Abstract
Nanoparticles are being developed as delivery vehicles for therapeutic pharmaceuticals and contrast imaging agents. Polymersomes (mesoscopic polymer vesicles) possess a number of attractive biomaterial properties that make them ideal for these applications. Synthetic control over block copolymer chemistry enables tunable design of polymersome material properties. The polymersome architecture, with its large hydrophilic reservoir and its thick hydrophobic lamellar membrane, provides significant storage capacity for both water soluble and insoluble substances (such as drugs and imaging probes). Further, the brush-like architecture of the polymersome outer shell can potentially increase biocompatibility and blood circulation times. A further recent advance is the development of multi-functional polymersomes that carry pharmaceuticals and imaging agents simultaneously. The ability to conjugate biologically active ligands to the brush surface provides a further means for targeted therapy and imaging. Hence, polymersomes hold enormous potential as nanostructured biomaterials for future in vivo drug delivery and diagnostic imaging applications.
Keywords: Polymersome, Diblock copolymer, Vesicle, Drug delivery, Cancer diagnosis, Cancer therapy, Nanoparticle, Targeting, Optical imaging, Near-infrared
1. Background
Nanosized carriers are prime candidates for the delivery of highly toxic and/or hydrophobic therapeutic agents. These delivery vehicles have the potential to augment the pharmacodynamic and pharmacokinetic profiles of drug molecules, thereby enhancing the therapeutic efficacy of the pharmaceutical agents [1]. Further, encapsulating the drug molecule in a delivery system can increase in vivo stability, extend its blood circulation time, and further provide a means for controlling the release of the agent [1]. Moreover, the delivery system can alter the biodistribution of the drug molecule by allowing the agent to accumulate at the tumor site, either passively or actively with targeting [1]. In addition to therapeutic drug delivery, serving as diagnostics tools, nanosized carriers can deliver imaging agents to detect and non-invasively diagnose disease.
Polymersomes, polymer vesicles self-assembled from a diverse array of synthetic amphiphilic block copolymers containing hydrophilic and hydrophobic blocks [2-4], have been shown to possess superior biomaterial properties, including greater stability and storage capabilities [5-7], as well as prolonged circulation time, as compared to liposomes (vesicles derived from phospholipids) [8]. A particularly attractive storage feature, highlighted in Fig. 1, is the large hydrophobic core of the polymersome membrane, which follows from the membrane-forming amphiphilic polymers being larger than conventional phospholipids [9]. Further, block copolymer chemistries can be tuned through polymer synthesis to yield polymersomes with diverse functionality [10]. A vast majority of vesicles made of synthetic copolymers have dense polyethylene oxide (PEO) outer shells, which affords them “stealth” like character that may lead to increased circulation times and in vivo biocompatibility [5]. Thus, although liposomes are presently used in various biotechnological and pharmaceutical applications to improve therapeutic indices and enhance cellular uptake [4], it appears that polymersomes can offer superior advantages for future clinical therapeutic and diagnostic imaging applications.
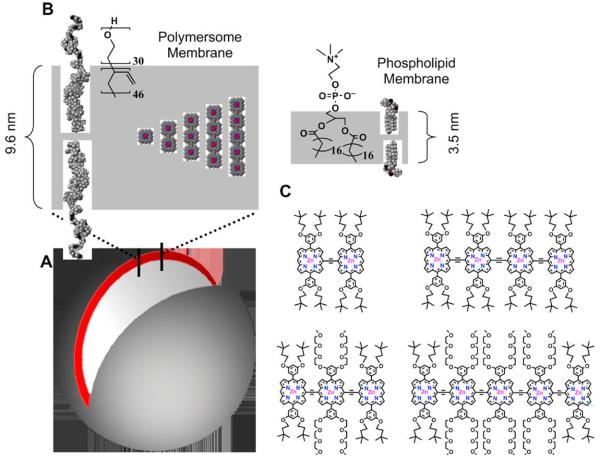
Fig. 1.
Schematic representations of NIR-emissive polymersomes. (A) In aqueous solution, amphiphilic diblock copolymers of polyethyleneoxide-1,2 polybutadiene (PEO30–PBD46) self-assemble into polymer vesicles (polymersomes) with the hydrophobic PBD tails orienting end-to-end to form bilayer membranes. The depicted unilamellar polymersome displays an excised cross-sectional slice illustrating the bilayer PBD membrane (gray) containing the hydrophobic (porphinato)zinc(II) (PZn)-based near-IR fluorophores (NIRFs, red). (B) CAChe-generated sectional schematic of the NIR-emissive polymersome membrane indicating the molecular dimensions of: (i) the PBD component of the bilayer (9.6 nm); (ii) the large, dispersed PZn-based NIRFs (2.1–5.4 nm); and, (iii) a typical liposome membrane (3–4 nm) comprised of phospholipids (1-stearoyl-2-oleoyl-sn-Glycero-3-Phosopho-choline—SOPC). (C) Chemical structures of NIR fluorophores PZn2–PZn5. [This image was reproduced from Ghoroghchian et al. [9] with permission from Copyright (2005) National Academy of Sciences, USA.]
In aqueous solutions, amphiphilic block copolymers can self-assemble into mesoscopic structures (≤200 nm-50 μm in diameter) [3]. The ratio of hydrophilic to hydrophobic block volume fraction determines whether micelles (spherical, prolate, or oblate), or vesicles (polymersomes) will form [2,11-13]. As a general rule, however, a ratio of hydrophilic block to total polymer mass of approximately ≤35% ± 10% yields membrane structures, while copolymers with ratios greater than 45% generally form micelles; those with ratios less than 25% form inverted microstructures [14]. Micellar structures have been used as intracellular and systemic delivery systems [15-18] but present significant limitations when compared to polymersomes. In aqueous solutions, they can only encapsulate hydrophobic molecules unless strong binding or covalent linking strategies are incorporated for sequestering aqueous-soluble components.
