Abstract
An LC/MS/MS assay we published for tenofovir (TFV) plasma levels is a useful tool for monitoring the pharmacotherapy of HIV-positive individuals (J. Chromatography B 830, 6–12, 2006). A new combination therapy consisting of the TFV pro-drug (300 mg) and another reverse transcriptase inhibitor, emtricitabine (FTC, 200 mg) has become available in a convenient once-daily dosage form (Truvada). This widely used medication has prompted us to develop and validate a convenient assay to determine simultaneously TFV and FTC plasma concentrations. In view of their chemical similarity to the analytes, stable isotope internal standards (IS) were chosen. These consisted of TFV labeled uniformly with 13C in the adenine moiety (Iso-TFV) and FTC labeled with 13C and 15N in the cytosine moiety (Iso-FTC). Trifluoroacetic acid was added to the patient’s EDTA plasma (containing the IS) to produce a de-proteinated extract after high speed centrifugation. The extracts were directly injected into the mobile phase (3% acetonitrile/1% acetic acid, aq.) stream flowing at 200 μL/min. A Synergi Polar-RP, 2.0 x 150mm, reversed-phase analytical column was used to achieve the chromatographic separation. Detection of the analytes was achieved by ESI positive ionization tandem mass spectrometry. The precursor/product transitions (m/z) in the positive ion mode were 288/176 and 293/181 ions for TFV and Iso-TFV, respectively and the precursor/ product transitions (m/z) were 248/130 and 251/133 ions for FTC and Iso-FTC, respectively. When the analyte/IS abundance ratios were plotted against the specified concentrations, the linearity of the concentration curves were in the range 10 ng/mL to 1500 ng/mL for both analytes (250 μL plasma extracted), with a minimum quantifiable limit of 10 ng/mL for both analytes. The inter- and intra-day accuracy and precision for both TFV and FTC were within ±20% at the LLOQ and ±15% at the other QC levels. We have expanded the method originally designed for the assay of TFV alone to incorporate the simultaneous determination of the latter and FTC using stable isotope IS. This assay has been successfully used for the periodic monitoring of 678 HIV-positive patients being treated with the combination therapy.
Keywords: Tenofovir (TFV), Tenofovir disoproxil fumarate (TDF), Emtricitabine (FTC), LC/MS/MS, Isotopic Internal Standards (IS), Selective Reaction Monitoring (SRM), Pharmacokinetics
Introduction
Tenofovir disoproxil fumarate (TDV, Viread) is a widely used nucleotide analog pro-drug administered to combat HIV infection. Since the disoproxil moiety is hydrolyzed following absorption to generate the viral reverse transcriptase inhibitor, tenofovir (TFV), pharmacotherapy is monitored by quantitation of the latter in plasma. The TFV pro-drug is currently combined with another nucleoside reverse transcriptase inhibitor, emtricitabine (FTC) in a single tablet (Truvada) for the convenience of HIV-positive patients and to improve medication adherence. FTC is similar to the established drug, lamivudine (3TC) but is clinically more effective. Since these anti-viral drugs do not interact pharmacokinetically, and have moderate elimination half-lives in plasma, a once-daily combination therapy is possible [1]. Furthermore, the combination of TDF and FTC can result in a greater HIV RNA suppression than either drug alone [2]. TDF and FTC administration to pregnant women has also been shown to contribute to a reduced viral resistance to non-nucleoside reverse transcriptase inhibitor drugs after nevirapine treatment [3]. Since the LC/MS/MS method we originally validated [4] has proven to be a useful technique for monitoring TFV plasma concentrations in pharmacokinetic studies [5], we redesigned the original method to permit the simultaneous assay of TFV and FTC. This new method utilizes stable isotope IS for both drugs whose chemical and chromatographic properties are negligibly different from that of the analytes. Our earlier TFV assay used adefovir [4] and others have used dideoxyuridine [6] and deoxyfluorocytidine [7] for FTC as internal standards. However, these internal standards have different chromatographic and chemical properties from that of the analytes, which could potentially lead to errors in the calculation of analyte concentrations[8]. An earlier version of the method described herein which used 8-azido adenosine as the FTC IS has been published as an abstract [9]. Since this manuscript was originally submitted, three separate research groups have published LC/MS methods for the simultaneous assay of TFV and FTC without the use of stable isotope IS (10,11,12).
Materials and Methods
Chemicals
TFV (MM: 287.2, 99.9% pure), the internal standards, Iso-TFV (MM: 292.2, 99 % pure) and Iso-FTC (MM: 250.2, 98.9% pure) were purchased from Moravek Biochemicals Inc., Brea CA. FTC (MM: 247.2) was obtained from the NIH AIDS Research and Reference Reagent Program, cat# 10071, NIAID. The Iso-TFV contained adenine uniformly labeled with 13C and the Iso-FTC was labeled in the urea portion of the cytidine moiety with 13C (position 2) and 15N (positions 1 and 3). No unlabelled analytes were present in these de novo synthesized IS. Trifluoroacetic acid, acetic acid, methanol, acetonitrile, (HPLC grade) and saturated ammonium hydroxide (14.8 M, A.C.S. certified) were obtained from Thermo-Fisher (Fairlawn, NJ). The ultra-pure DI water (DI water) was produced from a Barnstead Diamond Nanopure System. Human EDTA-plasma was purchased from Biological Specialty Corporation (Colmar, PA).
Instruments
The HPLC autosampler/pump was a Surveyor (Thermo Fisher, San Jose CA). The analytical column was a Synergi 4μm Polar RP HPLC Column pore size, 80Å, 2.0 x 150 mm protected by a Polar RP guard column (Phenomenex, Torrance CA). The mobile phase consisted of 3% acetonitrile/1% acetic acid in DI water flowing at 200 μL/min. A second external LC pump was used to rinse the MS source with 100% methanol flowing at 0.5 mL/min (model 510, Waters Corporation, Milford MA) while the LC flow was diverted from the source. The MS/MS detector was a TSQ Quantum, operating in the ESI, positive polarity mode (Thermo Fisher, San Jose CA) and the data system was Xcalibur, version 1.3, also from Thermo Fisher.
