Abstract
Background and Purpose
A major limitation of tissue plasminogen activator (tPA) thrombolysis for ischemic stroke is the narrow time window for safe and effective therapy. Delayed tPA thrombolysis increases the risk of cerebral hemorrhage and mortality, which, in part, is related to neurovascular proteolysis mediated by matrix metalloproteinases (MMPs). We recently showed that normobaric hyperoxia (NBO) treatment reduces MMP-9 expression and blood brain barrier (BBB) disruption in the ischemic brain. Therefore, we hypothesized that NBO could increase the safety of delayed tPA thrombolysis in stroke.
Methods
Male Sprague-Dawley rats were exposed to NBO (95%O2) or normoxia (21%O2) during 5-hr filament occlusion of the middle cerebral artery, followed by 19-hr reperfusion. Thirty min before reperfusion, saline or tPA were continuously infused to rats over 1 hr. Outcome parameters were neurological score, mortality rate, brain edema, hemorrhage volume and MMP-9. Hemorrhage was quantified with a hemoglobin spectrophotometry method. Edema was evaluated as hemispheric enlargement. MMP-9 was measured by gelatin zymography.
Results
In normoxic rats, delayed tPA treatment at 4.5 hrs after stroke onset resulted in high mortality, more severe neurological deficits, increased hemorrhage volumes and augmented MMP-9 induction compared with saline. Rats treated with combined NBO and tPA showed significantly reduced tPA-associated mortality, brain edema, hemorrhage and MMP-9 augmentation, as compared with tPA alone.
Conclusions
Our results suggest that early NBO treatment may represent an important strategy to increase the safety of delayed tPA thrombolysis in ischemic stroke.
Keywords: tPA, cerebral hemorrhage, matrix metalloproteinases, oxygen, stroke, Brain Ischemia, Neuroprotective Agents, Thrombolysis, normobaric hyperoxia
Thrombolytic reperfusion with tissue-type plasminogen activator (tPA) is now an established stroke treatment but only for those patients presenting within 3 hrs of ischemic stroke onset,1 or within 4.5 hrs if the initial stroke is less severe.2 Delayed tPA therapy is associated with increased risk of serious neurovascular complications involving cerebral hemorrhage and edema, leading to high mortality in stroke patients.3–8 Any strategy that can safely extend the thrombolytic time window would allow more stroke patients to benefit from tPA treatment.
Although the mechanisms underlying tPA’s neurovascular complications are not fully understood, it has been suggested that they occur as a result of blood-brain barrier (BBB) disruption.9 Matrix metalloproteinases (MMPs), a family of zinc binding proteolytic enzymes, play an important role in mediating BBB disruption after cerebral ischemia by degrading the major components of basal laminar around the BBB microvasculature.10 Animal and human studies have provided strong evidence linking MMP-9 induction and tPA-induced hemorrhagic transformation in ischemic stroke. For example, in rodent experiments, tPA exacerbated ischemia-induced BBB damage by enhancing the proteolytic activity of MMP-9.11,12 MMP inhibitors significantly reduced the incidence of tPA-associated hemorrhage and mortality in ischemic stroke animal models.13,14 Human studies indicate that stroke patients with higher pretreatment plasma levels of MMP-9 are more likely to experience cerebral hemorrhagic complications after tPA.15–17 These data indicate that inhibition of MMP-9 may represent an important strategy to increase the safety of tPA thrombolysis for ischemic stroke.
We and others have recently shown that normobaric hyperoxia (NBO) treatment significantly reduced infarction volumes and improved neurological function in animal stroke models.18–23 Furthermore, NBO treatment was demonstrated to attenuate MMP-9 induction and BBB disruption in the ischemic brain.18,24 Because NBO is readily available, noninvasive, and can be initiated within minutes after stroke symptom onset, these neurovascular protective effects of NBO may allow it to serve as an effective strategy to improve the safety and efficacy of tPA thrombolysis. In this study, we tested the hypothesis that NBO treatment could reduce the neurovascular complications associated with delayed tPA thrombolysis in a filament suture model of middle cerebral artery occlusion (MCAO) in rats.
