Abstract
NKG2D is a receptor used by natural killer (NK) cells to detect virally infected and transformed cells. It recognizes ligands that are expressed constitutively on primary tumors and tumor cell lines. In this report, we have identified four microRNAs (miRNAs) that each was sufficient to reduce the expression of the NKG2D ligand major histocompatibility complex class I-related chain A (MICA). One of these miRNAs (miR-520b) was induced by IFNγ, leading to a reduction in MICA surface protein levels. Interestingly, miR-520b acted on both the MICA 3′UTR and promoter region and caused a decrease in the levels of MICA transcript. In contrast, an anti-sense oligonucleotide inhibitor of miR-520b increased the expression of a reporter construct containing the MICA 3′UTR but not the MICA promoter region. These findings demonstrate the novel regulation of an NKG2D ligand by an endogenous miRNA that is itself induced by IFNγ.
Keywords: NKG2D ligands, MICA, IFN, microRNA
Introduction
Various models of primary tumor formation have shown that NK cells and the IFN system participate in cancer immunoediting (1). As initiators of the anti-tumor response, NK cells utilize the activating receptor NKG2D to recognize and eliminate abnormal, damaged cells (2). NKG2D binds to a family of ligands whose expression is induced by viral infection, transformation, and cellular stress (3, 4). One NKG2D ligand is MICA, a highly polymorphic transmembrane protein that is regulated by transcriptional and post-transcriptional mechanisms (5, 6). Whereas MICA at the cell surface can enhance NK cell recognition of targets, many tumors cleave and release soluble forms of MICA which inhibit the immune response (7). Mechanisms that down-regulate soluble MICA may potentially be clinically useful (8).
microRNAs (miRNAs) represent a recently identified mechanism of post-trancriptional gene repression (9). miRNAs are a large family of noncoding RNAs that induce mRNA degradation or repress translation by base-pairing with the 3′ untranslated regions (3′UTRs) in the target genes. The possibility that miRNAs may regulate immune recognition is supported by a recent report by Stern-Ginossar et al., who showed that a virally encoded miRNA can down-regulate MICB expression during viral infection by human cytomegalovirus (10). This group also identified several host miRNAs that could inhibit the expression of both MICA and MICB (11).
We have shown that interferons (IFNs) can down-regulate the mouse NKG2D ligand H60 by decreasing H60 transcripts (12). Herein, we show that IFNγ and miRNAs can down-regulate the expression of the NKG2D ligand MICA. Notably, IFNγ increased the expression of miR-520b. This miRNA was sufficient to reduce MICA protein and transcript levels and acted on the MICA promoter and 3′UTR. Our findings identify miRNAs and IFNγ as novel targets for the immunotherapeutic modulation of MICA.
Materials and Methods
miRNA analysis
miRNAs were purchased in the stem-loop form and co-transfected using siPORT NeoFX reagent (Ambion, Austin, TX) with siGLO Red fluorescent oligonucleotide (Dharmacon, Boulder, CO).
Cell culture
HeLa, MDA-MB-231, A375, and HCT116 are from ATCC (Manassas, VA). MelJuSo is a cell line developed from a human melanoma (13). Recombinant IFNγ was obtained from eBioscience (San Diego, CA).
MICA cis elements
The MICA promoter was amplified from BAC Clone RP11-943l14 (BACPAC, Oakland, CA) (primer sequences: 5′aaggctgtgcagtaatctaggc and 5′ttaagcttcgacgtcgccac) and subcloned into the pGL3 vector (Promega, Madison, WI) to produce pGL3-MICA-pro. The MICA 3′UTR was amplified from total MelJuSo RNA using Superscript III (Invitrogen) (primer sequences: 5′cgactgtactctacagccagg, 5′cccaagcttaatatggtacagct) and subcloned into pMIR-luc (Ambion) to produce pMIR-MICA-3′UTR. All products were verified by sequencing.
Luciferase Assay
Transfection was done through Lipofectamine 2000 (Invitrogen). In all conditions, pRL-TK was co-transfected. Firefly luciferase light values were divided by renilla luciferase light values, and ratios were normalized against control plasmid (i.e. pMIR-MICA-3′UTR vs pMIR-luc; pGL3-MICApro vs PGL2-control).