Calibration, IS, and QC Solutions
Two, separately weighted,1.00 mg/mL stock solutions of TFV and FTC (Calibration Preparation stock, #1 and QC Preparation stock, #2) were prepared by dissolving approximately 1 mg aliquots of each drug , accurately weighed on a Mettler MX-5 micro balance (Mettler, Toledo Ohio), in the calculated volumes of DI water and methanol, respectively. The TFV and FTC Stocks # 1 and # 2 were used for the preparation of working calibration standards and quality controls (QCs), respectively. Preparation stocks # 1 and # 2 were compared for accuracy prior to the preparation of working calibration standard solutions and QC’s. Nine TFV/ FTC combined working calibration standard solutions were prepared in 50:50 methanol/water (v/v) from the stock solution # 1 using calibrated positive displacement pipets to give a final plasma concentration range of 10–1500 ng/mL for each analyte. All stock solutions were stored at −20 °C in glass screw-top test tubes tightly sealed with Teflon-lined caps to prevent evaporation. The stock 1.00 mg/mL internal standard (IS) solutions were prepared by dissolving the calculated quantity of the chemical in DI water (iso-TFV) or methanol (iso-FTC). A second preparation stock of the IS consisted of 10 μL (stock iso-TFV) and 50 μL (stock iso-FTC) per 3 mL of their respective solvents. The working IS solution was prepared daily by combining the appropriate volumes of the latter solutions in a 1:1 ratio. QCs were prepared from TFV/FTC preparation stocks # 2. These consisted of High QC (1200 ng/mL; 80% of the highest calibration standard), Medium QC (300 ng/mL), Low QC (30 ng/mL; 3x the lowest calibration standard) and LLOQ (10 ng/mL; equivalent to the lowest calibration standard). They were prepared with blank human plasma and the appropriate volumes of the preparation stock # 2 using volumetric flasks and the appropriate positive displacement pipets. Aliquots (600 μL) were stored in screw-top Cryovial tubes at −20 °C.
Sample Preparation
The preparation method for calibrators, QCs and patient samples followed that used for TFV alone (4). All plasmas used in this study were derived from EDTA treated human blood. Working calibration solutions, QCs and unknowns were allowed to equilibrate to ambient. Calibration standards for each run were prepared by pipetting 20 μL (calibrated positive displacement pipet) of each working calibration standard solution (methanol/water 50/50 %, v/v) to nine 250 μL aliquots of blank human plasma in 1.5 mL microcentrifuge tubes (SealRite, USA Scientific) resulting in a concentration range of 10–1500 ng/mL for the calibration curve. Aliquots (250 μL) of the plasma unknowns, blank and QCs were added to appropriately labeled 1.5 mL microcentrifuge tubes to which 20 μL of methanol/water (50/50 %, v/v) had been added to normalize the volume of unknown, blank and QC tubes to that of the calibration standard tubes. The working IS solution (20 μL) was added to each extraction tube using a calibrated positive displacement pipet and all were vortexed well. Trifluoroacetic acid (25 μL) was then added to all tubes, followed by prompt capping and vortexing. After standing at ambient for 15 minutes, all tubes were ultra-centrifuged in a Spectrafuge (Labnet International) at 13,000 rpm for 15 min. The deproteinated extracts were transferred to labeled autosampler vials containing 400 μL capacity flat-bottom inserts (ChromTech, Apple Valley, MN) to which 20 μL of concentrated ammonium hydroxide (14.8 M) had been previously added. After capping and vortexing, the vials were loaded in the autosampler tray which was maintained at 4 °C.
LC/MS/MS Procedure
Chromatography conditions: Ten μL aliquots of the extract were injected into the HPLC with the mobile phase (3% acetonitrile/1% acetic acid in DI water) flowing isocratically at 200 μL/min. Column temperature: 22–25ºC (ambient). Run time: 11 min. TSQ Quantum conditions: ESI, positive polarity. Spray voltage: 3200 V; capillary temperature: 300ºC; Gas Pressures: sheath (nitrogen); 30, Aux (argon);10, Finnigan arbitrary units. Collision gas pressure: 1.8 mTorr; collision energy: 28 eV (TFV and Iso-TFV), 11 eV (FTC and Iso-FTC). Precursor/Product ion transitions were used for selective reaction monitoring (SRM). [M+H] ions detected (m/z): TFV; precursor 288, product 176. FTC; precursor 248, product 130. Iso-TFV; precursor 293, product 181. Iso-FTC; precursor 251, product 133. Quadrupole resolution: Q1 and Q3; 0.7 FWHM. Scan width: 1m/z. Scan time: 100 msec. Source CID: 10 V. Divert Valve Program: Methanol flowing externally at 0.5 mL/min from the Waters pump rinsed the source while the HPLC flow was diverted. The HPLC eluate flow destination was switched alternatively between the detector and waste as follows. From 0–2 min: to waste. From 2–9 min: to detector. From 9–11 min: to waste.
Validation Methods
An FDA Bioanalytical Method Validation publication was used as a guide for this validation (13).
Accuracy and Precision
Calibration curves for this validation were run in separate analytical runs as previously described for TFV alone (4). They were created by plotting the peak area ratio of the TFV/Iso-TFV and FTC/Iso-FTC against the analyte concentration. A 1/X weighted linear regression equation of the type y=bx+a was used. Back-calculated concentrations for each calibration level were determined as well as slope and coefficient of determination data (n=3 for interday). Accuracy and precision of the method was determined by analysis of the QCs in 3 separate analytical runs with five replicates (see below). These results should be within ±20% at the LLOQ and ±15% at all other concentrations. Dilute specimens were also validated for accuracy as follows. A 3000 ng/mL highly concentrated sample was prepared for TFV and FTC. From this, 3-fold and 5-fold diluted samples were prepared and analyzed. Acceptable accuracy results should be within ±15%.
Stability
Stock solution stability included testing the stability of TFV and FTC in preparation solvents and storage at −20°C. Both preparation stocks in their respective solvents (TFV, water; FTC, 50:50 methanol/water) were re-analyzed periodically (see below). The stability of the analytes in plasma during 3 freeze/thaw cycles was also tested using spiked plasma samples at concentrations of 30.0 and 600 ng/mL (TFV) and 30.0 and 800 ng/mL (FTC). Samples were removed from freezer, allowed to equilibrate to ambient and placed back into −20°C storage. Room temperature stability was also assessed to account for potential shipping errors that may occur when plasma samples are in transit to the laboratory. The spiked plasma samples were allowed to equilibrate to ambient and held there for 6 days before being extracted and reanalyzed. The stability of the extracts in the autosampler at 4°C was also tested to show that subsequent analysis after LC malfunction would not jeopardize the analysis. The long term stability of the analytes in proficiency plasmas (ACTG proficiency testing program, Rd 15, March 2005) was determined after storage at −80°C. A comparison was then made to the nominal analyte concentrations of the tested samples (see below). Instability would be considered if a deviation from nominal of greater than 15% were observed.