Materials and Methods
Animal Model and Experimental Design
The Laboratory Animal Care and Use Committee at UNM approved all experimental protocols. Male Sprague–Dawley rats (Charles River Laboratories) weighing 290–325g were subjected to MCAO and reperfusion following the same surgical procedures as we previously decribed.24 Thirty six rats were randomly divided into 4 experimental groups: (1) Normoxia+saline (Normo/saline, n=8), (2) NBO+saline (NBO/saline, n=8), (3) Normoxia+tPA (Normo/tPA, n=10), (4) NBO+tPA (NBO/tPA, n=10). All rats were subjected to 5-hr MCAO with 19-hrs of reperfusion. 10 min after the onset of MCAO, anesthesia was discontinued, and rats were put into an anesthesia box which was ventilated (3 L/min) with medical air (21%O2, Normoxia) or a gas mixture of 95%O2 + 5%CO2 (NBO) until 30 min before the end of 5-hr MCAO. 95%O2 + 5%CO2 was chosen as the NBO treatment because our earlier study demonstrated that rats breathing this gas mixture were able to maintain the ischemic penumbral pO2 close to the preischemic level, and showed a relatively normal blood pH and breathing rhythm compared to rats breathing 95%O2 + 5%N2 or 100%O2.18
Physiologic saline (2.5 mL/kg body weight) with or without recombinant tPA (10 mg/kg body weight, Genetech) was administered (10% bolus, 90% continuous infusion) to rats via tail vein. The relatively high dose of tPA was necessary to achieve a fibrinolytic effect in rats similar to that of thrombolytic therapy in humans.25 The drug or saline treatment was started 30 min before reperfusion and continued for 30 min after withdrawal of the suture. This regimen assured that the reperfused tissue was exposed to the agent. After saline or tPA administration, rats were returned to their cages.
Analysis of Neurological Deficits and Confirmation of Successful MCAO
At the end of 19-hrs reperfusion, the neurological deficit was assessed with Rogers' eight-point neurologic scale as described previously.18,26 The surviving rats (27/36 rats) were transcardially perfused under deep anesthesia with 250ml cold PBS to remove intravascular blood. Brains were removed and processed for assessing infarction, brain edema, hemorrhage and MMP-9 expression as described below.
To verify successful MCAO, a 1-mm thick brain coronal section 6-mm away from the tip of the frontal lobe was stained with 2,3,5-triphenyltetrazolium chloride (TTC) as described in our recent study.24 Animals that died during reperfusion were excluded for measuring brain edema, hemorrhage and MMP expression, however, mortality rate was calculated for each group and was used as an important parameter for the beneficial effect of NBO. No incidence of subarachnoid hemorrhage which resulted from perforation of the intracranial carotid occurred on postmortem examination for all the rats including those that died during reperfusion.
Postischemic Tissue Processing
Brains were sectioned into five 2-mm thick and one 1-mm thick coronal slices from an 11-mm thick region 3 mm away from the tip of the frontal lobe. After digitally photographing the 2-mm thick brain slices, they were carefully cleaned of meninges. Nonischemic and ischemic hemispheric tissue were then collected, and homogenized in 1.5 mL ice-cold PBS. The resulting homogenates were divided into two parts: 150 µL for measuring hemoglobin contents, and the rest for cerebral microvessel isolation. The 1-mm thick brain section was used for TTC staining as described above.
Measurement of Brain Edema
Brain edema was assessed by measuring the hemispheric areas of each 2-mm thick brain slice on the digital photographs obtained as described above using ImageJ software (NIH). Edema was quantitated as relative increase of the brain area in the ischemic hemisphere versus the nonischemic hemisphere as described previously.24
Isolation of Cerebral Microvessels
Isolation of cerebral microvessels was performed exactly as described in our previous study.24 Briefly, the hemispheric homogenates were filtered through a 41-µm nylon mesh (Spectrum). Microvessels retained on the mesh were then purified with dextran T-500 and stored at −80 °C until further analysis.
Spectrophotometric Assay of Cerebral Hemorrhage
Cerebral hemorrhage was quantified as previously described.27 Hemispheric brain tissue from normal rats was processed with exactly the same procedure as described above. Incremental volumes of homologous blood were added to each hemispheric sample. After homogenization, an aliquot of 150µL from each sample was sonicated on ice for 30s, followed by a 15-min centrifugation at 14,000g at 4°C. Then 80µL of supernatant were added to 320µL reaction reagent (QuantiChrom Hemoglobin Assay Kit; BioAssay Systems). After 5 min, optical density of each reaction was measured with a microplate reader at 400nm. These procedures yielded a linear relationship between hemoglobin concentrations in hemispheric brain tissue and the volume of added blood. Measurements from 150µL hemispheric tissue homogenate obtained from ischemic rats were compared with this standard curve to obtain data in terms of hemorrhage volume (µL).