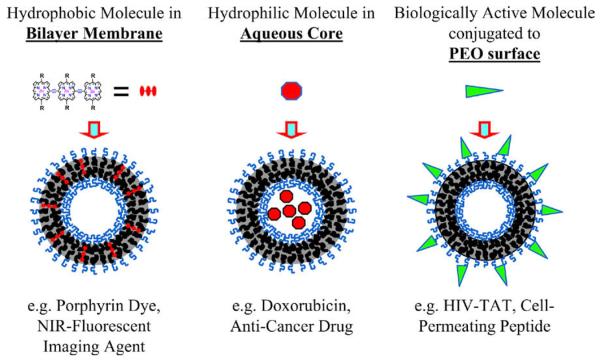
In contrast, polymersomes can simultaneously encapsulate hydrophilic components in their aqueous interior and hydrophobic molecules within their thick lamellar membranes [10]. In addition, biologically active ligands, such as antibodies, can be readily conjugated to the exterior brush surface to target the vesicles or to provide a therapeutic response [19-22]. These properties of the vesicle architecture effectively create a multimodal platform, which can be used for therapeutic (drug delivery) and/or diagnostic (imaging) applications (Fig. 2).
Fig. 2.
General application of polymersome architecture in therapeutics. Schematic representation of polymersome assembly illustrating three possible applications, namely optical imaging, drug delivery, and targeted-therapy.
Although vesicles can be targeted to specific sites using biologically active ligands, the anatomical and pathophysiological abnormalities of the tumor tissue alone can be utilized to aid in the localized delivery of macromolecules [23]. The tumor vasculature, characterized by irregularly shaped, dilated, defective, and/or leaky blood vessels, disorganized endothelial cells with fenestrations, as well as other abnormalities, allows for the passive accumulation of macromolecules at the tumor site [24]. Further, due to the poor lymphatic drainage, nanoparticles can accumulate and remain at the tumor site even in the absence of a targeting moiety [25]. This phenomenon is known as the enhanced permeability and retention (EPR) effect and makes it possible to achieve high local concentrations of macromolecules at the tumor site without specific targeting [24]. However, a question that has yet to be addressed with polymersomes is how much additional accumulation is possible with targeting.
2. Diblock copolymers forming vesicles and release mechanisms
In this section, we highlight some of the polymer formulations, which have led to the formation of polymersomes, that have demonstrated promise for controlled release of pharmaceuticals.
Initial polymersome research by Hammer and Discher used poly(ethylene oxide)-block-poly(ethylethylene) (PEO-b-PEE) diblock copolymers to demonstrate the formation of polymersomes in aqueous solution, as well as to characterize the vesicles material and physical properties [3]. Additional work in the field has led to the synthesis of a number of biocompatible PEO-based amphiphilic block copolymers that form aqueous vesicles dispersions, including poly(ethylene oxide)-block-poly(butadiene) (PEO-b-PBD) [7].
A significant limitation of these polymers for in vivo therapeutics is that they are not biodegradable and likely not fully biocompatible. In an effort to create vesicles that degrade and release their contents in vivo, PEO-b-PBD polymers have been blended with hydrolysable block copolymers, such as poly(ethylene oxide)-block-poly(lactic acid) (PEO-b-PLA) or poly(ethylene oxide)-block-poly(caprolactone) (PEO-b-PCL); these vesicles have been shown to undergo hydrolytic degradation intracellularly (in the acidic environment of the endolysosomal compartment) leading to release of the polymersomes' encapsulates [26-28]. Cryo-TEM images and dynamic light scattering measurements serve to demonstrate that nanoscale phase transitions occur in these blends as the polyester backbone of the vesicles' hydrolytic components degrade over time; the intact vesicle begins to form pores, which leads to the transition to worm-like micelles and ultimately leads to the formation of spherical micelles [27]. Further, it has been shown that the release rate of encapsulates in blended polymersomes increases linearly with increasing mole ratio of hydrolysable polymer [26]. While these studies represent a reasonable first step in the development of polymersomes for therapy, it is critical to overcome the hurdle of in vivo toxicity presented by the residual PEO-b-PBD in these structures.
Recently, efforts in our group have focused on the development of self-assembled polymersomes from fully-biodegradable synthetic amphiphiles. The ability to generate self-assembled, fully-bioresorbable vesicles comprised of an amphiphilic diblock copolymer consisting of two previously FDA-approved building blocks, poly(ethylene oxide) (PEO) and poly(caprolactone) (PCL), has been demonstrated by Ghoroghchian and coworkers [10]. Unlike polymersomes formed from the blending of “bio-inert” and hydrolysable block copolymers [26], these fully-bioresorbable PEO-b-PCL vesicles undergo acid catalyzed hydrolysis of their ester linkages and degrade without leaving any potentially toxic byproducts [10,29]. We have demonstrated the release of doxorubicin from these systems with time-constants of 18–24 h, depending on pH; in vivo testing of these polymersomes for delivery is underway.
In contrast to acid catalyzed hydrolysis of the polymer backbone, which occurs on the order of hours to days, pH triggered contents release, using block copolymers whose solubility in aqueous solutions is dependent upon solution pH, can occur much more rapidly [30]. Borchert and colleagues generated polymersomes comprised of poly(2-vinylpyridine)-block-poly(ethylene oxide) (P2VP-b-PEO) copolymers and showed that the resultant vesicles disassemble in acidic solutions and quickly and completely release their contents; this dissolution is due to the protonation of the P2VP block in acidic solutions (below pH 5) which converts the previously hydrophobic block into a water soluble polymer [30].