Sensitivity and Selectivity
Seven different blank plasma lots were checked for any erroneous positive or negative MS responses. Any positive response would need to have a peak area <10% of the LLOQ or the IS peak areas. These plasma lots were also used to generate separate calibration curves which were compared to determine if different plasma lots could affect the response of the calibrators. The possibility that concomitant drugs such as protease inhibitors (PI), other nucleoside reverse transcriptase inhibitors (NRTI) and non-nucleoside reverse transciptase inhibitors (NNRTI) might have an effect on the TFV/FTC analysis was also assessed. Several antiviral drugs likely to be present in the plasma of HIV-positive patients at concentrations significantly higher than what would be expected in clinical practice were added to blank plasma. Aliquots of this plasma were used to prepare the 250 ng/mL calibrator and a blank. The following drugs were used: IDV, DLV, APV, NFV, M8, ATV, SQV, EFV, RTV and LPV (20,000 ng/mL for each drug) and 3TC, d4T, AZT, ABC and DDI (10,000 ng/mL for each drug). [Definitions of these drug acronyms can be found in the footnotes]. After analysis, a comparison was made to the nominal concentration. A difference of greater than 15% would be indicative of a potential effect from these concomitant drugs. Since SRM detection was employed, we did not investigate where these concomitant drugs eluted on the chromatogram.
Suppression Effects
The possibility that the trifluroacetic acid and salts present in the extracts could by themselves result in a suppression of the MS signal was assessed as follows.
Set A: Five aliquots (250 μL) of mobile phase in microcentrifuge tubes were spiked with 20 μL of standard solution (300 ng/mL level) and 20 μL IS. Then 25 μL trifluoroacetic acid and 20 μL ammonium hydroxide were mixed in and the solutions transferred to vials for analysis as described above.
Set B: These solutions were prepared as described for Set A, except that 45 uL of mobile phase was used instead of the acid and base.
Sets A and B were analyzed and the results compared to assess suppression.
Matrix Effects
The method used for testing matrix effects (ME) was a modification of that used by Matuszewski, et al. [8] which involved the preparation and analysis at 3 concentration levels: 30, 300, and 600 ng/mL for TFV and 30, 300, and 800 ng/mL for FTC, constituted as follows.
Set 1: Analytes and IS were dissolved in the mobile phase at the appropriate concentration and analyzed (used for comparison).
Set 2: Post-extraction spike of analytes and IS into the appropriate volume of supernatant from extracted blank plasma, followed by analysis (used to calculate suppression and losses due to protein precipitation).
Set 3: Pre-extraction spike of analytes and IS into plasma followed by extraction as described above under Sample Preparation. (used to calculate overall process efficiency, PE).
Five different lots of plasma were used at each concentration level for Sets 2 and 3. Set 1 (mobile phase only) was performed in 5 replicates. Recovery and suppression were assessed by comparing the peak areas between the different Sets.
Results and Discussion
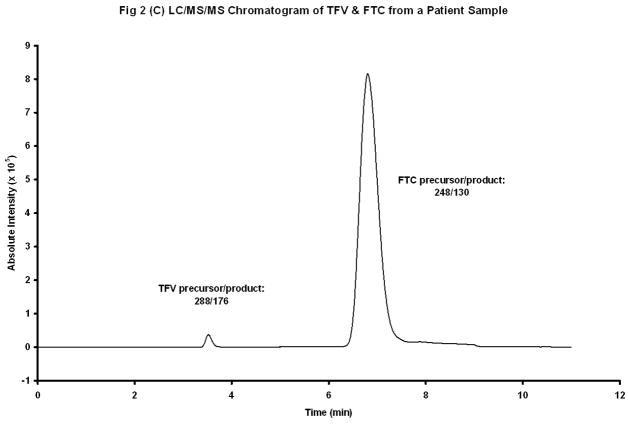
The chemical structures of TFV and Iso-TFV are shown in Fig 1(A). The nucleoside analog, TFV is a phosphonomethoxypropyl adenine derivative. Fig 1(B) depicts the structures of FTC and Iso-FTC. FTC is a dideoxyfluorothia derivative of cytidine, closely related chemically to 3TC (Lamivudine). The position of the isotopic elements in the structure of the Iso-TFV and the Iso-FTC molecules is indicated by their atomic mass in their respective structures. In Fig 2(A) and 2 (B) are depicted typical chromatograms showing the TFV and FTC response from an extracted calibration standard (TFV and FTC= 250 ng/mL) and their corresponding internal standard responses (Iso-TFV and Iso-FTC), respectively. The monitored m/z values for the respective precursor/ products were: TFV, 288/176; FTC, 248/130; Iso-TFV, 293/181; Iso-FTC, 251/133. The peak patterns and retention times obtained when TFV and FTC precursor/products were specified were identical to that seen when the corresponding isotopic precursor/products were specified. Retention time variability within a run: TFV; mean±SD, 3.51 ±0.013 min; FTC; mean±SD, 7.05 ±0.039 min; N= 35. Fig 2(C) depicts the chromatogram obtained when a patient’s plasma extract was analyzed for the drugs (TFV= 61.9 ng/mL, FTC= 475 ng/mL, IS’s not shown). It should be noted that the peak area for FTC was always considerably greater than that of the TFV due to the greater response of the former in the MS source. The absolute abundances are plotted in the figures and the various peak areas are also shown in the legends for comparison. Fig 2(D) depicts a typical chromatogram obtained when the LLOQ calibrator (10ng/nL) was analyzed. The tracing obtained when blank plasma was analyzed using the specified precursor/product ions for TFV/FTC is also depicted in Fig 2(D), illustrating the low abundance of the blank tracing relative to the LLOQ. Calibration curves were obtained by plotting the peak area ratios of TFV and FTC, relative to the corresponding IS, against the assigned concentrations of the spiked plasma calibration standards (analytical range; 10–1500 ng/mL).
Figure 1.
