Finer (1963) verified and extended Lehner's findings for the abdominal and leg flexion reflexes in humans. Crampton and Schwam (1961) have shown that dishabituation of postrotatory nystagmus in the cat by audotory or cutaneous habituates in a similar fashion.
“In reviewing the behavioral habituation literature, it is striking to find virtually complete agreement on the parametric characteristics of the phenomenon in such a wide variety of animals and responses. These nine common characteristics may consequently serve as the detailed operational definition of habituation, replacing the more general definition given above. The extent to which any other response decrements satisfy these characteristics will thus determine whether they can be called habituation.” (Thompson and Spencer, 1966, pp.18-20).
These nine defining properties of habituation were a major issue for discussion in this symposium and will be treated later (Rankin??). I note that Davis and Wagner (1968) challenged the parameter concerning stimulus intensity. Recall that Sherrington, describing this effect that weaker stimuli yielded more rapid “fatigue” than stronger stimuli, felt it to be paradoxical. Davis and Wagner used the acoustic startle response in the rat and found that habituation to an intense stimulus caused a greater degree of absolute response decrement when tested with a weak stimulus than habituation to the weak stimulus. However, they also noted that if habituation and test stimuli were of identical intensities, then the relative degree of habituation increases as stimulus intensity is decreased, in accordance with Thompson and Spencer's characteristic number 5. So the key is absolute vs. relative measures of habituation. As Groves and Thompson (1970) stressed, characteristic number 5 must refer to relative rather than absolute measures of response strength.
In an ingenious and rather complex study Davis and Wagner (1969) showed that a group given gradually increasing intensity of tone (rat-startle response) showed the greatest habituation, the constant loud intensity showed less habituation and a constant intermediate intensity group showed the least habituation and a marked rebound when tested at a loud intensity. As they noted, these results could not be accounted for by a single-process theory. However, Groves and Thompson (1970) were able to account for these results with their Dual-Process theory, and actually reproduced these results using the hind limb flexion reflex of the acute spinal cat (see below).
Using the spinal flexion reflex Thompson and Spencer were able to rule out changes in skin receptors, cutaneous afferent nerve terminals and in motor neurons as loci of the decremental process underlying habituation, in accordance with Sherrington's earlier speculations. The decremental process must occur in interneurons. Perhaps their most important discovery was the fact that dishabituation was not a disruption of habituation but rather an independent superimposed process of sensitization. The decremental process underlying habituation was not disrupted at all by dishabituation. Indeed, in the flexion reflex, dishabituation (sensitization) always produce an increase in excitability of motor neurons. To the extent tested in mammalian systems, dishabituation is in fact a separate process of sensitization, but an exception has been noted in Aplysia (Rankin and Carew, 1988). These observations led Groves and Thompson (1970) to develop the dual process theory (see below).
A number of theories, or at least hypotheses, concerning the process of habituation have been proposed over the years. A few examples are: Stimulus satiation (Glanzer, 1953); Reactive inhibition (Hull, 1943); Afferent neuronal inhibition (Hernández-Peón, 1960); Cholinergic inhibition (Carlton, 1968); Classical conditioning (Stein, 1966). Actually, many of these theories were more generally concerned with processes of learning and not developed specifically to deal with habituation. These and other views are treated at length in a number of publication, e.g., Groves and Thompson, 1970; Peeke and Herz, 1973 a&b; Thompson and Spencer, 1966. In most cases these theories collided with uncooperative facts. However, three theories have been relatively successful and are still prominent today: Eugene Sokolov's (1960; 1963a, b) Stimulus-Model Comparator Theory; Allan Wagner's (1979) Revision of Konorski's Gnostic Hypothesis; and Groves and Thompson's (1970) Dual Process Theory. I treat each of these briefly here.