Flow Cytometry
Cells were harvested without trypsin and stained with antibodies to MICA, MICB, ULBP1-3 (R&D Systems), HLA-ABC-FITC, or appropriate isotype controls (eBioscience). Secondary antibodies used were either goat anti-mouse IgG-APC (eBioscience) or rat anti-mouse IgG2a/b-PE (BD, San Jose, CA).
RT-PCR
MICA and miRNA Taqman primers and cDNA synthesis kits were purchased through Applied Biosystems. Total RNA was extracted using RNABee (Tel-Test, Friendswood, Texas). RT reactions were normalized to GAPDH for MICA transcript or RNU6B for microRNA transcript.
Results and Discussion
miRNA- mediated regulation of cell surface MICA expression
To identify miRNAs that regulate the expression of MICA, we used the Sanger MicroCosm Search Engine and retrieved a list of 61 human miRNAs that could bind MICA sequences (http://microrna.sanger.ac.uk/cgi-bin/targets/v4/detail_view.pl?transcript_id=ENST00000376222). We manually scanned the miRNAs on this list and found 5 miRNAs (106b, 302b, 372, 520b, 615) that displayed characteristics (14) suitable for regulating MICA. Interestingly, 4 of the 5 miRNAs were specific for a 5-base pair motif (CACTT) that was duplicated in the MICA 3′UTR and overlapped with the seed sequence of the viral miRNA that regulated MICB (10) (Fig. S1).
We next examined whether these miRNAs could affect MICA surface protein levels. Tumor cells were transiently transfected with the individual stem-loop miRNAs, a control miRNA, or an siRNA cocktail specific for MICA, and the surface expression of MICA was assessed by FACS. Interestingly, four of the five miRNAs significantly down-regulated MICA surface expression to an extent similar to a cocktail of siRNA designed to down-regulate MICA (60% reduction in median fluorescence intensity, Figs. 1A-B). The effect was specific for MICA, since MHC class I levels were not affected (data not shown). These data suggest that the miRs 106b, 302b, 372, and 520b may represent a novel molecular mechanism by which host cells may regulate MICA expression.
Figure 1. miRNA-mediated down-regulation of MICA surface protein expression.
MelJuSo and HeLa cell lines were transiently transfected with a control scrambled miR, the indicated miR, or a pooled siRNA cocktail and stained with anti-MICA-APC or isotype control-APC. The expression of MICA surface protein was quantitated by subtracting median fluorescence intensity (MFI) of isotype control staining from MICA staining and then (A) plotted as normalized values or (B) depicted in the histograms (anti-MICA, thick lines; isotype control, thin lines). These experiments are representative of 3 independent experiments
Our findings are consistent with the recent studies of Stern-Ginossar et al., who also showed that these miRs could regulate MICA and MICB (11). Using a different search engine, these authors additionally observed that miR-20a and miR-373 could down-regulate MICA surface protein levels. The study also confirmed using site-directed mutagenesis that the duplicated seed sequences in the MICA 3′UTR that we identified (Fig. S1) was required for miRNA-mediated regulation, and furthermore, the down-regulation had functional consequences in limiting NK cell target recognition.
Interestingly, the miRs 372 (15) and 106b (16) have been implicated in tumorigenesis. Our findings that these miRNAs regulate a ligand for the immunoreceptor NKG2D suggests that tumor promoting miRNAs may also promote the escape of tumor cells from immunosurveillance. As other miRNAs become functionally implicated in tumor progression, it will be noteworthy to examine their regulation of tumor immunogenicity.
IFNγ downregulates surface MICA expression on tumor cell lines
We have previously shown that IFN significantly down-regulates the NKG2D ligand H60, but not RAE1 or MULT1, on murine tumor cell lines (12). Similarly, in human cell lines, we found that IFNγ significantly reduced the level of MICA and ULBP2 expression in the MelJuSo melanoma cell line without affecting ULBP3 levels (Fig. 2A). The down-regulation of MICA was also seen with several other tumor cell lines, although the extent of reduction varied between 30 to 70% (Fig. 2B). The decrease in surface MICA protein reached saturating levels at a dose of 10 U/mL (Fig. 2C), was maximal by two days of treatment (Fig. 2D), and occurred concomitantly with the up-regulation of MHC class I. These data establish that IFNγ can down-regulate the NKG2D ligand MICA on various tumor cells at physiologically relevant doses. Similar to the down-regulation of H60 by IFNγ (12), the down-regulation of MICA by IFNγ was not associated with its anti-proliferative properties (data not shown). A recent study has also shown that IFNγ can down-regulate MICA expression on tumor cell lines (17).
