Abstract
The ovine sexually dimorphic nucleus (oSDN) is characterized by high levels of aromatase mRNA expression which can be used to delineate its boundaries. The volume of the oSDN is approximately 2 to 3-fold larger in rams that mate with ewes (female-oriented rams) than in rams that mate with other rams (male-oriented rams) and ewes. The sex difference in oSDN volume is present in late gestation fetuses and can be eliminated before birth by exposing genetic females to exogenous testosterone during midgestation, suggesting that early exposure to androgen masculinizes volume of the oSDN. The present study was performed to determine whether differences in oSDN volume are influenced by the adult hormonal environment. Adult rams, behaviorally characterized as female-oriented or male-oriented, and ewes were gonadectomized and treated with subcutaneous implants of testosterone to achieve physiologic concentrations of serum testosterone. Three weeks after implant placement brain tissue was prepared for histological assessment of oSDN volume using in situ hybridization for detection of aromatase mRNA expression. Quantitative analysis revealed that despite similar serum testosterone levels among the groups, the volume of the oSDN was greater in female-oriented rams than in male-oriented rams and ewes (P < 0.05). Differences in oSDN volume were specific and not reflective of differences in preoptic area height or brain size. These results suggest that differences in the size of the oSDN in adult sheep were not influenced by adult exposure to testosterone.
1. Introduction
The ovine sexually dimorphic nucleus (oSDN) is a sexually dimorphic region occupying the central medial preoptic area – anterior hypothalamus of the sheep brain (Roselli et al., 2004). The dense cluster of Nissl-stained neurons that comprise the oSDN is characterized by high levels of aromatase mRNA expression that can also be used to delineate its boundaries. The volume of the oSDN is approximately 2 to 3-fold larger in rams that mate with ewes (female-oriented rams; FORs) than in rams that mate with other rams (male-oriented rams; MORs) and ewes. The structural differences among the brains of FORs, MORs, and ewes have been interpreted as evidence that the behavioral differences among these groups are likely caused by differences in the early, i.e. fetal, development of the brain. Indeed, the sex difference in oSDN volume is present in late gestation fetuses and can be eliminated before birth by exposing genetic female fetuses to exogenous testosterone (T) during midgestation suggesting that early exposure to androgen masculinizes the volume of the oSDN (Roselli et al., 2007). However, there are several reports showing that sexually dimorphic nuclei in the mammalian brain are affected by adult hormone manipulations. In the gerbil, adult castration reduces the size of the sexually dimorphic preoptic nucleus, an effect that was prevented by the implantation of T capsules (Commins and Yahr, 1984). In contrast, adult castration and T replacement can completely reverse a sexual dimorphism in the medial amygdala of the rat (Cooke et al., 1999). Therefore, the present study sought to evaluate whether size differences in the volume of the oSDN as revealed by aromatase mRNA expression are influenced by adult concentrations of systemic T. Because the sexual dimorphism in oSDN appears to be nearly complete by birth, we hypothesized that size differences in oSDN volume among rams with differing sexual partner preference and ewes would be unaltered when the adult sheep were gonadectomized and given subcutaneous implants to clamp T at equivalent physiological levels.
2. Results
The rams designated as FORs in this study mated exclusively with estrous ewes in the sexual performance tests (2.5 ± 0.26 ejaculations per 30 min), while rams designated as MORs never copulated in the performance test. Table 1 shows the results of the sexual partner preference tests for the FORs and MORs used in this study. Rams categorized as FORs exhibited significantly more precopulatory behaviors, mounts, and ejaculations with ewes than with stimulus rams, whereas rams identified as MORs directed their pre-mount, mount, and ejaculatory behaviors toward stimulus males. Precopulatory behaviors were combined for statistical analysis, but in general, FORs showed more genital sniffs, vocalization and foreleg kicks directed at females and MORs showed more sniffs and foreleg kicks directed at males. Flehmen responses were low in both groups (data not shown).
Table 1.