Gelatin Zymography Analysis for MMP-9
Gelatin zymography was performed to evaluate MMP-9 levels in the isolated cerebral microvessels as we described.24 Briefly, microvessel lysates (30µg protein) were loaded onto 10% SDS-polyacrylamide gels co-polymerized with 1 mg/mL gelatin (Sigma) for electrophoresis. After electrophoresis, gels were washed in 2.5% Triton X-100 and then incubated for 72-hrs at 37°C with a developing Tris buffer before staining with Coomassie blue R-250. Gels were then destained and MMP-9 band intensity was quantified. A mixture of human MMP-2/9 (Chemicon) was used as gelatinase standards.
Statistical Analysis
The data are presented as means±SEM. Statistical analysis was carried out using χ2 test, paired t test or ANOVA, as indicated in the text. A value of P≤0.05 was considered statistically significant.
Results
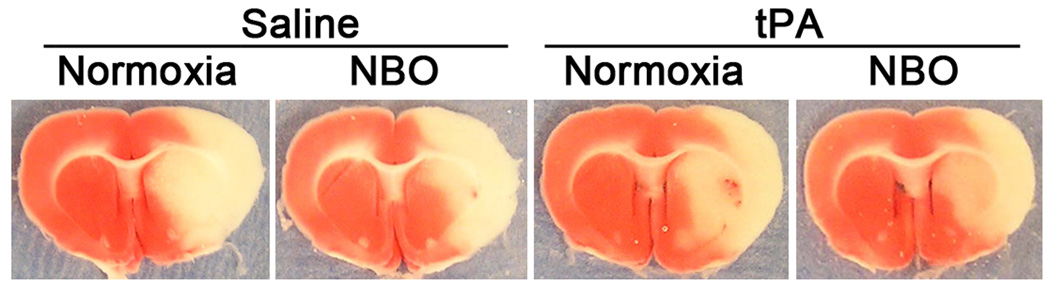
TTC-staining of the 1-mm thick brain section showed that 5-hr MCAO with 19-hr reperfusion induced significant infarction in the ischemic hemispheres of all surviving rats (n=27) except one NBO/tPA rat, which showed a very small infarction (6% of the ischemic hemisphere), indicating unsuccessful MCAO, and was excluded from this study. As shown in the TTC-stained sections (Figure 1), regardless of saline or tPA treatment, NBO-treated rats showed a relatively smaller infarct area than normoxic rats. However, this reduction in lesion size by NBO was much less impressive than our previous results obtained from rats subjected to less severe ischemia (90-min MCAO).24
Figure 1.
Typical TTC-stained brain sections for each group, verifying successful MCAO. NBO was initiated 10min after the onset of MCAO and lasted until 30 min before the end of 5-hr MCAO. Saline or tPA was administered at 4.5-hrs after MCAO onset.
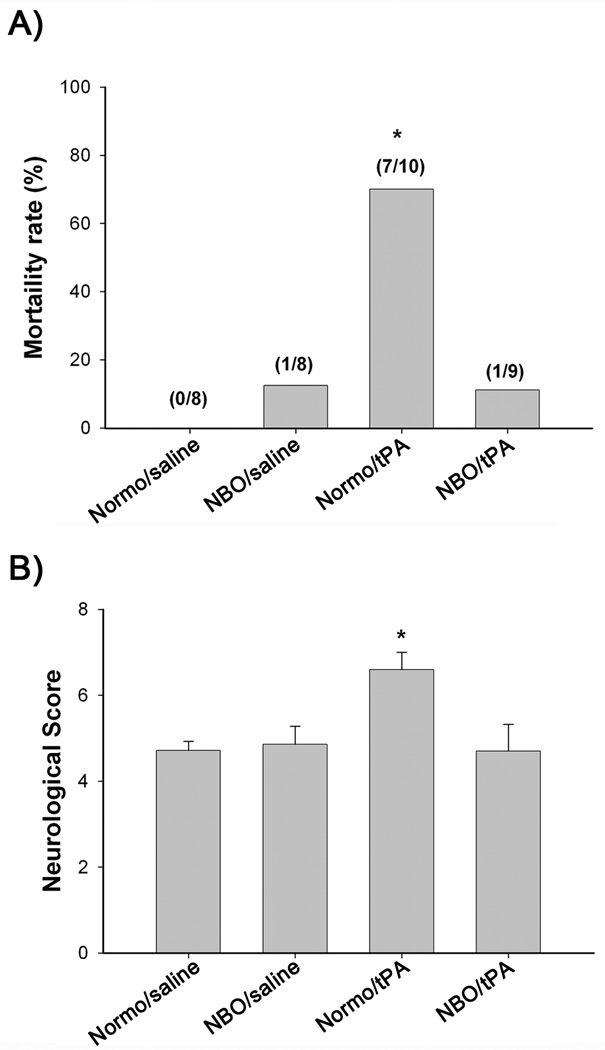
As shown in Figure 2A, the Normo/tPA group showed dramatically higher mortality (70%; 7/10 rats) than the Normo/saline (0%; 0/8 rats), NBO/saline (12.5%; 1/8 rats) and NBO/tPA groups (11.1%; 1/9 rats) (P<0.05, χ2 test). Similar results were obtained for Rogers’ neurological score assessment, in which Normo/tPA rats showed more severe neurological deficits than the other three groups (P<0.05; ANOVA) (Figure 2B). No significant difference in mortality and neurological score was noted among Normo/saline, NBO/saline and NBO/tPA groups.