Stimulus-Model Comparator Theory
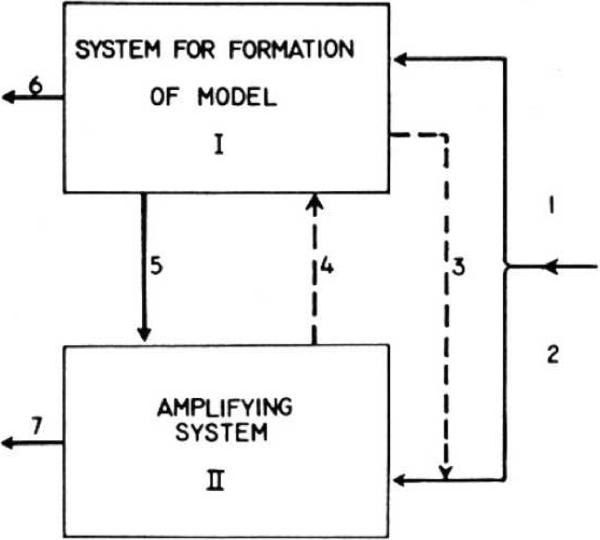
Evgeny Sokolov (1960; 1963) developed a most influential Stimulus-Model Comparator theory of habituation (see Fig. 1). It was based primarily on his observation of the orienting response, often measured as arousal in EEG activity. The basic notion is that as a result of repeated stimulation a stimulus model is formed in the brain, specifically in the cerebral cortex. In addition, there is an amplifying system that normally subverses behavioral output. A novel stimulus will result in a large orienting response, mediated by the amplifying system, identified with the ascending reticular activity system in lower brain regions. As the same stimulus is repeated, the stimulus model develops and as it develops, it exerts increasing inhibition on the amplifying system via descending corticofugal influences, thus yielding habituation. If a new or altered stimulus occurs which does not match the model, then inhibition is released and response strength recovers accordingly.
Figure 1.
Sokolov's model of habituation. Sensory input through lines 1 and 2 project to both the model formation and the amplfying systems. With repeated stimulation, the model develops and inhibits the amplifying system. See text for details (From Sokolov, 1960).
Wagner-Konorski Gnostic Unit Theory
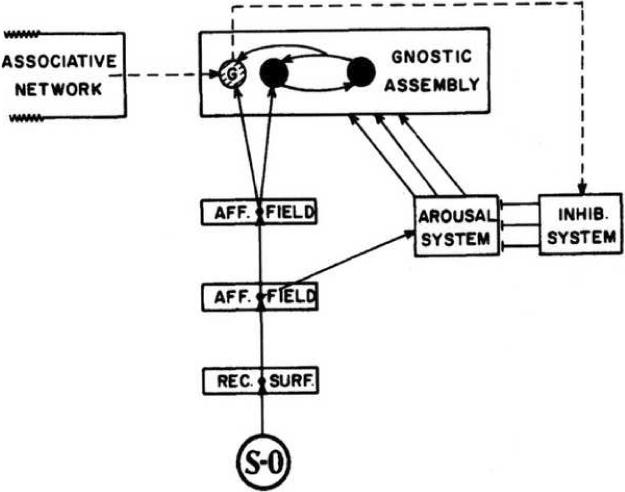
Konorski briefly developed a theory of habituation that is in many ways analogous to Sokolov's theory (Konorski, 1967). Allan Wagner (1979) elaborated Konorski's notion with greater emphasis on the roles of short-term memory and the existing associative network. This model is shown in Fig. 2. A stimulus (SO = stimulus object) is processed via afferent fields to project to a memory system, the Gnostic assembly, and to the arousal system. As the stimulus is repeated, a gnostic unit is formed, an increasingly accurate neuronal model or memory of the stimulus. As this model develops it increasingly activates an inhibitory system that inhibits the arousal system, resulting in habituation. Wagner added two processes to Konorski's model, a reverberating circuit of transient memory (short-term memory - the two solid circles in the gnostic assembly) and the influence of the preexisiting associative network. Indeed, Wagner's reformation of Konorski's model is in the main stream of information-processing theory (e.g., Anderson and Bower, 1973; Estes, 1975; Schiffrin and Schnieder, 1977). A key notion introduced by Wagner is that contextual cues may act via the associative network to excite stimulus representations in memory, i.e., in the Gnostic assembly. An important implication of this view is that some response systems should show context-specific long-term habituation.
Figure 2.
Wagner-Konorski's model of habituation. Sensory input forms a memory model in the Gnostic assembly. As the model develops it inhibits the arousal system. See text for details (From Wagner, 1979).