Cerritelli and colleagues have designed and characterized a diblock copolymer of poly(ethylene glycol) (PEG) and poly(propylene sulfide) (PPS) with a reduction sensitive disulfide link between the two blocks (PEG-SS-PSS); they demonstrated the ability of this block copolymer to form polymer vesicles which burst within a few minutes of endocytosis due to the reductive environment in the endosome [31]. In addition to diblock copolymers, various other polymeric amphiphiles can form vesicles in aqueous solutions. Napoli et al. synthesized a triblock copolymer of poly(ethylene glycol)-block-poly(propylene sulfur)-block-poly(ethylene glycol) (PEG-b-PPS-b-PEG) [32], which at dilute concentrations forms polymeric vesicles [33,34]. Napoli and colleagues then demonstrated that vesicles comprised of this triblock copolymer could be destabilized by the oxidation of the hydrophobic PPS block; when oxidized, PPS is first converted to poly(propylene sulphoxide) and subsequently converted to poly(propylene sulfone), both of which are more hydrophilic than PPS [35]. This change in hydrophobicity of the “hydrophobic” block alters the ratio of hydrophobic block to total polymer mass, leading to changes in morphology of the self-assembled structures from vesicles, to worm-like micelles, to spherical micelles, and finally to unimolecular micelles [35]. These polymers present the promise of biodegradability, due to oxidation of the hydrophobic chain into small molecules solutes that can be readily cleared [32].
Another possibility to generate fully-biodegradable vesicles is to utilize polypeptides as their composite amphiphiles. Vesicles and micelles comprised of polypeptide block copolymers can mimic the shape and biological performance of natural vesicles and micelles [36]. Sun et al. synthesized various diblock copolypeptides of poly(L-lysine)-block-poly(L-phenylalanine) (PLL-b-PPA) which spontaneously self-assemble into giant vesicles in aqueous solutions [36].
3. Therapeutic applications of polymersomes
Currently, many compounds with toxic side effects and/or low bioavailability hold extraordinary promise as potential therapeutic agents. However, limited bioavailability of hydrophobic compounds and/or toxic side effects of these molecules can render their therapeutic value ineffective. Further, the ability of the therapeutic agents to reach the target site can be limited by the body's clearance. Thus, the development of a polymeric delivery vehicle with specifically tuned pharmacokinetics, which can encapsulate and release highly toxic therapeutic agents for concentrated local delivery, should greatly increase therapeutic efficacy.
Doxorubicin (DOX) is an amphipathic anti-neoplastic agent that shows much promise in cancer therapy, both alone and in conjunction with antibodies and peptides [37]. One of the major limitations associated with administration of this chemotherapeutic agent is cardiac myocyte toxicity [38]. However, utilizing drug carriers to deliver doxorubicin can alleviate some of the associated cardio-toxicity by altering the pharmacodistribution of the drug, thereby reducing the drug concentration in the heart [38]. Delivery of doxorubicin in liposomes has been shown to extend the circulation time and alter the pharmacodynamics of doxorubicin in such a way as to decrease its toxicity while still maintaining its anticancer activity [38]. Using active loading methods originally developed for liposomes, doxorubicin can be efficiently loaded into the aqueous center [10,26,39] of polymer vesicles.
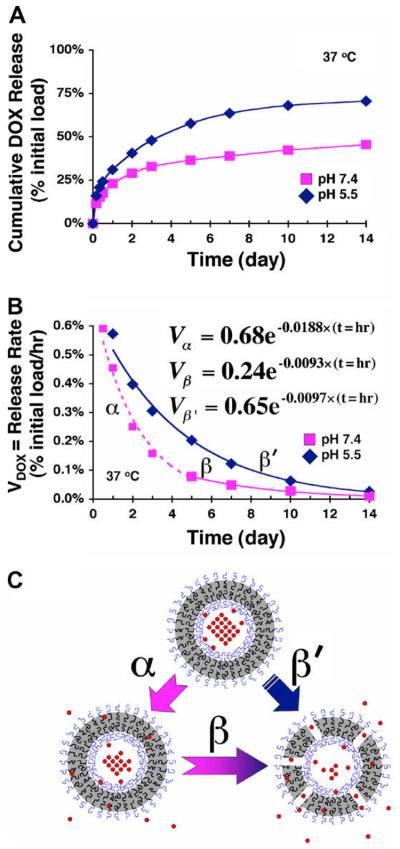
Ghoroghchian et al. have successfully loaded doxorubicin into the hydrophilic reservoir of PEO-b-PCL polymersomes [10] by using an ammonium sulfate gradient [40-42]. Using doxorubicin, as a model system, the mechanism by which PEO-b-PCL based polymersomes release a physiologically relevant encapsulant was assessed under various conditions (pH 7.4 and pH 5.5 at 37 °C) [10]. In-situ doxorubicin release from polymersomes was monitored spectro-fluorometrically (λex 480 nm, λem 590 nm) over 14 days. While the kinetics of release varied at the two pHs, an initial burst release phase (approx. 20% of the initial payload within the first 8 h) was observed at both pH conditions followed by a more controlled, pH-dependent release over the following several days [10]. At a pH of 7.4, kinetic release studies suggest that the drug molecules initially escape the polymersome through passive diffusion of the drug across the intact PCL membrane (days 1–4) and subsequently through hydrolytic matrix degradation of PCL (days 5–14) [10] (Fig. 3). At a pH of 5, however, it appears that the dominant mechanism of release at both short and long times is acid catalyzed hydrolysis of the PCL membrane [10].
Fig. 3.
Kinetics of doxorubicin loaded polymersomes. (A) Cumulative in situ release of doxorubicin, loaded within 200 nm diameter PEO(2 K)-b-PCL(12 K)-based polymersomes, under various physiological conditions (pH 5.5 and 7.4; T = 37 °C) as measured fluorometrically over 14 days. N = 4 samples at each data point; individual data points for each sample varied by less than 10% of the value displayed at each time interval. (B) Release rates of DOX (Vdox) from 200 nm diameter PEO(2 K)-b-PCL(12 K)-based polymersomes vs. time. Dotted and solid lines represent exponential fits obtained by regression analysis (R2 = 0.99 for each curve), and the displayed equations correspond to the respective release regimes (α, β, β′). (C) Schematic illustrating differing regimes of DOX release via (α) intrinsic drug permeation through intact vesicle membranes vs. (β and β′) release predominantly by PCL matrix degradation. [This image was reproduced from Ghoroghchian et al. [10] with permission from Copyright (2006) American Chemical Society.]