Figure 2.
Effects of NBO and tPA on mortality and neurological scores. A) Mortality was increased markedly in Normo/tPA rats (*P < 0.05) when compared with the other three groups. The number of rats that died and the total number of rats studied in each group are shown in parentheses above the bars. B) Neurological function. Normo/tPA-treated rats showed a more severe neurological deficit than the other three groups (*P < 0.05).
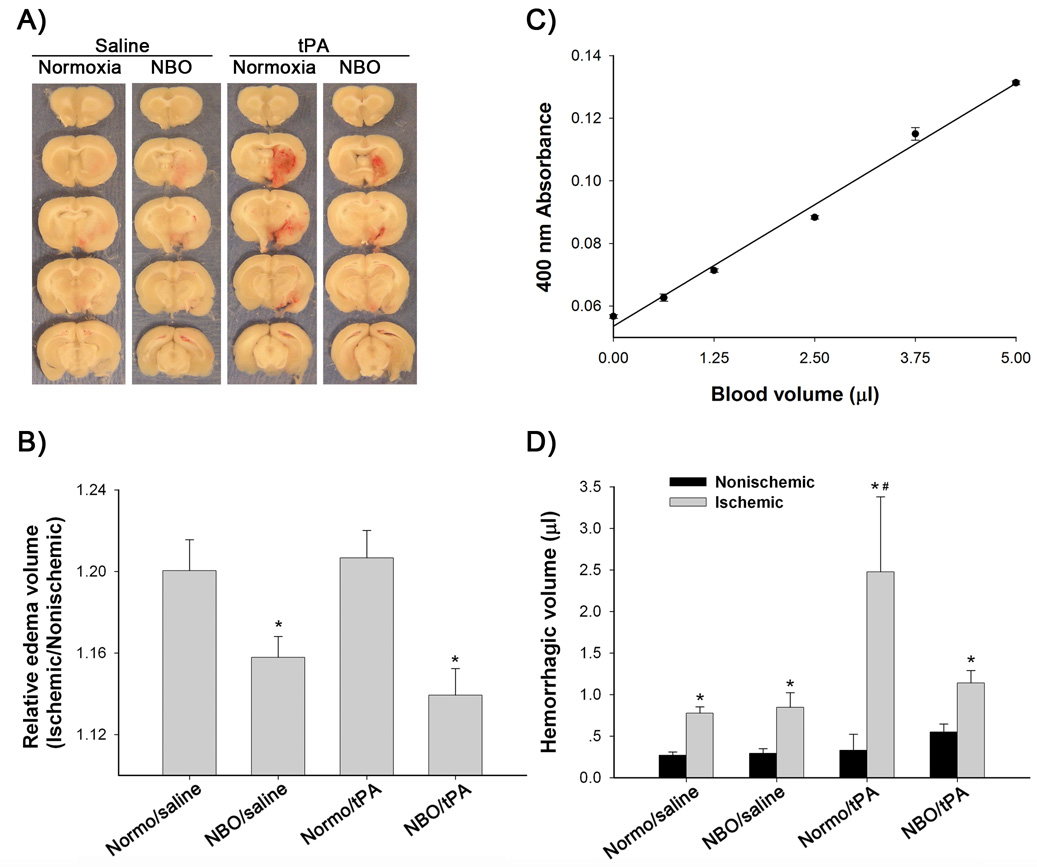
Brain edema and cerebral hemorrhage were assessed in surviving rats. As expected, brain swelling was observed in the ischemic hemispheres of all groups (Figure 3A). In both saline- and tPA-treated rats, NBO treatment significantly reduced hemispheric enlargement (P<0.05 versus normoxia; ANOVA). In the normoxic rats, tPA did not significantly increase brain edema compared to saline (P>0.05, Figure 3B). Hemorrhage, identified as blood evident at the macroscopic level (Figure 3A), was clearly visible in the ischemic hemispheres of all surviving rats except one Normo/saline and one NBO/saline rat. As expected, Normo/tPA rats showed larger hemorrhage size than the other three groups. None of the rats displayed any grossly visible hemorrhage in their nonischemic hemispheres (Figure 3A). Spectrophotometric measurement of whole blood showed a linear response between blood volume and hemoglobin absorbance (Figure 3C), which validated our method for quantifying hemorrhage. As shown in Figure 3D, a low value of hemorrhage volume was detected in the nonischemic hemispheres, which may reflect the residue blood left in the cerebral vasculature after transcardial perfusion with PBS. Rats in all 4 groups had larger blood volumes in the ischemic hemisphere (P<0.05 versus nonischemic hemisphere, paired t-test). As predicted, Normo/tPA rats showed much larger hemorrhage volume than Normo/saline rats (P<0.05; ANOVA). NBO treatment significantly reduced bleeding volumes in tPA-treated rats (P<0.05), but not in saline-treated rats (P>0.05; ANOVA).