Figure 2. IFNγ down-regulated MICA surface protein expression.
(A) MelJuSo and HeLa cell lines were treated with 100 U/ml of IFNγ and stained with reagents listed on the x-axis (thick lines) or corresponding isotype controls (thin lines). (B) Various tumor cell lines were treated with either 100 U/ml of IFNγ (black bars) or medium alone (white bars), and assessed for MICA (left panel) and HLA expression (right panel). (C-D) MelJuSo cells were treated either with (C) various doses of IFNγ and incubated over two days or (D) treated with 100 U/ml IFNγ and incubated for the time indicated. Shown is the MFI of MICA (left axis) or MHC class I (right axis) staining. The results were reproduced once with MelJuSo and twice with HeLa cells.
IFNγ induces miR-520b
Having shown regulation of MICA by various miRNAs and by IFNγ, we sought to integrate our findings by examining whether IFNγ could regulate the expression of miRs-106b, 302b, 372b, and 520b. Among the panel of miRNAs, only miRs-106b and 520b were detectable in our tumor cell lines. This result is not surprising considering that many tumors have global decreases in miRNA levels (18). Notably, miR-520b displayed a two- or three-fold induction after IFNγ treatment in several tumor cell lines and primary human fibroblasts (Fig. 3A-left panel). IFNγ did not significantly affect the levels of miR-106b in either cell line (Fig. 3A-right panel). These findings suggest that IFNγ specifically up-regulates certain miRNAs to mediate gene regulation. Further identification of IFNγ-induced miRNAs, as exemplified by the elegant approach recently described by Pedersen et al. (19) should provide deeper insight into the genes that are regulated by IFNγ.
Figure 3. Regulation of the MICA 3′UTR by IFNγ-induced miR-520b.
(A) miRNA transcripts indicated on the x-axis were quantified by qPCR from total RNA isolated from cells treated with 100 U/ml IFNγ for 1 or 24 hours. Shown are 95% confidence intervals. All results were reproduced at least once for each cell line. (B) MelJuSo and HeLa cells were transfected with pMIR-MICA-3′UTR or pMIR-luc along with 5 nM of mir-520b, anti-520b, control scrambled miR, or control scrambled anti-miR. Values are the result of raw firefly luciferase values over raw renilla values which were normalized to control vector (pMIR-luc). Error bars represent standard deviation. All results were reproduced at least once for each cell line and once with MDA-MB231 cells.
miR-520b regulates the MICA 3′UTR
Short-hairpin RNAs can regulate genes through binding of the 3′UTR of the target gene or by repressing transcription factors involved in target gene expression (20). Therefore, we examined whether miR-520b can act on the MICA 3′UTR. The full length MICA 3′UTR was subcloned into a luciferase reporter construct and transiently transfected in combination with miR-520b, control scrambled miRNA, an antisense oligonucleotide inhibitor (anti-miR) of miR-520b (anti-520b), or control scrambled anti-miR. We observed a significant reduction (70% inhibition in HeLa, 40% inhibition in MelJuSo) in luciferase activity in cells transfected with miR-520b as compared to cells transfected with the scrambled miRNA (Fig. 3B). Moreover, in cells that were blockaded for endogenous miR-520b function by transfection of anti-520b, the luciferase activity was increased by 20-50% compared to transfection with a control anti-miR (Fig. 3B). These results show that miR-520b acts on the MICA 3′UTR and suggest that endogenous levels of miR-520b in tumor cells are functioning to regulate MICA expression.