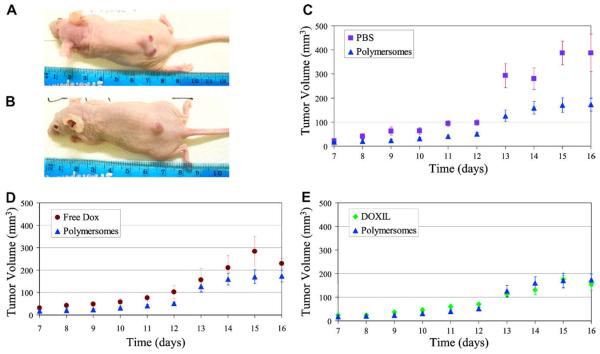
Moreover, Levine and colleagues have recently demonstrated the therapeutic potential of doxorubicin loaded PEO-b-PCL polymersomes in vivo; drug loaded bioresorbable polymersomes were administered to xenotransplanted (T6-17 cells) tumor-bearing mice and their capability to retard tumor growth was assessed using such metrics as tumor size and body weight (Levine, unpublished data). As demonstrated in Fig. 4, doxorubicin loaded PEO-b-PCL polymersomes were able to retard tumor growth in a live animal on a par with the commercially available agent DOXIL® (a clinically administered liposomal formulation of doxorubicin) (Levine, unpublished data). Further, mouse weights remained within ±1.5 g of the initial weight, for all treatment groups throughout the study (Levine, unpublished data).
Fig. 4.
Anti-tumor effects of doxorubicin loaded polymersome in mice. Mice were inoculated with tumor cells on day 0, were administered drug (free dox, dox loaded polymersome, or DOXIL) or PBS on day 7, and sacrificed on day 16. Images of tumor bearing mice administered PBS (A) and DOX polymersomes at the culmination of the study, day 16. (B); (C–E) Average tumor Volume vs. Time, Tumor volumes of the 5 mice per group averaged. Error bars are reported as standard error.
Paclitaxel (taxol) (TAX), an anticancer agent, whose therapeutic efficacy is limited by its poor aqueous solubility [43] is currently administered in a mixture of Cremophor EL (polyoxyethylated castor oil) and dehydrated ethanol [44] to increase bioavailability. Systemic administration of taxol is associated with several negative side effects in patients including dyspnea, hypotension, broncho-spasm, urticaria, and erythematous rashes [44]. In addition, the formulation agent (Cremophor EL) used to solubilize the hydrophobic taxol is believed to be responsible for inducing the hypersensitivity reactions observed in patients [44]. As a result, various aqueous formulations of taxol have been examined to decrease toxic side effects and increase water solubility. Li et al. demonstrated the ability to load taxol into the hydrophobic bilayer of PEO-b-PBD polymer vesicles and thus increase the water solubility of this drug while maintaining its cytotoxic properties [45].
Combination therapy involves the administration of different classes of chemotherapeutics to a patient in order to treat their disease; this approach has been shown to be generally effective and many cancer treatment regimes employ such multi-drug therapy. A combination regime of DOX and TAX has been shown clinically to retard tumor growth more effectively in comparison to the administration of a single agent alone [46]. A reasonable hypothesis is that the synergistic effect of these two drugs would be increased when both drugs are administered in the same delivery vehicle, as this would ensure delivery of the drug molecules in prescribed ratios to a given target at the same time; Ahmed et al. demonstrated the ability to co-encapsulate DOX and TAX into polymer vesicles and showed the increased synergistic effect when DOX and TAX are in the same polymersome [27,28]. PEG-b-PLA/PEG-b-PBD blended polymer vesicles were loaded with DOX in their hydrophilic reservoir and TAX in their hydrophobic bilayer, and were administered in vivo; the results demonstrate a higher maximum tolerated dose (MTD), as well as increased tumor shrinkage and maintenance, when both agents are administered in vesicles rather than as free drugs [27]. Since there are a wide variety of both hydrophilic and hydrophobic pharmaceuticals, this paradigm is generally applicable to creating other polymersome-formulations for combination therapy. Ultimately, as mentioned before, further work to combine these pharmaceuticals within a safe and fully-biodegradable formulation is necessary.
In addition to small molecules, peptides, proteins, and nucleic acids have been encapsulated in block copolymer assemblies. Lee et al. successfully encapsulated myoglobin, hemoglobin, and albumin in PEO-b-PBD based polymer vesicles at varying degrees of encapsulation efficiency [5]. Arifin and Palmer further demonstrated that bovine hemoglobin (Hb) could be encapsulated inside PEO-b-PBD polymer vesicles with oxygen affinities similar to those of human red blood cells; they demonstrated that these “polymersomes-encapuslated hemoglobin” (PEH) dispersions could store and transport Hb and potentially act as in vivo oxygen therapeutics [47]. The ability to encapsulate proteins within polymersomes provides a promise for future protein therapies, which are currently facing delivery obstacles.
4. Diagnostic applications of polymersomes
The ability to non-invasively image nanoparticles in vivo is a major advantage in determining their biodistribution and developing these delivery vehicles for both therapeutic and diagnostic applications. Biodistribution studies with polymersomes, in particular, would be greatly aided by the encapsulation of an imaging agent in the vesicles; this would enable non-invasive monitoring of the location of vesicles during drug delivery without the need to sacrifice the animal. Although nanoparticles have been used with a spectrum of different imaging modalities including PET [48,49] and MRI [50-52], here we will focus on polymersomes that encapsulate fluorescent agents for optical imaging. Because light scattering decreases with increasing wavelength, and hemoglobin and water absorption spectra have their nadir in the near infrared (NIR) spectral region, much work has been focused on developing NIR contrast agents for in vivo imaging studies [9]. To this end, Ghoroghchian et al. have successfully loaded porphyrin-based near infrared fluorophores (NIRFs) into the hydrophobic bilayer membranes of PEO-b-PBD [9,10,53,54], PEO-b-PCL [54], PEO-b-PEE [54], and poly(ethylene oxide)-block-poly(methylcaprolactone) (PEO-bPmCL) [54] polymersomes.