Groves and Thompson Dual Process Theory
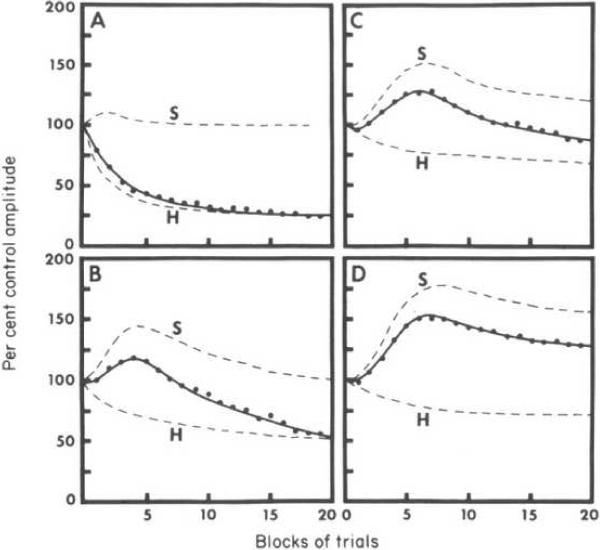
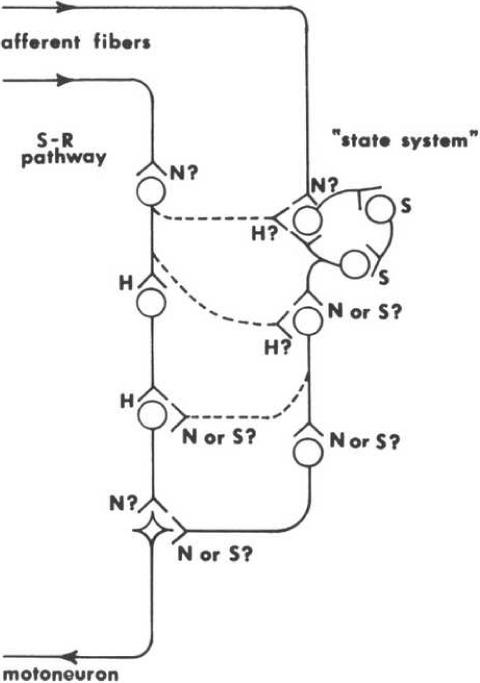
The basic assumption is that any effective stimulus will result in two independent processes in the central nervous system, one decremental (habituation) and one incremental (sensitization) that interact (see Fig. 3). It is further assumed that habituation develops in the stimulus-response (S-R) pathway for whatever stimulus-evoked response is being habituated and that sensitization develops in a separate state system which then acts on the S-R pathway to yield the final behavioral outcome (see Fig. 4). Strong supporting evidence for this theory came from studies of the activity of interneurons in the spinal cord (see e.g., Glanzman et al., 1972; Groves et al., 1969; Groves and Thompson, 1970; 1973).
Figure 3.
Basic notion of the dual process theory of habituation. Two processes (dashed lines) elicited by stimuli, one sensitizing and one habituating, interact to yield the behavioral response (solid line) (From Groves and Thompson, 1970).
Figure 4.
Neuronal model of the dual process theory. The S-R pathway develops habituation and the state pathway may develop sensitization. They interact to yield behavior. See text for details (From Groves and Thompson, 1970).
It is not my purpose here to provide a detailed critique of these theories although my bias is obvious. Instead I will simply note a few observations.
All three theories have much in common. First, they all propose that a model of the stimulus develops as a result of repetition. This model is viewed more-or-less as a memory trace in the Sokolov and Wagner-Konorski models and simply as a decrement in synaptic transmission in the dual-process model. Such a synaptic decrement can of course qualify as a memory. Second, all three theories have an arousal system (“amplifying” in Sokolov; “arousal” in Wagner-Konorski; “state” in dual-process).
In terms of differences, Both Sokolov and Wagner postulate a descending inhibitory system activated by the memory trace that inhibits the arousal system. The dual-process theory postulates no such inhibitory system. Instead, both the S-R pathway and the state system activated by stimulus repetition will habituate. In my view this is a key difference. Another difference is that Sokolov, and particularly Wagner-Konorski, focus on long-term or between session habituation whereas much of the focus in the Dual-Process theory is on short-term or within session habituation.