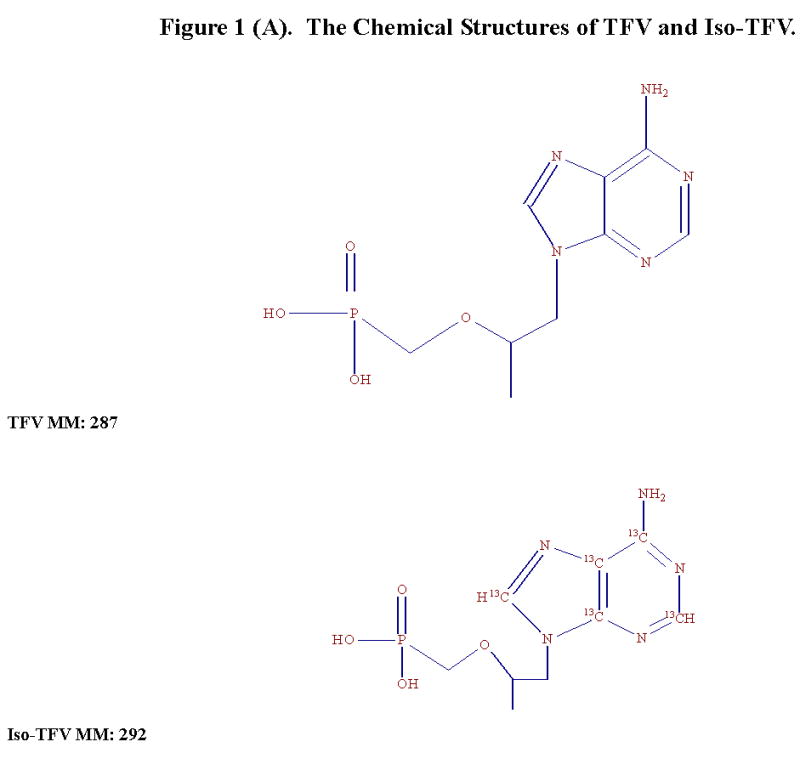
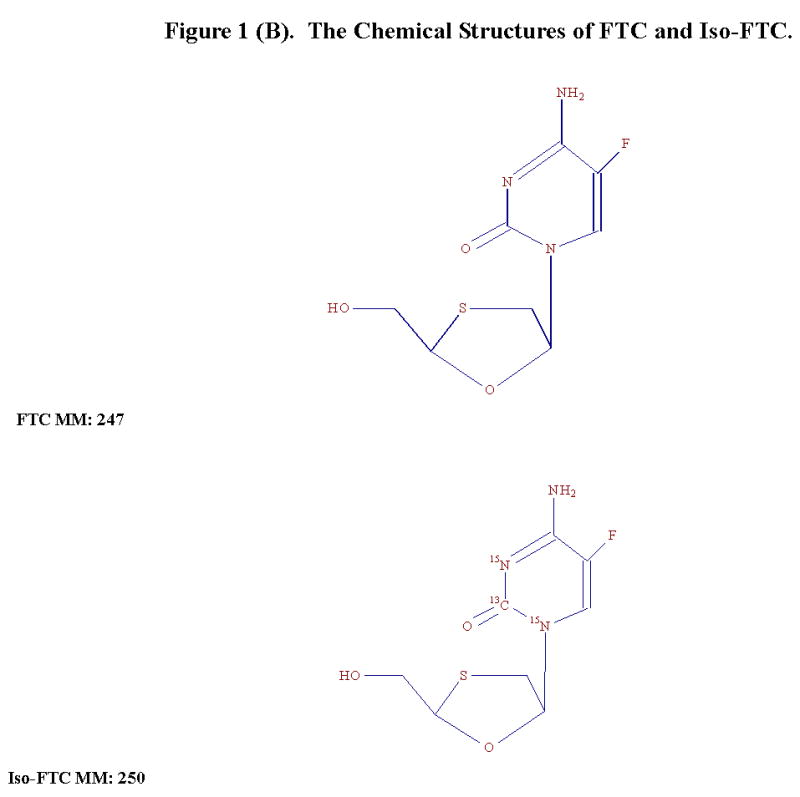
Figure 1(A). The Chemical Structures of TFV and Iso-TFV.
Figure 1(B). The Chemical Structures of FTC and Iso-FTC.
Figure 2.
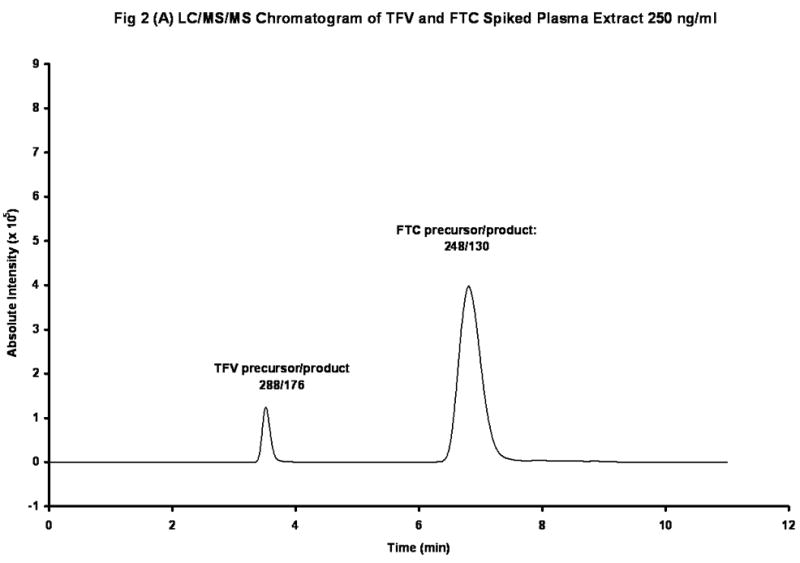
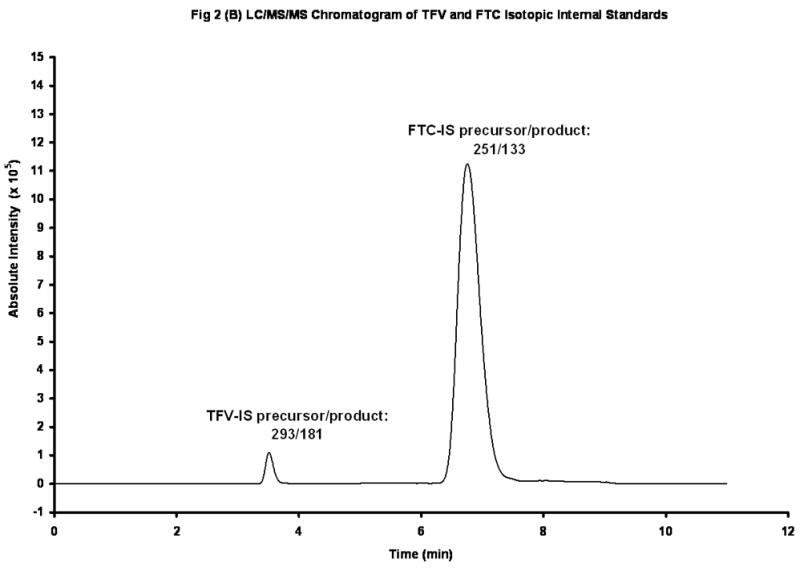
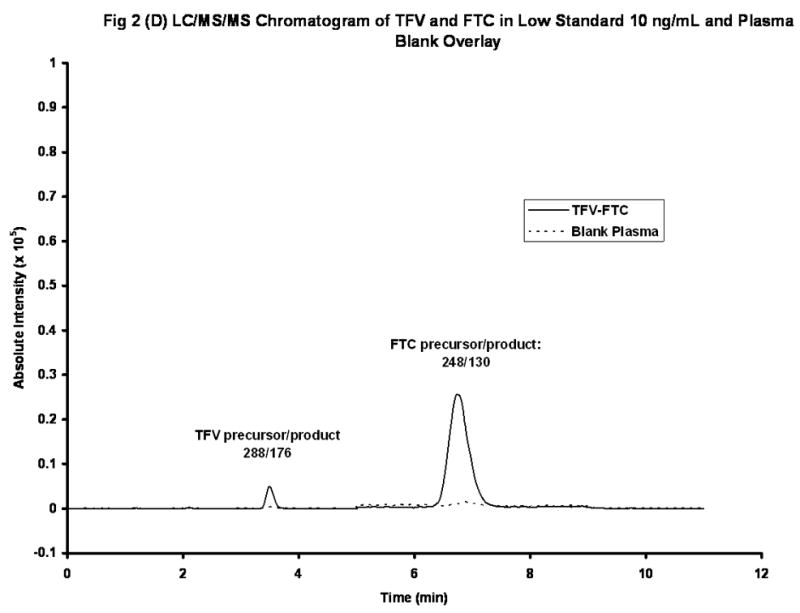
Figure 2(A). LC/MS/MS Chromatogram of TFV and FTC in Spiked Plasma Extract (250 ng/mL). Peak Areas: TFV, 1.16 X 106; FTC, 10.1 X 106.