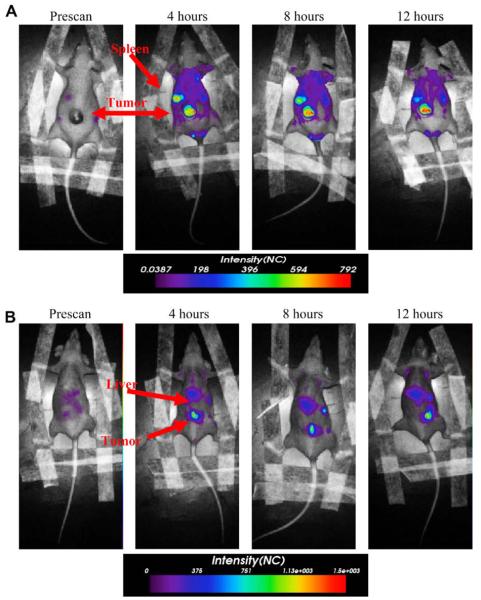
Studies using PEO-b-PBD polymersomes have shown that porphyrin-based NIRFs, when encapsulated in polymersomes, are able to generate a signal with enough intensity to penetrate through 1 cm of a solid tumor [9]. Further, when these NIR-emissive nanopolymersomes are injected into the tail-vein of mice, the biodistribution of the nanoparticles can be tracked in vivo via non-invasive NIR fluorescence-based optical imaging [55]. Fig. 5 demonstrates the ability to track PEO-b-PBD polymer vesicles in tumor bearing mice over 12 h (Levine, unpublished data). Combining drug delivery with imaging will allow for the continuous noninvasive monitoring of drug-loaded nanopolymersomes in vivo, obviating the need to sacrifice animals at each time point to determine basic pharmacokinetic and biodistribution profiles, thereby greatly reducing animal load.
Fig. 5.
Tumor imaging by NIR-emmisive polymersome. Fluorescence images obtained using eXplore Optix instrument of the same mouse taken prior to administration of NIR-emissive polymersomes, and at 4, 8, and 12 h post tail-vein injection. (A) Prone position, (B) supine position (λex = 785 nm, λem = 830–900 nm). The arrows in the prone and supine positions suggest location of organs. In the supine position, the arrow suggests the fluorescence emanating from the lower portion of the mouse body is from the tumor; it may also be emanating from the gut of the mouse due to break down of food.
In addition to developing drug delivery applications, NIR-emissive polymersomes have also been shown to be useful for ex vivo cellular labeling and in vivo cellular tracking. Dendritic cells (DCs) play an important role in the immune response and have shown potent anticancer activity leading to DC-based vaccines research [56]. Current progress in DC-based vaccines has been, however, limited by various factors [56], some of which could be overcome by the development of imaging methods for in vivo DC tracking [19]. Christian et al. have demonstrated the ability to label DCs ex vivo with polymersomes encapsulating porphyrin-based NIRFs; the TAT peptide, as will be discussed in greater detail below, was conjugated to these NIR-emissive polymersome to facilitate efficient uptake of polymer vesicles by DCs [19]. Christian and colleagues determined that DC surfaceassociated polymersomes shed over the first 24–48h; but, polymer vesicles that were fully internalized by the DCs remained stably incorporated over 3 days [19]. They further showed that the NIR-emissive-polymersome-labeled DCs, when administered into the foot pad of mice, traffic to the nearest lymph node (popliteal lymph node) and could be tracked in vivo via optical imaging over 33 days [57]. They further showed that dendritic cells are sequestered in the liver when the cells are delivered intravenously [42], indicating that the mode of dendritic cell delivery will be critical for the effectiveness of cancer immunotherapy. These results suggest that polymer vesicles can be employed for cell tracking in longitudinal studies and could thus assist in the further development of cell-based vaccines. Overall, the results in this section demonstrate that the loading of imaging agents, such as porphyrin-based NIRFs, into the polymersome bilayer creates soft matter optical imaging agents suitable for in vitro diagnosis and deep-tissue imaging, non-invasive biodistribution and pharmacokinetic studies, as well as in vivo cellular tracking.
An alternative imaging modality that can be used to image polymer vesicles is diagnostic ultrasound. Zhou et al. prepared air-encapsulated polymersomes via lyophilization and rehydration of previously formed polymer vesicles [58]. The polymer bubbles were imaged using a Pie Medical Scanner 350 and were visualized as bright spots, validating the acoustic activity of air-encapsulated polymersomes [58]. These results show that polymer vesicles hold promise in the realm of ultrasound imaging as well as optical imaging.
5. Polymersome surface modifications for delivery and therapy
Biologically-active molecules conjugated to the surfaces of polymersomes can be used to direct these nanoparticles to sites of disease and inflammation. Modifying polymer vesicles with biological ligands enables targeting of upregulated receptors and molecules on affected cells in vitro and in vivo, thereby enhancing the nanoparticles' EPR effect and further mitigating the potential toxic side effects of systemic delivery. Additionally, chemotherapeutics, when used in conjunction with molecular targeting agents, can have a synergistic effect [59]. In addition to therapeutic applications, over the past two decades the use of anticancer antibodies against molecular targets has been developed for tumor imaging applications [37]. Polymer vesicles can be directed to specific sites in vivo by conjugation of targeting moieties to the end group of their hydrophilic polymer block (usually PEO). It is important to recognize that the conjugation of ligands to the polymersome surfaces can alter the composite polymer amphiphiles' hydrophilic-block-to-total-mass ratio leading to a change in structural morphology (e.g. from vesicles to micelles).
Using a modular biotin-avidin chemistry, Lin and colleagues functionalized polymer vesicles with anti-ICAM-1 antibody to target ICAM-1 (intercellular adhesion molecule-1) [21], a molecule that is upregulated on endothelial cells during inflammation. Using micropipette aspiration, they measured the adhesiveness of these functionalized polymer vesicles to ICAM-1 immobilized on the surface of polystyrene beads and determined that the adhesion strength is linearly proportional to the surface density of the anti-ICAM-1 molecules on the polymersome [21]. This finding is in contrast to their earlier adhesion experiments carried out with functionalized biotinylated polymersomes and avidin coated beads [22], suggesting that the adhesiveness of functionalized vesicles is not only dependent on surface density, but also upon the presentation/orientation of the targeting molecules on the vesicle surface [21].