IFNγ and miR-520b reduce the level of MICA transcript
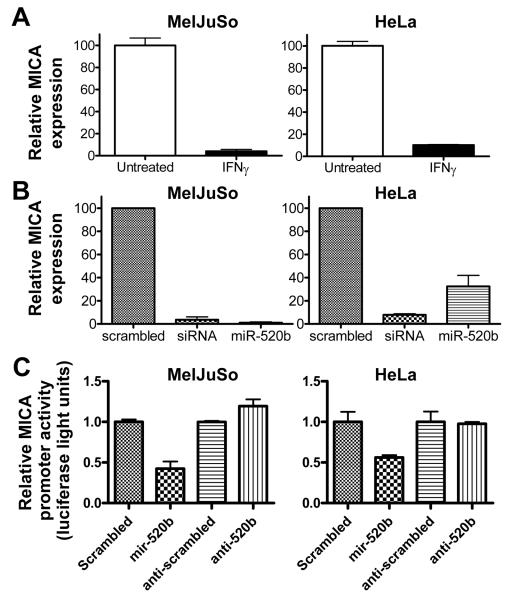
We have previously shown that IFNs regulate the level of transcript of the NKG2D ligand H60 (12). Therefore, we examined whether IFNγ and miR-520b could regulate MICA mRNA levels. The MelJuSo and HeLa cell lines were treated with IFNγ or transfected with miR-520b or control miRNA, and MICA transcript levels were measured by qPCR. Fig. 4A shows that IFNγ significantly reduced (>85%) the level of MICA transcript in the MelJuSo and HeLa cell lines compared to untreated cells. Similarly, transfection of miR-520b significantly reduced the level of MICA transcript (>95% in MelJuSo, >60% in HeLa) compared to transfection of control miRNA (Fig. 4B). Notably, the down-regulation of MICA transcript by a single endogenous miRNA (miR-520b) occurred to an extent that was similar to a cocktail of siRNAs which was designed to down-regulate MICA. The potent down-regulation of MICA transcript (Fig. 4) did not translate to a similar down-regulation of MICA protein (Fig. 1), suggesting that either the half-life of MICA protein is long in our cell lines or there is highly efficient translation of minimal MICA transcripts.
Figure 4. IFNγ and miR-520b down-regulate MICA transcripts.
MICA transcripts were measured from total RNA isolated from cells (A) treated with 100 U/ml IFNγ (closed bars) or medium alone (open bars) or (B) transfected with control scrambled miR, an siRNA pool specific for MICA, or miR-520b. Error bars represent standard deviation of real-time measurements. These results were reproduced at least twice for each cell line. (C) HeLa cell lines were transfected with a luciferase construct containing the 1kb promoter region of MICA (pGL3-MICApro-luc) or a control reporter construct (pGL2-control) along with the reagents specified in the figure. Values are the result of raw firefly luciferase values over raw renilla values normalized to control vector (PGL2-control). Error bars represent standard deviation. These results were reproduced at least once for each cell line and once with the MDA-MB231 cell line.
A recent study also showed that IFNγ down-regulated MICA transcript levels but further showed that IFNγ induced the cleavage of MICA (17). To begin to dissect the mechanism by which IFNγ down-regulated MICA surface protein, we inhibited miR-520b function in IFNγ-treated MelJuSo cells using anti-520b. We did not detect any change in IFNγ-mediated down-regulation of MICA in MelJuSo cells transfected with anti-520b vs control miRNA (Fig. S2). Thus, although miR-520b may be sufficient to mediate down-regulation of MICA, this effector pathway was not necessary for IFNγ's actions. This result is consistent with the fact that IFNγ activates multiple, possibly redundant, pathways to modulate gene expression.
miR-520b down-regulates the MICA promoter
Since miR-520b dramatically reduced MICA transcripts, we sought to determine whether miR-520b could regulate MICA gene expression by acting on the promoter region. MelJuSo and HeLa cells were transiently transfected with a luciferase reporter gene construct containing 1 kb of the MICA promoter region in combination with control miRNA, miR-520b, control anti-miR, or anti-miR-520b. Fig. 4C shows a reduction (2-3 fold) in luciferase activity in cells transfected with miR-520b as compared to cells transfected with the scrambled control miRNA. Interestingly, there was a slight but reproducible increase in MICA promoter activity in MelJuSo but not HeLa cells transfected with anti-520b compared to control anti-miR. Taken together, these data indicate that miR-520b inhibits MICA gene expression not only via target sequences in the 3′UTR but also by acting on the promoter region.