Averages (Mean ± SEM) of behaviors exhibited by male-oriented (MOR) and female-oriented (FOR) rams during exposure to 2 estrous ewes and 2 rams in four separate 30-min sexual partner preference tests.
| MORs (n = 6) | FORs (n = 5) | |||
|---|---|---|---|---|
| Behavior | Estrous ewe stimulus |
Ram stimulus |
Estrous ewe stimulus |
Ram stimulus |
| Pre-mounta | 2.6 ± 0.5 | 36.9 ± 10.3b | 29.1 ± 4.1 | 8.6 ± 2.2b |
| Mounts | 0 | 11.1 ± 0.6b | 8.4 ± 1.2 | 0.4 ± 0.2b |
| Ejaculations | 0 | 0.6 ± 0.3b | 2.8 ± 0.3 | 0b |
See Material and Methods for details of behavior testing.
Pre-mount behaviors include: number of genital sniffs, foreleg kicks, vocalizations, and Flehmens (curling of the upper lip associated with pheromone detection).
P < 0.05 compared with response in an estrous ewe Behavior data were analyzed by Mann-Whitney U tests.
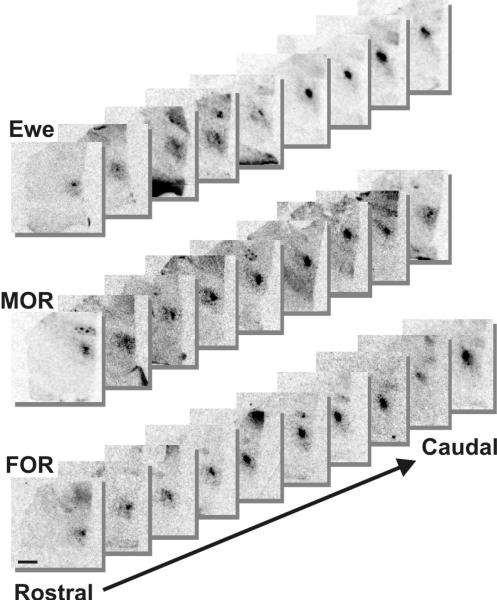
Serum concentrations of T (Fig. 1) in ewes and rams after gonadectomy and treatment with T were not significantly different (F2, 15 = 2.92; P = 0.09). Autoradiograms of in situ hybridization showed heavy expression of aromatase mRNA in a cell group corresponding to the oSDN among all subjects (Fig. 2). Quantitative analysis of the volume of aromatase mRNA expression (Fig. 3) revealed significant differences among groups (F2, 15 = 8.08; P = 0.005). The mean volume of the oSDN was significantly larger (P < 0.05) in FORs than in MORs and ewes, while MORs and ewes were not different. There were no significant group differences in the level of aromatase mRNA when expressed as total integrated optical density (IOD) in the oSDN (F2,15 = 0.83; P = 0.46; data not shown). No significant differences were found in mean brain weights or POA heights among groups (Table 2), suggesting that there were no group differences in brain size that could account for the volume differences in oSDN. Mean body weights were also equivalent between FORs and MORs (Table 2).
Figure 1.
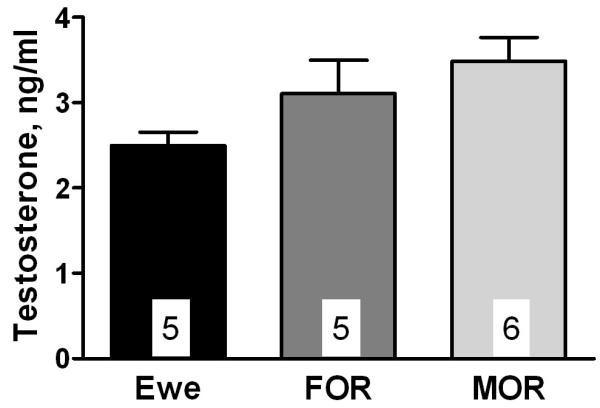
Serum concentrations of T in ewes, MORs, and FORs that were gonadectomized and given subcutaneous Silastic packets containing crystalline T as described in Materials and Methods. Data are presented as mean ± SEM; n = 5-6 per group.