Additionally, sialyl lewisx (sLex), a selectin ligand, has been conjugated to polymer vesicles using similar biotin-avidin modular chemistry as previously described (Hammer et al., in press, Faraday-discussions 139). In addition to ICAM-1 molecules, selectins are also upregulated at sites of inflammation [21]. In an effort to create “leukopolymersomes,” i.e. polymersomes that mimics the adhesive properties of leukocytes, dual functionalized vesicles of sLex and anti-ICAM-1 have been made by the Hammer lab. These investigators were able to measure firm and rolling adhesion of anti-ICAM-1-, sLex-, and anti-ICAM-1/sLex conjugated polymersomes under flow along ICAM-1, P-selectin, and ICAM-1/P-selectin coated surfaces, respectively, at venous shear rates. It is believed that dual functionalized leukopolymersomes will be able to serve as targeting agents to bring both therapeutics (drugs) and diagnostics (imaging agents) to sites of inflammation [21].
Meng and co-workers functionalized polymersomes comprised of PEG-block-poly(ester) and PEG-block-poly(carbonate) diblock copolymers with anti-human IgG (a-HIgG) or anti-human serum albumin (a-HSA) [6]. a-HIgG and a-HSA were either conjugated to the polymersome through covalent attachment to carboxyl groups on the vesicle surface or by attachment to protein G, which was covalently attached to the polymersome surface via the carboxyl groups; using imaging surface plasmon resonance (iSPR), they determined that immobilization of antibodies on the vesicle surface through protein G is preferred for targeting [6]. iSPR was further used to demonstrate the potential of antibody functionalized vesicles for targeting antigens [6].
In addition to targeting, these biologically active ligands can aid in cellular uptake [60]. As previously mentioned, Christian et al. demonstrated that the highly cationic HIV-derived TAT peptide, when coupled to NIR-emissive polymersomes, enhances cellular delivery of polymer vesicles to dendritic cells while moderately affecting cell viability [19]. Intracellular uptake of polymersomes was dependent upon their concentration and incubation time in solution; viability was affected by these factors as well [19].
We have recently attempted to conjugate small anti-HER2/ neu peptidomimetics, designed by Murali and coworkers [59], to polymersomes in order to further develop these nanoparticles for both clinical breast cancer diagnosis (NIR-emissive polymer-somes) and therapy (e.g. with and without doxorubicin incorporation). In comparison to normal epithelial tissues, over-expression of the HER2 protein, a member of the epidermal growth factor (EGFR) or HER family, has been seen in approximately 30% of breast, ovarian, and colon cancers [37,59]. A family of anti-HER2/ neu peptides (AHNPs) designed by Murali et al. has a potency on par with that of the full-length monoclonal antibody (Herceptin®; Genentech, San Francisco, CA) and demonstrates biochemical and biological properties predictive of clinical therapeutic response [59]. It has been demonstrated that AHNP prevents tumor growth of transformed T6-17 cells, in which HER2/neu is over-expressed, in vivo and in vitro [59]. However, the relatively short half-life of peptides and proteins in vivo is one challenge that still remains to be overcome when using such agents for therapeutic applications [61]. To overcome the challenge of rapid clearance, “stealth” or “sterically stabilized” nanoparticles, such as pegylated liposomes, have been employed to deliver peptides [62]. Thus, linking AHNP to a nanoparticle surface can greatly improve the pharmacokinetics of the small peptide and allow for targeting as well as improved therapeutic efficacy.
Ghoroghchian et al. observed changes in polymersome morphology from vesicles to micelles post-conjugation of the AHNP peptides to PEO-b-PBD vesicles [55]. Vesicles, as well as small spherical micelles, not present in aqueous suspensions of the functionalized and unfunctionalized diblock copolymer without peptide, were observed in the polymersome suspension post AHNP conjugation [55]. Since these micelles were not seen in cryoTEM images of the pure or unfunctionalized polymer, it is probable that they are comprised of peptide-conjugated polymer; furthermore, it is hypothesized that the vesicles in the suspension consist of polymer not conjugated to AHNP [55]. Peptide-conjugated vesicle generation with less hydrophobic AHNP family members were also attempted and again resulted in phase separation of the diblock copolymer-peptide “triblock” from the diblocks [55]. Our interpretation of these results is that the underlying polymer material needs to be redesigned to accommodate peptides and preserve vesicular structure in order to develop AHNP polymersomes fit for clinical diagnostic and therapeutic applications.
6. Future directions
Polymersomes are new and valuable tools for both disease diagnosis and therapy. Our view is that the enhanced stability and tunability of polymersomes will ultimately lead to the development of effective carriers for in vivo drug delivery, molecular imaging, and cellular mimicry that extend well beyond what has thus far been achieved with phospholipid vesicles.
In drug delivery, the potential to co-encapsulate two drug molecules in the same polymersome enables combination therapies and eliminates the need to individually administer two separate drug formulations. As such, polymersome may not only be more effective in treating recurrent, resistant, or residual tumors, but may also be more convenient for patient administration and treatment tolerance. It is also possible to make separate polymersome-formulations, each with different drugs or with different dosing that deliver drugs in a sequence, as needed for the particular type of disease that is being treated. Additionally, localizing therapeutics to the site of intent, either through passive accumulation (EPR effect) or with targeting ligands, can enable administration of higher doses of drug while minimizing the toxic side effects of systemic delivery. Further, the ability to image polymer vesicles during delivery will offer numerous advantages for understanding the mechanisms of therapy as well as efficiently designing drug delivery regimens in small animal models. Aside from the demonstration of the activity of multi-modal polymersomes with existing block copolymers, we believe that further developments in polymer design will extend the applicability of polymersomes to different drugs and imaging modalities.