Figure 3.
Effects of NBO and tPA on brain edema and hemorrhage. A) Representative brain sections showing significant hemispheric enlargement and hemorrhage in the ischemic brains of all groups. B) Quantitative image analysis showed a significant reduction of hemispheric enlargement in the NBO-treated rats when compared to normoxic rats, regardless of saline or tPA treatment (*P < 0.05). C) Spectrophotometric measurement shows a linear response between blood volume and hemoglobin absorbance. D) Hemorrhage volumes in the ischemic hemispheres are significantly higher than that in the nonischemic hemispheres in all animal groups (*P < 0.05). Normo/tPA rats showed larger hemorrhage volumes in the ischemic hemispheres than the other three groups (#P < 0.05). Data are expressed as mean±SEM.
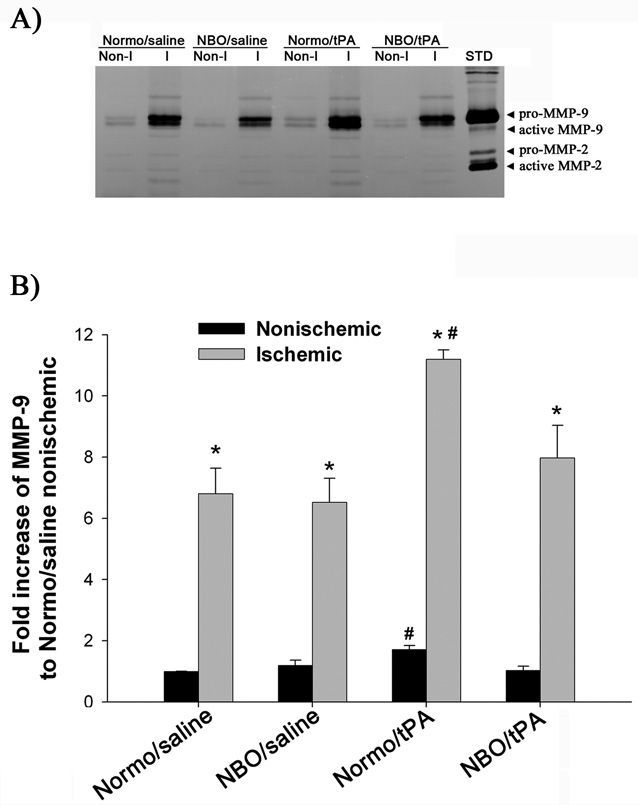
Since the “tPA-MMP-9 hypothesis” has been proposed as an important mechanism underlying tPA’s neurovascular complications,13, 15, 16 we investigated whether 5-hr MCAO with 19-hr reperfusion would lead to increased MMP-9 expression in the cerebral microvessels of the ischemic brain, and more importantly, whether tPA treatment would further enhance MMP-9 expression. As shown in Figure 4, the proform of MMP-9 was dramatically upregulated in the ischemic hemispheric microvessels of all rats (Figure 4B). Furthermore, Normo/tPA rats showed significantly higher levels of MMP-9 in both ischemic and nonischemic hemispheric microvessels than the other three groups (P<0.05; ANOVA). No significant difference in MMP-9 levels was observed for both ischemic and non-ischemic hemispheric microvessels among Normo/saline, NBO/saline and NBO/tPA rats.