Figure 2.
Representative autoradiographic images of aromatase mRNA expression showing coronal sections through the preoptic area at 120 μm intervals. Images are of the right side of the diencephalon and are arranged in a rostral to caudal direction proceeding from left to right. The boarder of the third ventricle is on the right side of each individual image. Image contrast was enhanced to highlight the position of aromatase mRNA expression demarcating the oSDN. Bar = 2 mm.
Figure 3.
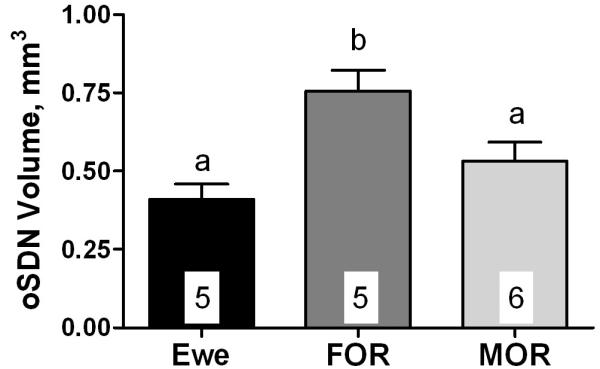
Differences in oSDN volume among gonadectomized T-treated ewes, MORs, and FORs. Data are presented as mean ± SEM; n = 5-6 per group. Bars marked with different letters differ significantly (P < 0.05).
Table 2.
Differences in POA height, brain weight, and body weight among ewes, MORs, and FORs (Mean ± SEM).
| Measure | Group |
||
|---|---|---|---|
| Ewes (n = 5) | MORs (n = 6) | FORs (n = 5) | |
| POA hgt (mm) | 107.8 ± 1.8 | 106.9 ± 2.2 | 109.3 ± 1.6 |
| Brain wgt (g) | 108.0 ± 2.5 | 118.2 ± 4.8 | 114.8 ± 3.3 |
| Body wgt (kg) | nd | 102.0 ± 3.6 | 105.2 ± 4.1 |
3. Discussion
This study indicates that the observed differences in oSDN volume among FORs, MORs, and ewes are not the result of differences in adult systemic levels of T. The volume differences reported previously for gonad-intact rams and luteal phase ewes (Roselli et al., 2004) were also apparent in the current study using animals that were gonadectomized and given equivalent physiological replacement levels of T. These results, along with our previous report that fetal exposure to T can completely masculinize the volume of the oSDN before birth (Roselli et al., 2007), strongly support the hypothesis that variations oSDN volume are largely dictated by prenatal hormone exposure, and as such, constitute a permanently differentiated feature of sheep brains.
In other vertebrate species sexually dimorphic preoptic nuclei vary in the degree to which their features are altered by adult hormone exposure. The sexually dimorphic preoptic nuclei in rats and guinea pigs are not altered by adult gonadectomy or gonadectomy and treatment with T (Gorski et al., 1978; Hines et al., 1985; Bloch and Gorski, 1988a). However, gonadectomy and treatment of adult males with estrogen and progesterone did decrease the absolute volume of the rat SDN-POA, in particular its medial and anteroventral components (Bloch and Gorski, 1988b). In the Mongolian gerbil, the sexually dimorphic pars compacta (SDApc) portion of the preoptic area found primarily in males is reduced by 35-40% in volume after adult gonadectomy, an effect that was prevented by the T administration (Commins and Yahr, 1984). The dorsal nucleus in the caudal preoptic area of the ferret is discernable in adult males but not females regardless of hormone status (Tobet et al., 1986). Cells in this nucleus are larger in intact males and gonadectomized males given T, dihydrotestosterone, or estradiol than in gonadectomized males given no hormone treatment or progesterone. Hormone treatments, however, do not alter the cross-sectional area and length of the dorsal nucleus in adult ferrets. In the adult quail, T treatment of females causes the sexually dimorphic preoptic nucleus to reach male volumes after 21 days, whereas male castration leads to significant reductions in volume (Panzica et al., 1987). In all of these examples of sexually dimorphic preoptic nuclei, their formation is determined by perinatal exposure to T or its metabolites. Moreover, it appears generally that the more complete perinatal T is in masculinizing the preoptic area morphology the less effective adult treatments are in inducing morphological changes (Dohler et al., 1982; Dohler et al., 1984).