Perhaps that most significant difference between dual-process and both Sokolov and Wagner-Konorski concerns the response measures used. The dual-process theory emphasizes discrete muscle responses whereas the other two focus on nonspecific or “state” measures, e.g., EEG arousal, GSR, vasoconstriction, etc. One of the most dramatic predictions from Sokolov's theory (and also, I believe, the Wagner-Konorski theory) is the missing stimulus effect - if a stimulus is repeatedly presented at a fixed interval until the response has habituated, and if now the stimulus omitted on one trial, the response will recur full-blown. Voronin and Sokolov (1960) reported this for the EEG alpha-blocking response (arousal) in humans.
Note that the missing stimulus effect is really an example of temporal conditioning. In general, temporal conditioning can be obtained most readily with nonspecific responses, salivation (Pavlov, 1927), EEG arousal (Jasper and Shagass, 1941), galvanic shin response (Lockhart, 1966). Hull (1934) found that regularly repeated shocks yielded temporal conditioning (in humans) of the galvanic response but not finger withdrawal, suggesting that in contrast to “state” measures, discrete muscle responses do not show temporal conditioning.
In 1973, when I was at Harvard, Sokolov joined me for two weeks and we attempted to establish temporal conditioning of the eyeblink response in rabbits. Animals were given a corneal airpuff every minute for 100 trials per day. On occational trials the US was omitted and there was no response - no evidence of temporal conditioning. So temporal conditioning seems primarily to be a phenomenon of “state” variables. In contrast to the classical conditioning of discrete muscle responses, it is easy to establish operant temporal avoidance conditioning of discrete motor responses (Herrnstein, 1969; Lockhart & Steinbrecher, 1963; Sidman, 1962).
Mechanisms of Habituation
In animals with nervous systems, the underlying processes yielding behavioral habituation are due to alteration in neurons and synapses (see below). But single cell animals that behave, e.g., amoeba and paramecium, also show at least some phenomena of habituation (see Harris, 1943; Jennings, 1906) and they of course have no neurons. Perhaps the most simplified or reduced preparation used for habituation is the PC12 cell line, studied by Daniel Koshland and associates (McFadden & Koshland, 1990 a & b). The cells in this cell culture do not behave but they do secrete. The PC12 cell culture is a pheochromocytoma (cancer) cell line from the adrenal medulla. The cells are immortal in that they stop dividing until activated by nerve growth factor. When appropriately stimulated they secrete norepinephrine (NE) and other neurotransmitters. Koshland stimulated PC12 cells by pulses of potassium ions (K+) and measured release of 3HNE from the culture. The amount released showed clear habituation and exhibited both the frequency effect (more rapid and pronounced habituation with more rapid frequency of stimulation) and the intensity effect (the weaker the stimulus the more rapid and pronounced the habituation). Further, they showed both within session and between session habituation, i.e., both short and long term “memory.” Interestingly, NE release was also stimulated by acetycholine (ACh) pulses and this also habituated. The habituation to K+ pulses and ACh pulses were independent of each other, and treatment by PMA (a phorbal ester) dishabituated the habituated response to K+ but not to ACh. So NE release by K+ and ACh seems to involve differing mechanisms. The PC12 cell culture appears to be a most promising model for analysis of the physical-chemical bases of “habituation” like pheonomena.
In animals with nervous systems, a consistent picture appeared to emerge in the 1970's of the basic synaptic mechanism of habituation. In a series of elegant experiments, Eric Kandel and associates analyzed within-session habituation of the monosynaptic gill-withdrawal response in Aplysia (see e.g., Kandel, 1975, Pinsker et al., 1970). In brief, repeated stimulation resulted in a decrease in the probability of neurotransmitter release at the sensory-motor synapse, a presynaptic process, due in turn to a decrease in calcium ion influx at the sensory nerve terminals.
Thompson and Spencer, working with the polysynaptic spinal flexion reflex, were unable to establish this but their results were certainly consistent with synaptic depression as a mechanism (see, e.g., Spencer et al., 1966). Later, using the descending monosynaptic pathway from lateral column to motor neuron in the isolated frog spinal cord, the Thompson group were able to show habituation was due to a presynaptic decrease in synaptic efficacy, consistent with Kandel's earlier findings (Farel et al., 1973; Farel and Thompson, 1976; Glanzman and Thompson, 1979). So a clear picture seemed to emerge, at least for within-session habituation. Unfortunately, more recent work suggests that the mechanisms of both short and long-term habituation are far more complex than earlier believed (see, e.g., Ezzeddine & Glanzman, 2003; Li, Roberts & Glanzman, 2005; Rose and Rankin, 2001).