Figure 4.
Effects of NBO and tPA on MMP-9 induction in ischemic cerebral microvessels. A) Representative gelatin zymogram showing MMP-9 expression in the nonischemic (Non-I) and ischemic (I) hemispheric cerebral microvessels for each group. STD is a mixture of standard MMP-2 and MMP-9. B) Quantified MMP-9 band intensity for each group. A significant increase in MMP-9 levels was observed in the ischemic hemispheric microvessels in all animal groups (*p<0.05, versus Nonischemic; paired t-test). Normo/tPA rats showed higher levels of MMP-9 in both ischemic and nonischemic hemispheric microvessels than the other three groups (#P<0.05). Data are expressed as mean±SEM.
Discussion
Thrombolysis with tPA is currently the only FDA-approved treatment for acute ischemic stroke.1 However, the clinical use of tPA is constrained to <5% of ischemic stroke patients mainly due to its narrow therapeutic time window.28 Delayed tPA treatment is associated with elevated risks of edema and cerebral hemorrhage, leading to increased mortality in stroke patients.3–8 tPA augmented-ischemic damage to the BBB through upregulating MMP-9 is implicated in these complications.11,29 Therefore, inhibiting MMP-mediated BBB disruption is a potential strategy to prevent tPA’s neurovascular complications and safely extend its therapeutic time window.13,30–32 The present study demonstrates that NBO treatment reduced tPA-associated mortality, brain edema and hemorrhage in a rat model of cerebral ischemia. Moreover, inhibition of tPA-augmented-MMP-9 induction in the BBB microvasculature may be an important underlying mechanism for this protection.
Studies have demonstrated that NBO can effectively reduce infarction volume and improve neurological outcome following transient cerebral ischemia.18–23 These neuroprotective effects are important for slowing down the evolution of ischemic damage to the brain and may therefore "buy time" for reperfusion.20 The potential for combined NBO treatment with tPA thrombolysis was recognized several years ago.19 Recently, Henninger et al showed that early NBO treatment in conjunction with tPA at a later time point significantly reduced infarct volume and did not increase hemorrhage risk,33 providing important initial evidence to support the combination therapy with NBO and tPA in ischemic stroke. However, it remains unclear about NBO’s potential to decrease tPA-associated hemorrhage,33 a potentially fatal complication of tPA. Our present study was designed to address this critical issue by investigating the effects of NBO on mortality, brain edema and hemorrhage associated with delayed tPA treatment.
To mimic delayed tPA thrombolysis in the clinical setting, we used a rat stroke model of 5-hr MCAO with 19 hrs of reperfusion, and administrated tPA to rats after 4.5 hrs of ischemia, which represented a time point outside the established 3-hr time window of tPA treatment. Although using a filament suture to mechanically occlude the MCA was not directly relevant to thromboembolic ischemia in patients, this stable model would allow us to compare the different effects of NBO and normoxia on tPA’s complications under identical and controlled ischemia and reperfusion conditions. In contrast, the more clinically relevant stroke model by injection of a pre-formed clot into the MCA is at present still problematic in terms of stability because we cannot predict how long and which part the clot will occlude the MCA. Our results showed that delayed tPA treatment resulted in high mortality in the normoxic rats, which is consistent with previous studies with mechanical or embolic stroke animal models.14,32 Surprisingly, although NBO treatment reduced the mortality rate from 70% to 11% in tPA-treated rats, it did not decrease neurological deficits in the saline-treated rats. The lack of effect on neurological deficits in the saline-treated rats differs from our earlier report with rats subjected to a much shorter ischemic duration of 90 min.18 This discrepancy is most likely due to the significantly different severities of ischemia associated with 90-min versus 5-hr MCAO. Consistent with this is the finding (Figure 1) that the reduction in infarct area by NBO was much less impressive in rats subjected to 5-hr than 90-min MCAO (quantitative data of infarct size on those TTC-stained brain sections are not shown). Reports that the neuroprotective effects of short-term NBO alone cannot be sustained with permanent or prolonged ischemia20, 23 further corroborate our results.