Figure 2(B). LC/MS/MS Chromatogram of Iso-TFV and Iso-FTC Internal Standards. Peak Areas: Iso-TFV, 1.24 X 106; Iso-FTC, 33.1 X 106.
Figure 2(C). LC/MS/MS Chromatogram of TFV and FTC in Patient’s Plasma Extract. Peak Areas: TFV, 0.347 X 106; FTC, 21.4 X 106
Figure 2(D). LC/MS/MS Chromatograms of TFVand FTC in a Low Spiked Plasma (10 ng/mL) and a Blank Plasma (overlaid).
Since the back calculated results for the validation showed good accuracy and precision, it was concluded that the TFV/FTC calibration curves produced by this method could be used to determine unknown drug concentrations in plasma. In addition, twenty-eight consecutive calibration analyses were performed on different days with freshly prepared calibration standards and the back-calculated values for each level recorded. The % CV at each level varied from 1.01 to 8.19 (TFV) and from 2.5 to 7.6 (FTC) while the % deviation from the theoretical value varied from −3.79 to 6.09 (TFV) and from −2.5 to 7.6 (FTC). The % CV of the twenty-eight slopes was 11.1 for TFV and 12.2 for FTC and the lowest coefficient of determination (R2) among the twenty-eight calibration curves was 0.9958 for TFV and 0.9955 for FTC.
Accuracy/Precision
The assay was assessed for inter-day and intra-day accuracy and precision using the High, Medium, Low and LLOQ QCs. Tables 1 and 2 depict the within-run (N=5) and day-to-day (N=15) results obtained when the QCs were analyzed for TFV and FTC, respectively. Each level was run five times sequentially on three different days. Inter-day and intra-day accuracy was tested by calculating the percent deviation from the theoretical concentration. Precision was determined by calculating the coefficient of variation (%CV). As can be seen, the inter-day and within-run precision and accuracy were acceptable for TFV and FTC at each level for the five replicates.
Table 1.
Inter- and Intra-Assay Accuracy and Precision of TFV in Plasma.
| TFV Sample # | LLOQ | Low QC | Middle QC | High QC | Run ID | Intraassay Statistics | LLOQ | Low QC | Middle QC | High QC |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.9 | 27.2 | 289 | 1146 | 1 | Mean | 11.5 | 29.1 | 300 | 1181 |
| 2 | 11.2 | 29.3 | 293 | 1196 | SD | 0.6 | 1.2 | 9.2 | 20 | |
| 3 | 12.0 | 29.0 | 301 | 1189 | %CV | 5.3 | 4.1 | 3.1 | 1.7 | |
| 4 | 12.3 | 30.4 | 305 | 1187 | %dev | 15.0 | −2.9 | 0.0 | −1.6 | |
| 5 | 11.1 | 29.7 | 312 | 1187 | n | 5 | 5 | 5 | 5 | |
|
| ||||||||||
| 1 | 9.26 | 29.1 | 326 | 1251 | 2 | Mean | 10.4 | 28.6 | 321 | 1295 |
| 2 | 9.93 | 28.5 | 326 | 1287 | SD | 1.4 | 0.4 | 14.5 | 43 | |
| 3 | 12.9 | 28.2 | 327 | 1286 | %CV | 13.8 | 1.5 | 4.5 | 3.3 | |
| 4 | 9.77 | 28.2 | 330 | 1284 | %dev | 3.9 | −4.7 | 6.9 | 7.9 | |
| 5 | 10.1 | 29.0 | 295 | 1366 | n | 5 | 5 | 5 | 5 | |
|
| ||||||||||
| 1 | 9.18 | 28.1 | 289 | 1148 | 3 | Mean | 9.31 | 28.0 | 299 | 1144 |
| 2 | 9.04 | 27.0 | 290 | 1102 | SD | 0.2 | 0.8 | 9.2 | 29 | |
| 3 | 9.53 | 27.5 | 303 | 1153 | %CV | 2.3 | 2.8 | 3.1 | 2.6 | |
| 4 | 9.53 | 28.6 | 308 | 1133 | %dev | −6.9 | −6.6 | −0.2 | −4.7 | |
| 5 | 9.25 | 28.9 | 307 | 1182 | n | 5 | 5 | 5 | 5 | |
|
| ||||||||||
| Interassay Statistics | ||||||||||
| Theor. Conc. | 10 | 30 | 300 | 1200 | ||||||
| mean | 10.4 | 28.6 | 307 | 1206 | ||||||
| SD | 1.3 | 0.9 | 14.7 | 73 | ||||||
| %CV | 12.0 | 3.2 | 4.8 | 6.0 | ||||||
| %dev | 4.0 | −4.7 | 2.2 | 0.5 | ||||||
| n | 15 | 15 | 15 | 15 | ||||||
Table 2.