In addition to targeted therapeutic drug delivery, targeting ligands can be used to direct diagnostic agents to tumors sites, assisting in in vivo diagnostic imaging. Air-encapsulated polymeric vesicles facilitate nanodiagnostics using ultrasound. Further, the encapsulation of both porphyrin-based near-infrared fluorophores and air into the same vesicle should yield a multi-modal polymersome, where both ultrasound and optical imaging can be performed concurrently thereby enhancing tumor imaging. Finally, we see promise for simultaneous clinical diagnostic imaging and in vivo therapeutic drug delivery with the correct polymer formulations.
Acknowledgments
This work is funded, in part, under a grant through the Health Research Formula Funds, Commonwealth of Pennsylvania to R.M., M.J.T. and D.A.H. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions. This work is also supported, in part, under grants from the National Institutes of Health (EB003457-01 and CA115229-01A1) to D.A.H. and M.J.T. The authors thank N.A. Christian, and G. Robbins for sharing unpublished results and/or recently submitted data.
References
- 1.Cegnar M, Kristl J, Kos J. Expert Opinion on Biological Therapy. 2005;5:1557–1569. doi: 10.1517/14712598.5.12.1557. [DOI] [PubMed] [Google Scholar]
- 2.Antonietti M, Forster S. Advanced Materials. 2003;15:1323–1333. [Google Scholar]
- 3.Discher BM, Won YY, Ege DS, Lee JCM, Bates FS, Discher DE, Hammer DA. Science. 1999;284:1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 4.Discher DE, Eisenberg A. Science. 2002;297:967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 5.Lee JCM, Bermudez H, Discher BM, Sheehan MA, Won YY, Bates FS, Discher DE. Biotechnology and Bioengineering. 2001;73:135–145. doi: 10.1002/bit.1045. [DOI] [PubMed] [Google Scholar]
- 6.Meng F, Engbers GHM, Feijen J. Journal of Controlled Release. 2005;101:187–198. doi: 10.1016/j.jconrel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Bermudez H, Brannan AK, Hammer DA, Bates FS, Discher DE. Macromolecules. 2002;35:8203–8208. [Google Scholar]
- 8.Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Journal of Controlled Release. 2003;90:323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 9.Ghoroghchian PP, Frail PR, Susumu K, Blessington D, Brannan AK, Bates FS, Chance B, Hammer DA, Therien MJ. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2922–2927. doi: 10.1073/pnas.0409394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoroghchian PP, Li GZ, Levine DH, Davis KP, Bates FS, Hammer DA, Therien MJ. Macromolecules. 2006;39:1673–1675. doi: 10.1021/ma0519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zupancich JA, Bates FS, Hillmyer MA. Macromolecules. 2006;39:4286–4288. [Google Scholar]
- 12.Hillmyer MA, Bates FS, Almdal K, Mortensen K, Ryan AJ, Fairclough JPA. Science. 1996;271:976–978. [Google Scholar]
- 13.Hillmyer MA, Bates FS. Macromolecules. 1996;29:6994–7002. [Google Scholar]
- 14.Discher DE, Ahmed F. Annual Review of Biomedical Engineering. 2006;8:323–341. doi: 10.1146/annurev.bioeng.8.061505.095838. [DOI] [PubMed] [Google Scholar]
- 15.Savic R, Luo LB, Eisenberg A, Maysinger D. Science. 2003;300:615–618. doi: 10.1126/science.1078192. [DOI] [PubMed] [Google Scholar]
- 16.O'Reilly RK, Hawker CJ, Wooley KL. Chemical Society Reviews. 2006;35:1068–1083. doi: 10.1039/b514858h. [DOI] [PubMed] [Google Scholar]
- 17.O'Reilly RK, Joralemon MJ, Hawker CJ, Wooley KL. Journal of Polymer Science Part a—Polymer Chemistry. 2006;44:5203–5217. [Google Scholar]
- 18.Sun XK, Rossin R, Turner JL, Becker ML, Joralemon MJ, Welch MJ, Wooley KL. Biomacromolecules. 2005;6:2541–2554. doi: 10.1021/bm050260e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christian NA, Milone MC, Ranka SS, Li GZ, Frail PR, Davis KP, Bates FS, Therien MJ, Ghoroghchian PP, June CH, Hammer DA. Bioconjugate Chemistry. 2007;18:31–40. doi: 10.1021/bc0601267. [DOI] [PubMed] [Google Scholar]
- 20.Lin JJ, Bates FS, Hammer DA, Silas JA. Physical Review Letters. 2005;95 doi: 10.1103/PhysRevLett.95.026101. [DOI] [PubMed] [Google Scholar]
- 21.Lin JJ, Ghoroghchian P, Zhang Y, Hammer DA. Langmuir. 2006;22:3975–3979. doi: 10.1021/la052445c. [DOI] [PubMed] [Google Scholar]
- 22.Lin JJ, Silas JA, Bermudez H, Milam VT, Bates FS, Hammer DA. Langmuir. 2004;20:5493–5500. doi: 10.1021/la036417a. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura Y, Maeda H. Cancer Research. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 24.Iyer AK, Khaled G, Fang J, Maeda H. Drug Discovery Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Duncan R. In: Polymer-Drug Conjugates: Targeting Cancer. Muzykantov VR, Torchlin VP, editors. Kluwer Academic Publishers; Boston: 2002. pp. 197–199. [Google Scholar]
- 26.Ahmed F, Discher DE. Journal of Controlled Release. 2004;96:37–53. doi: 10.1016/j.jconrel.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Journal of Controlled Release. 2006;116:150–158. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed F, Pakunlu RI, Srinivas G, Brannan A, Bates F, Klein ML, Minko T, Discher DE. Molecular Pharmaceutics. 2006;3:340–350. doi: 10.1021/mp050103u. [DOI] [PubMed] [Google Scholar]