In this brief review I have attempted to highlight important historical developments in the study of habituation. But habituation is by no means just an historical curiosity. From 2000-2005, over 50,000 publications were concerned with habituation (pub-med citations).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson J, Bower G. Human associative memory. Winston; New York: 1973. [Google Scholar]
- Carlton PL. Brain acetylcholine and habituation. In: Bradley PB, Fink M, editors. Progress in brain research. Vol. 28. Anticholinergic drugs and brain functions in animals and man. Elsevier; Amsterdam: 1968. [DOI] [PubMed] [Google Scholar]
- Coombs CH. Adaptation of the galvanic response to auditory stimuli. Journal of Experimental Psychology. 1938;22:244–268. [Google Scholar]
- Crampton GH, Schwam WJ. Effects of arousal reaction on nystagmus habituation in the cat. American Journal of Physiology. 1961;200:29–33. doi: 10.1152/ajplegacy.1961.200.1.29. [DOI] [PubMed] [Google Scholar]
- Davis RC. Modification of the galvanic reflex by daily repetition of a stimulus. Journal of Experimental Psychology. 1934;17:504–535. [Google Scholar]
- Davis M, Wagner AR. Startle responsiveness after habituation to different intensities of tone. Psychonomic Science. 1968;12:337–338. [Google Scholar]
- Davis M, Wagner AR. Habituation of startle response under incremental sequence of stimulus intensisites. Journal of Comparative and Physiological Psychology. 1969;67:486–492. doi: 10.1037/h0027308. [DOI] [PubMed] [Google Scholar]
- Estes WK. Structural aspects of associative models for memory. In: Cofer CN, editor. The structure of human memory. Freeman; San Francisco: 1975. [Google Scholar]
- Ezzeddine Y, Glanzman DL. Prolonged habituation of the gill-withdrawal reflex in Aplysia depends upon protein synthesis, protein phosphatase activity ands postsynsptic glutamate receptors. Journal of Neuroscience. 2003;23:9585–9594. doi: 10.1523/JNEUROSCI.23-29-09585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farel PB, Glanzman DL, Thompson RF. Habituation of a monosynaptic response in the vertebrate central nervous system: Lateral column-motoneuron pathway in isolated frog spinal cord. Journal of Neurophysiology. 1973;36:1117–1130. doi: 10.1152/jn.1973.36.6.1117. [DOI] [PubMed] [Google Scholar]
- Farel PB, Thompson RF. Habituation of a monosynaptic response in frog spinal cord: Evidence for a presynaptic mechanism. Journal of Neurophysiology. 1976;39:661–666. doi: 10.1152/jn.1976.39.4.661. [DOI] [PubMed] [Google Scholar]
- Glanzer M. Stimulus satiation: An explanation of spontaneous alternation and related pheonomena. Psychological Review. 1953;60:257–268. doi: 10.1037/h0062718. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Groves PM, Thompson RF. Stimulus generalization of habituation to spinal interneurons. Physiology and Behavior. 1972;8:155–158. doi: 10.1016/0031-9384(72)90145-x. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Thompson RF. Evidence against conduction failure as the mechanism underlying monosynaptic habituation in frog spinal cord. Brain Research. 1979;174:329–332. doi: 10.1016/0006-8993(79)90856-4. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Feldman SM. Habituation of the arousal response to direct stimulation of the brainstem. Electroencephalography and Clinical Neurophysiology. 1961;13:703–709. [Google Scholar]
- Griffith CR. The effect upon the white rat of continued bodily rotation. American Naturalist. 1920;54:524–534. [Google Scholar]
- Groves PM, Demarco R, Thompson RF. Habituation and sensitization of spinal interneuron activity in acute spinal cat. Brain Research. 1969;14:521–525. doi: 10.1016/0006-8993(69)90129-2. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Dual-process theory of habituation: Neural mechanisms. In: Peeke HVS, Herz MJ, editors. Habituation: Behavioral Studies and Physiological Substrates. Chapter 6. Vol. II. Acadmic Press; New York: 1973. [Google Scholar]