In sheep fetuses at term, the volume of oSDN and the magnitude of the sex difference approximate that observed in adults, suggesting that sexual differentiation is complete or nearly complete before birth in this species (Roselli et al., 2007). We did not compare oSDN volumes among sheep that were gonadectomized to those that were gonadectomized and received T replacement and, thus, we cannot entirely exclude the possibility that adult hormone exposure can contribute in some degree to oSDN volume. T stimulates the expression of aromatase mRNA in the neurons of the adult preoptic area in a number of species, including sheep (Roselli et al., 1998). However, the fact that group differences in oSDN volume were present under conditions of T replacement argues that these differences were not due to differential induction of enzyme, but to long-lasting organizing effects of T on the number of cells expressing aromatase.
Conceptually, brain differences exhibited between sexes as well as individuals of the same sex can be explained by hormonal theory of sexual differentiation (Goy and McEwen, 1980; MacLusky and Naftolin, 1981; Arnold, 1984). Accordingly, the ‘within sex’ differences observed in the brain structure and behavior of rams are most likely due to a variation in MOR development that results in incomplete masculinization and defeminization. Theoretically, this could arise from either differences in the absolute levels or timing of T secretion by the fetal testis or in the responsiveness of neural target tissue to T or its active metabolites. Evidence for both mechanisms exist in rodents (Houtsmuller et al., 1994; Ward et al., 2002). The high level of aromatase within the oSDN led us to test the hypothesis that inhibition of brain estrogen synthesis during the critical period for sexual differentiation in fetal sheep is responsible for masculinizing/defeminizing sexual partner preference and oSDN morphology. Surprisingly, prenatal inhibition of brain aromatization did not interfere with defeminization of adult sexual partner preference (Roselli et al., 2006) leading us to speculate that, as in a number of other long-gestation species including primates (Wallen and Baum, 2002), androgen receptor activation is more important for male-typical sexual differentiation of the sheep brain. Future studies will be needed to test this possibility.
In summary, our results demonstrate that the differences in the volume of the oSDN among sheep that exhibit differences in sexual partner preferences are not altered by systemic concentrations of T in adults, but are most likely established by exposure to T during fetal development.
4. Experimental Procedure
4.1. Animals
Eleven adult rams (4 years old) and five age-matched ewes were used in this study. The sheep were of mixed Western breeds obtained from the United States Sheep Experiment Station (USSES) in Dubois, Idaho and reared as described previously (Resko et al., 1996). The care and use of the sheep was conducted in accordance with NIH guidelines and approved by the IACUC committee at OSU.
4.2. Ram Classifications
At approximately 18 mo of age, rams were given sexual behavior tests so that they could be classified according to their sexual preference. The complete testing procedure was described previously (Resko et al., 1996; Stellflug and Berardinelli, 2002; Roselli et al., 2004). Briefly, it consisted of 9 h of tests with estrous ewes followed by four sexual partner preference tests in which the rams were allowed to choose between two estrous ewes and two unfamiliar rams that were all restrained. The sexual partner preference tests were conducted at the US Sheep Experiment Station over two consecutive years (2 tests/yr) in order to identify rams that mated exclusively with males (i.e., male-oriented rams or MORs) and rams that mated exclusively with females (i.e., female-oriented rams or FORs). The numbers of precopulatory behaviors (i.e., the combined mean number of genital sniffs, foreleg kicks, vocalizations, and flehmen responses), mounts and ejaculations were recorded during testing. Age-matched experimental ewes were also obtained from the US Sheep Experiment Station. The rams and ewes were trucked to Oregon State University in the spring after testing was completed and behavioral assignments were made. They were housed in single sex groups in larger fenced pastures with free access to water.