- 29.Bei JZ, Li JM, Wang ZF, Le JC, Wang SG. Polymers for Advanced Technologies. 1997;8:693–696. [Google Scholar]
- 30.Borchert U, Lipprandt U, Bilang M, Kimpfler A, Rank A, Peschka-Suss R, Schubert R, Lindner P, Forster S. Langmuir. 2006;22:5843–5847. doi: 10.1021/la060227t. [DOI] [PubMed] [Google Scholar]
- 31.Cerritelli S, Velluto D, Hubbell JA. Biomacromolecules. 2007;8:1966–1972. doi: 10.1021/bm070085x. [DOI] [PubMed] [Google Scholar]
- 32.Napoli A, Tirelli N, Kilcher G, Hubbell JA. Macromolecules. 2001;34:8913–8917. [Google Scholar]
- 33.Valentini M, Napoli A, Tirelli N, Hubbell JA. Langmuir. 2003;19:4852–4855. [Google Scholar]
- 34.Napoli A, Tirelli N, Wehrli E, Hubbell JA. Langmuir. 2002;18:8324–8329. [Google Scholar]
- 35.Napoli A, Valentini M, Tirelli N, Muller M, Hubbell JA. Nature Materials. 2004;3:183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- 36.Sun J, Chen XS, Deng C, Yu HJ, Xie ZG, Jing XB. Langmuir. 2007;23:8308–8315. doi: 10.1021/la701038f. [DOI] [PubMed] [Google Scholar]
- 37.Berezov A, Zhang HT, Greene MI, Murali R. Journal of Medicinal Chemistry. 2001;44:2565–2574. doi: 10.1021/jm000527m. [DOI] [PubMed] [Google Scholar]
- 38.Waterhouse DN, Tardi PG, Mayer LD, Ballly MB. Drug Safety. 2001;24:903–920. doi: 10.2165/00002018-200124120-00004. [DOI] [PubMed] [Google Scholar]
- 39.Choucair A, Soo PL, Eisenberg A. Langmuir. 2005;21:9308–9313. doi: 10.1021/la050710o. [DOI] [PubMed] [Google Scholar]
- 40.de Menezes DEL, Pilarski LM, Allen TM. Cancer Research. 1998;58:3320–3330. [PubMed] [Google Scholar]
- 41.Haran G, Cohen R, Bar LK, Barenholz Y. Biochimica Et Biophysica Acta. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 42.Bolotin EM, Cohen R, Bar LK, Emanuel N, Ninio S, Lasic DD, Barenholz Y. Journal of Liposome Research. 1994;4:455–479. [Google Scholar]
- 43.Sharma US, Balasubramanian SV, Straubinger RM. Journal of Pharmaceutical Sciences. 1995;84:1223–1230. doi: 10.1002/jps.2600841015. [DOI] [PubMed] [Google Scholar]
- 44.Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR, Vanecho DA, Vonhoff DD, Leylandjones B. Journal of Clinical Oncology. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 45.Li SL, Byrne B, Welsh J, Palmer AF. Biotechnology Progress. 2007;23:278–285. doi: 10.1021/bp060208+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustafson DL, Merz AL, Long ME. Cancer Letters. 2005;220:161–169. doi: 10.1016/j.canlet.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Arifin DR, Palmer AF. Biomacromolecules. 2005;6:2172–2181. doi: 10.1021/bm0501454. [DOI] [PubMed] [Google Scholar]
- 48.Pressly ED, Rossin R, Hagooly A, Fukukawa KI, Messmore BW, Welch MJ, Wooley KL, Lamm MS, Hule RA, Pochan DJ, Hawker CJ. Biomacromolecules. 2007;8:3126–3134. doi: 10.1021/bm700541e. [DOI] [PubMed] [Google Scholar]
- 49.Sun G, Xu J, Hagooly A, Rossin R, Li Z, Moore DA, Hawker CJ, Welch MJ, Wooley KL. Advanced Materials. 2007;19:3157–3162. [Google Scholar]
- 50.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Circulation Research. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 51.Perez JM, Simeone FJ, Tsourkas A, Josephson L, Weissleder R. Nano Letters. 2004;4:119–122. [Google Scholar]
- 52.Tsourkas A, Shinde-Patil VR, Kelly KA, Patel P, Wolley A, Allport JR, Weissleder R. Bioconjugate Chemistry. 2005;16:576–581. doi: 10.1021/bc050002e. [DOI] [PubMed] [Google Scholar]
- 53.Ghoroghchian PP, Frail PR, Susumu K, Park TH, Wu SP, Uyeda HT, Hammer DA, Therien MJ. Journal of the American Chemical Society. 2005;127:15388–15390. doi: 10.1021/ja055571b. [DOI] [PubMed] [Google Scholar]
- 54.Ghoroghchian PP, Frail PR, Li GZ, Zupancich JA, Bates FS, Hammer DA, Therien MJ. Chemistry of Materials. 2007;19:1309–1318. doi: 10.1021/cm062427w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghoroghchian PP. Emissive Polymer Vesicles: Soft Nanoscale Probes for In Vivo optical imaging. University of Pennsylvania; Philadelphia: 2006. Dissertation Bioengineering. [Google Scholar]
- 56.Figdor CG, de Vries IJM, Lesterhuis WJ, Melief CJM. Nature Medicine. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 57.Christian NA. Development Application of Tat-Near-Infrared-Emissive Polymersomes for In Vivo Optical Imaging of Dendritic Cells, Bioengineering. University of Pennsylvania; Philadelphia: 2007. [Google Scholar]
- 58.Zhou W, Meng F, Engbers GHM, Feijen J. Journal of Controlled Release. 2006;116:e62–e64. doi: 10.1016/j.jconrel.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 59.Park BW, Zhang HT, Wu CJ, Berezov A, Zhang X, Dua R, Wang Q, Kao G, O'Rourke DM, Greene MI, Murali R. Nature Biotechnology. 2000;18:194–198. doi: 10.1038/72651. [DOI] [PubMed] [Google Scholar]
- 60.Brooks H, Lebleu B, Vives E. Advanced Drug Delivery Reviews. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Lee KY, Yuk SH. Progress in Polymer Science. 2007;32:669–697. [Google Scholar]
- 62.Nobs L, Buchegger F, Gurny R, Allemann E. Journal of Pharmaceutical Sciences. 2004;93:1980–1992. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]