Inter- and Intra-Assay Accuracy and Precision of FTC in Plasma.
| FTC Sample # | LLOQ | Low QC | Middle QC | High QC | Run ID | Intraassay Statistics | LLOQ | Low QC | Middle QC | High QC |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.40 | 27.3 | 281 | 1089 | 1 | Mean | 8.76 | 28.4 | 287 | 1122 |
| 2 | 8.80 | 28.2 | 281 | 1145 | SD | 0.3 | 0.7 | 5.9 | 21 | |
| 3 | 8.80 | 28.2 | 289 | 1129 | %CV | 2.9 | 2.6 | 2.1 | 1.9 | |
| 4 | 9.10 | 29.0 | 287 | 1117 | %dev | −12.4 | −5.5 | −4.5 | −6.5 | |
| 5 | 8.70 | 29.1 | 295 | 1131 | n | 5 | 5 | 5 | 5 | |
|
| ||||||||||
| 1 | 8.50 | 26.6 | 302 | 1158 | 2 | Mean | 9.22 | 27.5 | 302 | 1189 |
| 2 | 8.89 | 27.8 | 306 | 1148 | SD | 1.4 | 0.6 | 13.7 | 41 | |
| 3 | 11.7 | 27.6 | 308 | 1181 | %CV | 15.1 | 2.0 | 4.5 | 3.5 | |
| 4 | 8.49 | 28.1 | 315 | 1211 | %dev | −7.8 | −8.2 | 0.7 | −0.9 | |
| 5 | 8.52 | 27.6 | 279 | 1249 | n | 5 | 5 | 5 | 5 | |
|
| ||||||||||
| 1 | 9.42 | 27.2 | 280 | 1084 | 3 | Mean | 10.0 | 27.2 | 280 | 1083 |
| 2 | 10.3 | 27.7 | 273 | 1096 | SD | 0.3 | 0.44 | 5.0 | 19 | |
| 3 | 10.0 | 27.5 | 277 | 1092 | %CV | 3.4 | 1.6 | 1.8 | 1.8 | |
| 4 | 10.1 | 26.9 | 285 | 1050 | %dev | 0.0 | −9.4 | −6.7 | −9.8 | |
| 5 | 10.2 | 26.6 | 284 | 1093 | n | 5 | 5 | 5 | 5 | |
|
| ||||||||||
| Interassay Statistics | ||||||||||
| Theor. Conc. | 10 | 30 | 300 | 1200 | ||||||
| mean | 9.33 | 27.7 | 289 | 1132 | ||||||
| SD | 0.94 | 0.7 | 12.8 | 53 | ||||||
| %CV | 10.1 | 2.7 | 4.4 | 4.7 | ||||||
| %dev | −6.7 | −7.7 | −3.5 | −5.7 | ||||||
| n | 15 | 15 | 15 | 15 | ||||||
Overall, the assay was both accurate and precise between runs and within individual runs. The greatest mean inter-day percent deviation for TFV and FTC were 12.0 % and 10.1 % respectively for the LLOQ (10 ng/mL). All non-LLOQ QC levels had inter-day percent deviations which were less than 7 % for both analytes. The within-run precision for the non-LLOQ QCs was less than 6 % on each of the five days of the study (see Tables 1 and 2). The accuracy of measuring the analytes in diluted samples was also assessed. Aliquots of the 3000 ng/mL spiked plasma were diluted 5-fold (600 ng/mL) and 3-fold (1000 ng/mL) with blank plasma and analyzed along with the nine calibrators and two sets of QCs. The variation from nominal of the 5-fold and 3-fold dilution results were: TFV, −5.3 % and −7.3 % ; FTC, 3.0% and −4.6%, respectively. This study indicated that should a result exceed the upper limit of the calibration curve, the remaining plasma could be diluted up to 5-fold and reanalyzed to fit within the established parameters.
Stability of Stock Solutions
A comparison was made between freshly prepared stock solutions and those that had been stored at −20°C. TFV (water) and FTC (MeOH) preparation stock 1.00 mg/mL solutions were shown to be stable for at least one year and 18 months, respectively. The percent differences in peak response varied no more than ±6.1% between the freshly prepared and stored solutions (data not shown).
Stability in Plasma and Extracts
As can be seen in Table 3, both TFV and FTC were stable for three freeze-thaw cycles since the % deviation from the theoretical concentrations indicated that no decomposition had occurred. Similarly, the analytes were found to be stable in plasma when stored at ambient temperature for 6 days. (The % deviation from nominal for the low and high concentrations: TFV, 5.2 and 5.4; FTC, 5.1 and 0.1, respectively, n=3, Table 3). This finding is consistant with reports by others illustrating the remarkable stability of these antiviral drugs [4, 6, 10, 12], also various Gilead Sciences publications, not listed. The stability of the extracted samples stored in the autosampler for two days was also assessed. As Table 3 shows, the % deviation from theoretical for the two levels (n=8 and 9) was 2.30 and 2.17 for TFV and −4.00 and −5.58 for FTC, suggesting that there was no significant decline in the response ratio during that time. (The actual peak areas and retention times of the analytes did not drift from those of the corresponding freshly prepared extracts, data not shown). As depicted in Table 4, the TFV and FTC concentrations in samples stored at −80°C for nearly 3 years were within acceptable limits of the nominal, indicating that both analytes were stable in plasma for this period when stored at −80°C.
Table 3.
Stability of TFV and FTC Under Various Conditions
| TFV in Plasma Matrix | FTC in Plasma Matrix | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 F/T Cycles | # | Low | High | # | Low | Low | High | |||||
| 30 | 600 | theoretical conc. | 30 | 600 | 30 | 800 | theoretical conc. | 30 | 800 | |||
| 1 | 33.2 | 644 | mean | 35.5 | 652 | 1 | 31.8 | 817 | mean | 31.7 | 818 | |
| 2 | 39.5 | 630 | SD | 3.5 | 26.8 | 2 | 31.9 | 820 | SD | 0.3 | 1.7 | |
| 3 | 33.7 | 682 | %CV | 9.9 | 4.1 | 3 | 31.3 | 817 | %CV | 1.1 | 0.2 | |
| %dev | 18.3 | 8.7 | %dev | 5.5 | 2.2 | |||||||
| n | 3 | 3 | 3 | 3 | n | 3 | 3 | |||||
| TFV in Plasma Matrix | FTC in Plasma Matrix | |||||||||||
| 144 hr Room Temperature | Low | High | Low | High | Low | High | Low | High | ||||
| 30 | 600 | theoretical conc. | 30 | 600 | 30 | 800 | theoretical conc. | 30 | 800 | |||
| 1 | 32.1 | 639 | mean | 31.6 | 632 | 1 | 32.4 | 812 | mean | 32 | 801 | |
| 2 | 31.3 | 621 | SD | 0.5 | 9.6 | 2 | 32.2 | 793 | SD | 1.3 | 10.1 | |
| 3 | 31.3 | 636 | %CV | 1.5 | 1.5 | 3 | 30.0 | 798 | %CV | 4.1 | 1.3 | |
| %dev | 5.2 | 5.4 | %dev | 5.1 | 0.1 | |||||||
| n | 3 | 3 | 3 | 3 | n | 3 | 3 | |||||
| TFV in Extract | FTC in Extract | |||||||||||
| 2 Days in Autosampler 4°C | Low | High | Low | High | ||||||||
| theoretical conc. | 30 | 1200 | theoretical conc. | 30 | 1200 | |||||||
| mean | 30.69 | 1226 | mean | 28.8 | 1133 | |||||||
| SD | 1.30 | 11.3 | SD | 0.53 | 6.77 | |||||||
| %CV | 4.23 | 0.93 | %CV | 1.83 | 0.60 | |||||||
| %dev | 2.30 | 2.17 | %dev | 4.0 | 5.58 | |||||||
| n | 9 | 8 | n | 9 | 8 | |||||||
Note: All concentrations are ng/mL
Table 4.