4.3. Hormone Treatment
Rams were castrated and ewes were ovariectomized approximately 2 months before treatment with testosterone (T). All experimental animals were fasted for 12 h prior to surgery. Anesthesia was induced with xylazine (0.2 mg/kg i.v) and maintained with halothane and oxygen. Animals were placed in right lateral recumbency and a 10 cm by 10 cm area on the left lateral thorax just caudal to the triceps area was prepared for aseptic surgery. A 2.5 cm incision was made to create a subcutaneous pocket to retain the implants followed by skin closure with simple interrupted nonabsorbable sutures. Rams were given 3 constant-release Silastic packets (3 × 5 cm) containing crystalline T (4 g) and ewes received 2 packets.
4.4 Tissue Collection
Three weeks after hormone treatment began, jugular blood samples (10 ml) were taken after which the sheep were euthanized with an overdose (15 mg/kg) of sodium pentobarbital (Euthasol ; Delmarva Laboratories, Inc; Midlothian, VA). The head was perfused through the carotid arteries with 500 ml of RNase-free physiological saline containing heparin (150,000 U/liter), then with 3.5 liters of ice-cold 4% paraformaldehyde in phosphate buffer (pH 7.4). The brain was removed from the skull, dissected to obtain a diencephalic block of tissue and postfixed for 1 h in 4% paraformaldehyde. The tissue was then cryoprotected by submersion in 10% glycerol, followed by 20% glycerol, then frozen and stored at −80°C (Roselli et al., 2000).
4.5 In situ hybridization
Diencephalic tissue blocks were sectioned coronally (30-μm thick) into four parallel series and mounted onto microscope slides. One series of brain sections taken at 120 μm intervals was stained with thionin for anatomical verification. An adjacent series of tissue sections was processed for in situ hybridization accordingly to our previously published procedures (Roselli et al., 2000). Briefly, a sheep-specific 33P-labelled aromatase cRNA probe was synthesized, purified, and diluted in hybridization buffer to an activity of 1.0 × 107 dpm/ml. On the day of hybridization, tissue sections were treated with Proteinase K (10 μg/ml), acetylated, and dehydrated. The hybridization solution with probe was then pipetted onto the sections (80 μl/slide), covered with a glass coverslip and sealed with DPX before incubation for 18 h at 57°C. Following hybridization, the slides were subjected to RNase digestion (20 μg/ml for 30 min at 37°C), washed several times to a final stringency of 0.1x SSC at 65°C for 30 min and dehydrated. The slides were then dried under vacuum and exposed to Kodak Biomax MR film for three weeks.
4.6 Image analysis
The volume of the oSDN was determined from film autoradiograms of in situ hybridization for aromatase mRNA since we previously demonstrated in both the adult and fetus that aromatase mRNA labeling corresponds to the area of dense thionin-staining cells comprising the oSDN (Roselli et al., 2004; Roselli et al., 2007). Images of film autoradiograms were collected at 1200 dpi with an Epson (Long Beach, CA) 1640SU flatbed scanner. Digital images were imported into Adobe Photoshop CS2 software (Adobe Systems Inc. San Jose, CA) and saved as tiff files. The outlines of the oSDN were traced by hand from the digitized autoradiographic images and analyzed for integrated optical density and cross-sectional area by computer using NIH Image software (version 1.62). Volumes were estimated by multiplying cross-sectional area × number of sections in which oSDN appeared × distance between sections (120 μm). Two investigators unaware of the sex or sexual preference of the animals made measurements independently. The means of these measurements were used for statistical comparisons. Thionin stained sections were used to confirm the location and boundaries of the oSDN in each animal.
4.7 Steroid hormone measurements
Serum T was measured by radioimmunoassay after extraction with ethyl ether as described previously (Roselli et al., 2007). All samples were measured in the same assay. The recovery, water blanks, and interassay coefficient of variation were: 83.4%, 3.8 pg, and 8.5%, respectively.
4.8 Statistics
Behavioral data were analyzed by Mann-Whitney U tests. oSDN volumes were compared by one-way ANOVA followed by post hoc Newman-Keuls multiple comparison tests. P values less than 0.05 were considered significant for both parametric and nonparametric tests.