Thrombolysis with tPA can elevate the risk of intracranial hemorrhage, a major cause of death in delayed tPA treatment for ischemic stroke.3,8,34 Our results showed that tPA dramatically increased the amount of hemorrhage in the ischemic hemisphere of normoxic rats (Figure 3), which is likely responsible for the high mortality rate observed in the Normo/tPA group (Figure 2). As expected, NBO treatment significantly reduced tPA-exacerbated hemorrhage and mortality. Because we did not measure hemorrhage volume for those rats that died prematurely during reperfusion, most of which were tPA treated normoxic rats, we cannot draw a solid conclusion on the causal relationship between mortality and cerebral hemorrhage. Nevertheless these findings indicate that NBO can increase the safety of delayed tPA treatment.
NBO treatment significantly reduced brain edema in both NBO/saline and NBO/tPA groups (Figure 3B). Interestingly, unlike its effects on cerebral hemorrhage, delayed tPA treatment did not increase hemispheric enlargement in normoxic rats, which is consistent with previous studies showing no difference in brain edema assessed as brain water content14 or hemispheric swelling33 between tPA- or saline-treated rats. The lack of tPA effect on edema may be due to a maximal hemispheric enlargement already developed in the normoxic rats after prolonged 5-hr ischemia. It is worth pointing out that although brain edema and hemorrhage share one common mechanism (i.e. BBB disruption) for their occurrence in ischemic stroke, our results show that NBO reduced brain edema, but not hemorrhage in saline-treated rats. Besides BBB disruption-triggered vasogenic edema, cytotoxic edema also contributes to hemispheric enlargement in ischemic stroke. One possible explanation is that NBO might reduce cytotoxic edema through a separate mechanism. In addition, our finding that NBO-treated rats did not increase, but decreased, infarct or edema indicates that there is no significant toxicity of prolonged NBO treatment under our experimental conditions.
Animal and human studies have suggested a strong link between MMP-9 induction and tPA-induced hemorrhagic transformation in ischemic stroke.13,15–17,32 Our data indicate that delayed tPA treatment significantly amplifies MMP-9 in the ischemic cerebral microvessels of normoxic rats compared with the saline group, which was then inhibited by NBO treatment (Figure 4). Interestingly, NBO treatment did not affect MMP-9 induction in the ischemic cerebral microvessels in saline-treated rats, which differs from our previous report with a much shorter ischemic duration of 90-min.24 This discrepancy may also be due to the reduction in the neuroprotective effects of NBO under prolonged cerebral ischemia.20,23 The observation that NBO reduced MMP-9 induction in tPA-treated but not in saline-treated rats suggests that NBO may inhibit tPA-augmented MMP-9 induction, but not ischemia-triggered MMP-9 induction under our experimental conditions. Several mechanisms, such as Rho/Rock35 and low density lipoprotein receptor-related protein pathways,11 have been proposed to mediate tPA-induced MMP-9 expression. However, how NBO inhibits tPA-augmented MMP-9 induction remains to be elucidated. Our results support the idea that inhibition of tPA-augmented MMP-9 induction may be part of the mechanisms accounting for the reduction in hemorrhage and mortality by NBO. On the other hand, since MMP-9 assessment was only done at a single time point (24 hrs after ischemia), and no specific experiments were carried out to investigate the causal relationship between MMP-9 and tPA-augmented hemorrhage and mortality rate, further studies are required to ascertain that NBO improves the safety of delayed tPA treatment through interfering with the tPA-MMP-9 mechanism. Since oxygen therapy is rarely initiated within the first minutes of stroke onset, it is also necessary to investigate the neurovascular protection of NBO at different time points after ischemia.
In conclusion, our results indicate that early NBO treatment can reduce brain edema, cerebral hemorrhage and mortality in delayed tPA treatment for ischemic stroke. Inhibition of tPA- augmented MMP-9 increases in the ischemic BBB microvasculature may represent a mechanism for NBO’s protection. Our findings suggest that NBO may represent an important strategy to reduce hemorrhagic complications and mortality of tPA thrombolysis and safely extend its current narrow therapeutic time window.
Acknowledgements
The work was supported in part by grants from NIH (P20RR15636 and R01AG031725), and AHA (0555669Z and 0765461Z).