Long Term Stability of TFV and FTC in Plasma
| Long Term Plasma Stability of TFV and FTC at −80°C Proficiency Testing Samples ACTG PT Rd 15 (March 2005) | |||||
|---|---|---|---|---|---|
| 1/9/2008 | Nominal | 1/9/2008 | Nominal | ||
| TFV | Rd 15 | %DIFF | FTC | Rd 15 | %DIFF |
| 363 | 387 | −6.2 | 94.8 | 90.0 | 5.3 |
| 732 | 773 | −5.3 | 447 | 450 | −0.7 |
| 74.8 | 77.0 | −2.9 | 853 | 900 | −5.2 |
| 288 | 299 | −3.7 | 524 | 550 | −4.7 |
| 2301 | 2308 | −0.3 | 101 | 100 | 1.0 |
Elapsed Time: 34 months
Sensitivity and Selectivity
Since the CV at the 10 ng/mL level was < 15% for both analytes (Tables 1 and 2), it was decided to make this the lowest calibrator, although a lower concentration could probably have been achieved. This LLOQ was found suitable for the compliance and pharmacokinetic analyses performed at our institution. For comparison, the following LLOQs (ng/mL) were reported in recent publications: Gomes et al [10]: TFV, 10; FTC, 25. D’Avolio et al [11]: TFV, 15.6; FTC, 11.7. Nirogi et al [12]: TFV, 20; FTC, 2. When the seven different plasma lots were used as described above, no positive or negative response for either the analyte or the IS at the appropriate retention times and SRM conditions was noted. After spiking the different plasmas with the nine calibration standards and the resulting calibration curves assessed, the variation between the area ratios at each TFV/FTC level was < 5 %. For example, at the 250 ng/mL level, the % CV’s for TFV and FTC were 4.22 and 3.66, respectively (N=7). Furthermore, the slopes of the calibration curves were similar for the different plasma lots (TFV: 4.25–3.90 x 10−4; FTC: 1.65–1.30 x 10−3), suggesting that plasma obtained from different individuals will not significantly affect the assay.
When 250 μL aliquots of the plasma spiked with the anti-viral drugs listed above were analyzed, no TFV or FTC was detected, indicating that none of the drugs listed were likely to give a false positive result. When this plasma containing the concomitant drugs was also spiked with the appropriate TFV/FTC working calibration solution at the 250 ng/mL level and included in a typical analytical run, the calculated concentrations of the analytes were within ±6% of nominal (250 ng/mL). It was therefore concluded that the presence of the concomitant anti-viral drugs did not affect the assay described here, a finding we expected due to the specificity of the precursor/product transitions. We also determined that the IS peak areas and retention times were not affected by the presence of concomitant drugs in the extracts.
Matrix Effect
The presence of matrix effects in this assay was also investigated since suppression could theoretically cause undetected inaccuracies in the LC-MS-MS assay. Non-plasma effects: When the results of Set A and Set B (above) were compared, a 69% decrease in both TFV and Iso-TFV areas was detected, suggesting that the presence of trifluoroacetic acid and salts in the preparation results in a significant suppression. However, the FTC and its isotopic analogue, Iso-FTC, were only reduced by 16% in this experiment. This analyte-specific effect was presumably due to the early co-elution of the TFV and the acid and/or salts in the chromatogram. Plasma Effects: No significant variation in analyte areas was found between the 5 different plasma lots, suggesting that different endogenous plasma components did not affect the recovery. When Sets 1 and 2 were compared, the results showed that TFV and FTC abundances were suppressed by 73 % and 17 % at the three concentration levels, respectively. The abundances for the isotopic analogues were suppressed to the same degree as the analytes, illustrating the advantage of using stable labeled compounds as internal standards in this analysis. When Sets 2 and 3 were compared, the recoveries from the protein precipitation step were found to be 101.2% for TFV and 73 % for FTC. The overall recoveries for TFV and FTC were 37.7% and 66%, respectively (Set 3 compared to Set 1). Even though the abundances underwent a large drop in PE, the peak area ratios was unaltered due to the fact that the analyte and analogue abundances were equally affected. As the results from Sets A and B demonstrate, the primary agents suppressing the TFV abundance were the acid and salts in the extracts. On the other hand the FTC abundances were mostly affected by losses during the centrifugation step.
Patient Analysis
In one study, plasma samples from 228 HIV-positive patients receiving a daily dose of 300 mg TDF/600mg FTC (Truvada) were analyzed periodically for drug concentration using the method described herein. The TFV concentrations ranged from 28.9 to 513 ng/mL (N=360, median concentration, 92.6 ng/mL) and the FTC concentrations ranged from 41.9 to 2886 ng/mL (N=360, median concentration, 373 ng/mL). The elapsed time from the morning dosing to phlebotomy varied from one to twenty-four hours for these random visits. In another protocol, HIV-positive patients were enrolled in pharmacokinetic studies which involved phlebotomy at various times up to 24 hr after Truvada administration. A typical pharmacokinetic curve is shown in Fig 3 for a single patient from this study. Since this assay was designed, a total of 678 HIV-positive patient samples have been analyzed for TFV and FTC under the auspices of the ACTG.
Figure 3.
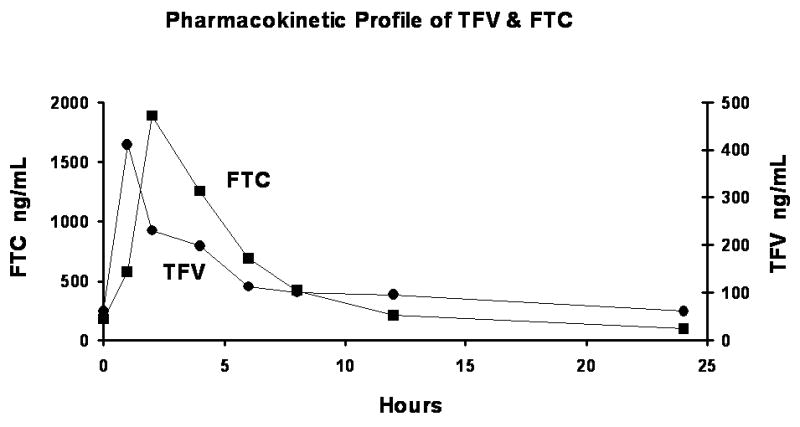
A Typical TFV/FTC Pharmacokinetic Profile.