- Hagbarth KE, Finer BL. The plasticity of human withdrawal reflexes to noxious skin stimuli in lower limbs. In: Moruzzi G, Fessard A, Jasper HH, editors. Progress in brain research, Vol. I. Brain mechanisms. Elsevier; New York: 1963. pp. 65–81. [Google Scholar]
- Hagbarth KE, Kugleberg E. Plasticity of the human abdominal skin reflex. Brain. 1958;81:305–318. doi: 10.1093/brain/81.3.305. [DOI] [PubMed] [Google Scholar]
- Harris JD. Habituatory response decrement in the intact organism. Psychological Bulletin. 1943;40:385–422. [Google Scholar]
- Hernández-Peón R. Neurophysiological correlates of habituation and other manifestations of plastic inhibition. Electroencephalography and Clinical Neurophysiology. 1960;13(Suppl):101–114. [Google Scholar]
- Hernández-Peón R, Scherrer H, Jouvet M. Modification of electrical activity in cochlear nucleus during “attention” in unanaesthetized cats. Science. 1956;123:331–332. doi: 10.1126/science.123.3191.331. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. Method and theory in the study of avoidance. Psychological Review. 1969;76:49–69. doi: 10.1037/h0026786. [DOI] [PubMed] [Google Scholar]
- Holmes SJ. Phototaxis in the sea-urchin, Arbacia ountulata. Journal of Animal Behavior. 1912;2:126–136. [Google Scholar]
- Hull CL. Learning II. The factor of the conditioned reflex. In: Murchison C, editor. Handbook of general experimental psychology. Clark University Press; Worchester, MA: 1934. [Google Scholar]
- Hull CL. Principles of behavior. Appleton Century Crofts, Inc.; New York: 1943. [Google Scholar]
- Humphrey G. The nature of learning in its relation to the living system. Harcourt, Brace; New York: 1933. [Google Scholar]
- Jasper HH, Shagass C. Conditioning the occipital alpha rhythm in man. Journal of Experimental Psychology. 1941;28:373–388. [Google Scholar]
- Jennings HS. Behavior of the lower organisms. Columbia University Press; New York: 1906. [Google Scholar]
- Kandel ER. An introduction to behavioral neurobiology. W.H. Freeman; San Francisco: 1975. The cellular basis of behavior. [Google Scholar]
- Konorski J. Conditioned reflexes and neuron organization. Cambridge University Press; New York: 1948. [Google Scholar]
- Konorski J. Integrative activity of the brain. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Lehner GFJ. A study of the extinction of unconditioned reflexes. Journal of Experimental Psychology. 1941;29:435–456. [Google Scholar]
- Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: dependence upon release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis and modulation of postsynaptic AMPA receptor efficacy. Journal of Neuroscience. 2005;25:5623–5637. doi: 10.1523/JNEUROSCI.5305-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart RA. Temporal conditioning of GSR. Journal of Experimental Psychology. 1966;71:438–446. doi: 10.1037/h0022979. [DOI] [PubMed] [Google Scholar]
- Lockhart RA, Steinbrecher DC. Temporal avoidance conditioning in the rabbit. Psychonomic Science. 1963;3:121–122. [Google Scholar]
- Lombard WP. The variations of the normal knee jerk and their relation to the activity of the central nervous system. American Journal of Physiology. 1887;1:5–71. [Google Scholar]
- Marsh JT, Worden FG, Hicks L. Some effects of room acoustics on evoked auditory potentials. Science. 1962;137:281–282. doi: 10.1126/science.137.3526.280. [DOI] [PubMed] [Google Scholar]
- McFadden PN, Koshland DE., Jr. Habituation in the single cell: diminished secretion of norepinephrine with repetitive depolarization of PC12 cells. Proceedings of the National Academy of Sciences. 1990a;87:2031–2035. doi: 10.1073/pnas.87.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden PN, Koshland DE., Jr. Parallel pathways for habituation in repetitively stimulated PC12 cells. Neuron. 1990b;4:615–621. doi: 10.1016/0896-6273(90)90119-z. [DOI] [PubMed] [Google Scholar]
- Mower OH. The modification of vestibular systems by means of repeated elicitation. Comparative Psychology Monographs. 1934:9. [Google Scholar]