References
- 1.The national institute of neurological disorders and stroke rt-pa stroke study group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Alberts MJ. Hyperacute stroke therapy with tissue plasminogen activator. Am J Cardiol. 1997;80:29D–34D. doi: 10.1016/s0002-9149(97)00582-1. [DOI] [PubMed] [Google Scholar]
- 4.Mohr JP. Thrombolytic therapy for ischemic stroke: From clinical trials to clinical practice. JAMA. 2000;283:1189–1191. doi: 10.1001/jama.283.9.1189. [DOI] [PubMed] [Google Scholar]
- 5.Larrue V, von Kummer R, del Zoppo G, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the european cooperative acute stroke study. Stroke. 1997;28:957–960. doi: 10.1161/01.str.28.5.957. [DOI] [PubMed] [Google Scholar]
- 6.Vivien D, Buisson A. Serine protease inhibitors: Novel therapeutic targets for stroke? J Cereb Blood Flow Metab. 2000;20:755–764. doi: 10.1097/00004647-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lapchak PA. Hemorrhagic transformation following ischemic stroke: Significance, causes, and relationship to therapy and treatment. Curr Neurol Neurosci Rep. 2002;2:38–43. doi: 10.1007/s11910-002-0051-0. [DOI] [PubMed] [Google Scholar]
- 8.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the ldl receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1996;16:1373–1378. doi: 10.1097/00004647-199611000-00036. [DOI] [PubMed] [Google Scholar]
- 10.Mun-Bryce S. Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18:1163–1172. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 12.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein c inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 13.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- 14.Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtpa-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- 15.Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, Davalos A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- 16.Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, Quintana M, Alvarez-Sabin J. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 17.Castellanos M, Leira R, Serena J, Blanco M, Pedraza S, Castillo J, Davalos A. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke. 2004;35:1671–1676. doi: 10.1161/01.STR.0000131656.47979.39. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- 19.Singhal AB, Dijkhuizen RM, Rosen BR, Lo EH. Normobaric hyperoxia reduces mri diffusion abnormalities and infarct size in experimental stroke. Neurology. 2002;58:945–952. doi: 10.1212/wnl.58.6.945. [DOI] [PubMed] [Google Scholar]
- 20.Kim HY, Singhal AB, Lo EH. Normobaric hyperoxia extends the reperfusion window in focal cerebral ischemia. Ann Neurol. 2005;57:571–575. doi: 10.1002/ana.20430. [DOI] [PubMed] [Google Scholar]
- 21.Shin HK, Dunn AK, Jones PB, Boas DA, Lo EH, Moskowitz MA, Ayata C. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130:1631–1642. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn EP, Auer RN. Eubaric hyperoxemia and experimental cerebral infarction. Ann Neurol. 2002;52:566–572. doi: 10.1002/ana.10322. [DOI] [PubMed] [Google Scholar]
- 23.Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2007;27:1632–1642. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Sood R, Chen Q, Sakoglu U, Hendren J, Cetin O, Miyake M, Liu KJ. Normobaric hyperoxia inhibits nadph oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem. 2008;107:1196–1205. doi: 10.1111/j.1471-4159.2008.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–565. [PubMed] [Google Scholar]
- 26.Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. discussion 2066. [DOI] [PubMed] [Google Scholar]
- 27.Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, Xi G. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- 28.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National us estimates of recombinant tissue plasminogen activator use: Icd-9 codes substantially underestimate. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 29.Lo EH, Broderick JP, Moskowitz MA. Tpa and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Zhang ZG, Ding GL, Jiang Q, Liu X, Meng H, Hozeska A, Zhang C, Li L, Morris D, Zhang RL, Lu M, Chopp M. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005;112:3486–3494. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]
- 31.Morris DC, Zhang L, Zhang ZG, Lu M, Berens KL, Brown PM, Chopp M. Extension of the therapeutic window for recombinant tissue plasminogen activator with argatroban in a rat model of embolic stroke. Stroke. 2001;32:2635–2640. doi: 10.1161/hs1101.097390. [DOI] [PubMed] [Google Scholar]
- 32.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henninger N, Bratane BT, Bastan B, Bouley J, Fisher M. Normobaric hyperoxia and delayed tpa treatment in a rat embolic stroke model. J Cereb Blood Flow Metab. 2009;29:119–129. doi: 10.1038/jcbfm.2008.104. [DOI] [PubMed] [Google Scholar]
- 34.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The european cooperative acute stroke study (ecass) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 35.Wang S, Lee SR, Guo SZ, Kim WJ, Montaner J, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced matrix metalloproteinase-9 by simvastatin in astrocytes. Stroke. 2006;37:1910–1912. doi: 10.1161/01.STR.0000226923.48905.39. [DOI] [PubMed] [Google Scholar]