The wide-spread prescribing of the single, once-a-day, Truvada combination tablet provided the incentive to develop a simple plasma assay for TFV and FTC which was sensitive, precise, accurate and not very labor intensive. Since these drugs each have unique pharmacokinetic characteristics, it is desirable to monitor both in a single assay. A recent publication by Sparidans et al [7], described a fast, sensitive LC/MS/MS method for FTC alone using deoxyfluorocytidine as the IS. However, in our view, the use of stable isotope labeled IS, chemically analogous to the analytes, is preferable in order to eliminate possible suppression effects which could potentially enhance or suppress the analyte/IS area ratio. Rezk et al. have published a simultaneous assay for TFV/FTC [6] using HPLC and ultra-violet detection. In this method, the plasma extracts were prepared using time-consuming solid phase extraction and evaporation with good recovery. An ion-pairing reagent was added to the mobile phase in order to achieve an adequate separation of the analytes. This method could be useful for those laboratories that are not equipped with MS/MS instrumentation. Gehrig et al. have recently characterized 18 anti-viral drugs from a single analytical run using LC/MS/MS [14]. The precursor/ product ions used to monitor TFV and FTC were identical to those described herein. However, the utility of this method for the routine quantitation of these two analytes remains to be established.
Conclusion
We have expanded an LC/MS/MS method, originally designed for the assay of TFV alone, to incorporate the simultaneous determination of TFV and FTC using stable-isotope labeled analogues of both analytes as IS with similar chromatographic and chemical properties to the administered drugs, avoiding potential problems with matrix effects. Although potentially expensive to duplicate in other laboratories, this assay could well be considered the method of choice for pharmacokinetic studies involving HIV-positive patients being treated with the combined TDF and FTC therapy.
Acknowledgments
This research was supported by the following grants to Courtney V. Fletcher: RO1 AI33835, UO1 AI68632, UO1 AI68636 and PO1 AI074340 from the National Institute of Allergy and Infectious Diseases
The authors would like to thank the following colleagues for assisting with this manuscript: Drs Peter Anderson, and Jonathan Weinhold BS.
Definitions of acronyms
- IDV
Indinavir
- DLV
Delavirdine
- APV
Amprenavir
- NFV
Nelfinavir
- M8
Nelfinavir metabolite
- SQV
Saquinavir
- EFV
Efavirenz
- RTV
Ritonavir
- LPV
Lopinavir
- ATV
Atazanavir
- 3TC
lamivudine
- d4T
Stavudine
- AZT
Zidovudine
- ABC
Abacavir
- DDI
Didanosine
- LLOQ
Lowest Level of Quantitation
- ACTG
AIDS Clinical Trials Group
- EDTA
Ethylenediaminetetraacetate
Footnotes
Ethics Considerations: The plasma samples analyzed in this study originated from institutions nation-wide under the auspices of the ACTG, a Federal agency. Before phlebotomy, that agency requires individual subjects to sign a form consenting to a plasma analysis which would result in the quantitation of any anti-HIV medication they may have been prescribed. No personal information which could be traced to an individual patient is presented here. Furthermore, this research was pre-approved by the University of Colorado’s Institutional Review Board to ensure that the project complied with the University’s ethical guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blum M, Chittick G, Begley J, Zong J. J Clinical Pharmacology. 2007;47:751–9. doi: 10.1177/0091270007300951. [DOI] [PubMed] [Google Scholar]
- 2.Gallant J, Dejesus E, Arribas J, Pozniak A, Gazzard B, Campo R, Lu B, McColl D, Chuck S, Enejosa J, Toole J, Cheng A. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 3.Chi B, Sinkala M, Mbewe F, Cantrell R, Kruse G, Chintu N, Aldrovandi G, Stringer E, Kankasa C, Safrit J, Stringer J. Lancet. 2007;370:1698–705. doi: 10.1016/S0140-6736(07)61605-5. [DOI] [PubMed] [Google Scholar]
- 4.Delahunty T, Bushman L, Fletcher CV. J Chrom B. 2006;830:6–12. doi: 10.1016/j.jchromb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Kiser J, Carten M, Aquilante C, Anderson P, Wolfe P, King T, Delahunty T, Bushman L, Fletcher CV. Clin Pharm Ther. 2008;83:265–72. doi: 10.1038/sj.clpt.6100269. [DOI] [PubMed] [Google Scholar]
- 6.Rezk N, Crutchley R, Kashuba A. J Chrom B. 2005;822:201–8. doi: 10.1016/j.jchromb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Sparidans R, Prins J, Schellens J. J Beijnen Biomed Chromatogr. 2007;21:621–7. doi: 10.1002/bmc.797. [DOI] [PubMed] [Google Scholar]
- 8.Matuszewski M, Constanzer C. M Chavez-Eng Anal Chem. 2003;75:3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 9.Delahunty T, Bushman L, Fletcher C. Clin Chem. 2007;53:A100. [Google Scholar]
- 10.Gomes N, Vaidya V, Putage A, Joshi S, Parekh S. J Pharm Biomed Anal. 2008;48:918–26. doi: 10.1016/j.jpba.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 11.D’Avolio A, Sciandra M, Siccardi M, Baietto L, Gonzalez de Requena D, Bonora S, Di Perri G. J Chromatograph Sci. 2008;46:524–528. doi: 10.1093/chromsci/46.6.524. [DOI] [PubMed] [Google Scholar]
- 12.Nirogi R, Bhyrapuneni G, Kandikere V, Mudigonda K, Komarneni P, Aleti R, Mukkanti K. Biomed Chromatogr. 2008:1–11. doi: 10.1002/bmc.1125. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. Guidance for Industry; Bioanalytical Method Validation. Rockville MD: US Department of Health and Human Services, CDER and CVM; May, 2001. www.fda.gov/cder/guideance/index.htm. [Google Scholar]
- 14.Gehrig A, Mikus G, Haefeli W, Burhenne J. Rapid Commun Mass Spectrom. 2007;21:2704–16. doi: 10.1002/rcm.3138. [DOI] [PubMed] [Google Scholar]