Notably, a recent study showed that miR-20a, -106b, -373, and -520d inhibited MICA protein expression but did not affect MICA transcript levels (11). This study did not examine miR-520b and did not study the MICA promoter. Our finding that miR-520b regulated the MICA promoter and the MICA 3′UTR provides a novel mechanism by which miRNAs can ensure that their target gene is inhibited. We speculate that miR-520b could affect MICA promoter activity by regulating transcription factors essential for MICA expression. The transcription factors Sp1, Sp3, and NF-κB have been found to up-regulate MICA promoter activity (21, 22), and Sp3 expression has been shown to be inhibited by IFNγ in monocytes (23). Interestingly, we have observed the 3′UTRs of Sp1 and Sp3 to have binding sites for miR-520b, suggesting that miR-520b may regulate the MICA promoter by targeting transcription factors essential for MICA expression. These findings set a precedence for the ability of miRNAs to control transcript levels by affecting transcription in addition to decreasing transcript stability.
In this report, we provide evidence for the regulation of a tumor recognition ligand by endogenous miRNAs. Furthermore, we show that the pleotropic cytokine IFNγ may control gene expression by regulating miRNAs. Since miRNAs are thought to regulate functionally similar genes, it is conceivable that the MICA miRNAs that we have identified also regulate other NKG2D ligands or even recognition ligands outside of the NKG2D family. Thus, the miRNAs that we have identified in this study can be used as probes to define and identify novel recognition proteins involved in tumor and viral immunosurveillance.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
This work was supported by grants to J.D.B. from the American Cancer Society (ACS-IRG #70-002), a grant from the Cancer Research Coordinating Committee (6-444951-34384), and the V Foundation Scholar Award.
REFERENCES
- 1.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nature reviews. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 2.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science (New York, N.Y. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 3.Cerwenka A, Lanier LL. Ligands for natural killer cell receptors: redundancy or specificity. Immunological reviews. 2001;181:158–169. doi: 10.1034/j.1600-065x.2001.1810113.x. [DOI] [PubMed] [Google Scholar]
- 4.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nature immunology. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 5.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12445–12450. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mistry AR, O'Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology. 2007;121:439–447. doi: 10.1111/j.1365-2567.2007.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 8.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9190–9195. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Stern-Ginossar N, Elefant N, Zimmermann A, Wolf DG, Saleh N, Biton M, Horwitz E, Prokocimer Z, Prichard M, Hahn G, Goldman-Wohl D, Greenfield C, Yagel S, Hengel H, Altuvia Y, Margalit H, Mandelboim O. Host immune system gene targeting by a viral miRNA. Science (New York, N.Y. 2007;317:376–381. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim O. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nature immunology. 2008;9:1065–1073. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 12.Bui JD, Carayannopoulos LN, Lanier LL, Yokoyama WM, Schreiber RD. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J Immunol. 2006;176:905–913. doi: 10.4049/jimmunol.176.2.905. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann JM, Holzmann B, Breitbart EW, Schmiegelow P, Riethmuller G, Johnson JP. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer research. 1987;47:841–845. [PubMed] [Google Scholar]
- 14.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS biology. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH, Agami R. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124:1169–1181. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson AL, Linsley PS, Cleary MA. MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote cell cycle progression. Molecular and cellular biology. 2008;28:2167–2174. doi: 10.1128/MCB.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Niu J, Zhang J, Wang Y, Zhou Z, Zhang J, Tian Z. Opposing effects of interferon-alpha and interferon-gamma on the expression of major histocompatibility complex class I chain-related A in tumors. Cancer science. 2008 doi: 10.1111/j.1349-7006.2008.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science (New York, N.Y. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molinero LL, Fuertes MB, Girart MV, Fainboim L, Rabinovich GA, Costas MA, Zwirner NW. NF-kappa B regulates expression of the MHC class I-related chain A gene in activated T lymphocytes. J Immunol. 2004;173:5583–5590. doi: 10.4049/jimmunol.173.9.5583. [DOI] [PubMed] [Google Scholar]
- 22.Venkataraman GM, Suciu D, Groh V, Boss JM, Spies T. Promoter region architecture and transcriptional regulation of the genes for the MHC class I-related chain A and B ligands of NKG2D. J Immunol. 2007;178:961–969. doi: 10.4049/jimmunol.178.2.961. [DOI] [PubMed] [Google Scholar]
- 23.Hughes TR, Tengku-Muhammad TS, Irvine SA, Ramji DP. A novel role of Sp1 and Sp3 in the interferon-gamma -mediated suppression of macrophage lipoprotein lipase gene transcription. The Journal of biological chemistry. 2002;277:11097–11106. doi: 10.1074/jbc.M106774200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.