- Patterson MM, Cegavske CF, Thompson RF. Effects of a classical conditioning paradigm on hindlimb flexor nerve response in immobilized spinal cat. Journal of Comparative and Physiological Psychology. 1973;84:88–97. doi: 10.1037/h0035021. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, translator. Oxford; London: 1927. [Google Scholar]
- Peeke HVS, Herz MJ, editors. Habituation: Behavioral Studies and Physiological Substrates. Vol. I. Academic Press; New York: 1973a. [Google Scholar]
- Peeke HVS, Herz MJ, editors. Habituation: Behavioral Studies and Physiological Substrates. Vol. II. Academic Press; New York: 1973b. [Google Scholar]
- Pinsker H, Kuppermann I, Catellucci V, Kandel E. Habituation and dishabituation of the gill withdrawal reflex in Aplysia. Science. 1970;167:1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Porter JM. Adaptation of the galvanic skin response. Journal of Experimental Psychology. 1938;23:553–557. [Google Scholar]
- Prosser CL, Hunter WS. The extinction of startle responses and spinal reflexes in the white rat. American Journal of Physiology. 1936;117:609–618. [Google Scholar]
- Rankin CH, Carew TJ. Dishabituation and sensitization emerge as separate processes during development in Aplysia. Journal of Neuroscience. 1988;8:197–211. doi: 10.1523/JNEUROSCI.08-01-00197.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Rankin CH. Analyses of habituation in Caenorhabditis elegans. Learning and Memory. 2001;8:63–69. doi: 10.1101/lm.37801. [DOI] [PubMed] [Google Scholar]
- Sharpless S, Jasper H. Habituation of the arousal reaction. Brain. 1956;79:357–388. doi: 10.1093/brain/79.4.655. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The intergrative action of the nervous system. Yale University Press; New Haven: 1906. [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic information processing: II. Perceptual learning, automatic attending, and a general theory. Psychological Review. 1977;84:127–190. [Google Scholar]
- Sidman M. Classical avoidance without a warning stimulus. Journal of the Experimental Analysis of Behavior. 1962;5:97–104. doi: 10.1901/jeab.1962.5-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. Neuronal models and the orienting influence. In: Brazier MA, editor. The central nervous system and behavior: III. Macy Foundation; New York: 1960. [Google Scholar]
- Sokolov YN. Higher nervous functions: The orienting reflex. Annual Review of Physiology. 1963a;25:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Sokolov YN. In: Perception and the conditioned reflex. Waydenfeld S, editor. Pergamon Press; Oxford: 1963b. [Google Scholar]
- Spencer WA, Thompson RF, Neilson DR., Jr. Responses decrement of the flexion reflex in the acute spinal cat and transient restoration by strong stimuli. Journal of Neurophysiology. 1966;29:221–239. doi: 10.1152/jn.1966.29.2.221. [DOI] [PubMed] [Google Scholar]
- Stein L. Habituation and stimulus novelty: A model based on classical conditioning. Psychological Review. 1966;73:352–356. doi: 10.1037/h0023449. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A Model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Voronin LG, Sokolov YN. Cortical mechanisms of the orienting reflex and its relation to the conditioned reflex. Electroencephalography and Clinical Neurophysiology. 1960;13(Suppl):335–346. [Google Scholar]
- Wagner AR. Habituation and memory. In: Dickinson A, Boakes RA, editors. Mechanisms of learning and motivation: A memorial volume for Jerry Konorski. Lawrence Earlbaum Assoc.; Hillsdale, NJ: 1979. pp. 53–82. [Google Scholar]
- Welker WI. An analysis of exploratory and play behavior in animals. In: Fiske DW, Maddi SR, editors. Functions of varied experience. Dorsey; Homewood, IL: 1961. pp. 175–226. [Google Scholar]
- Wendt GR. Vestibular functions. In: Stevens SS, editor. Handbook of experimental psychology. Wiley; New York: 1951. pp. 1191–1223. [Google Scholar]
- Worden FG, Marsh JT. Amplitude changes of auditory potentials evoked at cochlear nucleus during acoustic habituation. Electroencephalography and Clinical Neurophysiology. 1963;15:866–881. doi: 10.1016/0013-4694(63)90176-7. [DOI] [PubMed] [Google Scholar]