Acknowledgements
We wish to express our appreciation to Ms. Jessica Schrunk for her conscientious animal care and project management; and to Cecily Bishop, Tess Baumgartner, and Katie Dunlap for assistance with animal testing and tissue collection. This research was supported by NIH grant R01 RR014270
References
- Arnold AP. Gonadal steroid induction of structural sex differences in the central nervous system. Ann. Rev. Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Gorski RA. Cytoarchitectonic analysis of the SDN-POA of the intact and gonadectomized rat. J. Comp. Neurol. 1988a;275:604–612. doi: 10.1002/cne.902750408. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Gorski RA. Estrogen/progesterone treatment in adulthood affects the sized of several components of the medial preoptic area in the male rat. J. Comp. Neurol. 1988b;275:613–622. doi: 10.1002/cne.902750409. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Adult testosterone levels influence the morphology of a sexually dimorphic area in the Mongolian gerbil brain. J. Comp. Neurol. 1984;224:132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc. Natl. Acad. Sci. U S A. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is determined by the perinatal hormone environment. Neurosci. Lett. 1982;33:295–298. doi: 10.1016/0304-3940(82)90388-3. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Srivastava SS, Shryne JE, Jarzab B, Sipos A, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen antagonist. Neuroendocrinology. 1984;38:297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area or the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sexual Differentiation of the Brain. MIT Press; Cambridge: 1980. [Google Scholar]
- Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: A description and an investigation of their relationship to gonadal steroids in adulthood. J. Neurosci. 1985;5:40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtsmuller EJ, Brand T, De Jonge FH, Joosten RNJMA, Van De Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol. Behav. 1994;56:535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Calacagni M, Anselmetti GC, Schumacher M, Balthazart J. Sexual differentiation and hormonal control of the sexually dimorphic medial preoptic nucleus in the quail. Brain Res. 1987;416:59–68. doi: 10.1016/0006-8993(87)91496-x. [DOI] [PubMed] [Google Scholar]
- Resko JA, Perkins A, Roselli CE, Fitzgerald JA, Choate JVA, Stormshak F. Endocrine correlates of partner preference behavior in rams. Biol. Reprod. 1996;55:120–126. doi: 10.1095/biolreprod55.1.120. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Schrunk JM, Stadelman HL, Resko JA, Stormshak F. The effect of aromatase inhibition on the sexual differentiation of the sheep brain. Endocrine. 2006;29:501–512. doi: 10.1385/ENDO:29:3:501. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Stormshak F, Resko JA. Distribution and regulation of aromatase activity in the ram hypothalamus and amygdala. Brain Res. 1998;811:105–110. doi: 10.1016/s0006-8993(98)00995-0. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Stormshak F, Resko JA. Distribution of aromatase mRNA in the ram hypothyalamus: An In Situ hybridization study. J. Neuroendocrinol. 2000;12:656–664. doi: 10.1046/j.1365-2826.2000.00496.x. [DOI] [PubMed] [Google Scholar]
- Stellflug JN, Berardinelli JG. Ram mating behavior after long-term selection for reproductive rate in Rambouillet ewes. J. Anim. Sci. 2002;80:2588–2593. [PubMed] [Google Scholar]
- Tobet SA, Zahniser DJ, Baum MJ. Sexual dimorphism in the preoptic/anterior hypothalamic area of ferrets: effects of adult exposure to sex steroids. Brain Res. 1986;364:249–257. doi: 10.1016/0006-8993(86)90837-1. [DOI] [PubMed] [Google Scholar]
- Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 4. Elsevier Science (USA); San Diego: 2002. pp. 385–423. [Google Scholar]
- Ward OB, Ward IL, Denning JH, Hendricks SE, French JA. Hormonal mechanisms underlying aberrant sexual differentiation in male rats prenatally exposed to alcohol, stress, or both. Arch. Sex Behav. 2002;31:9–16. doi: 10.1023/a:1014018931977. [DOI] [PubMed] [Google Scholar]