Abstract
Human small nuclear (sn) RNA genes are transcribed by either RNA polymerase II or III depending upon the arrangement of their core promoter elements. Regardless of polymerase specificity, these genes share a requirement for a general transcription factor called the snRNA activating protein complex or SNAPC. This multi-subunit complex recognizes the proximal sequence element (PSE) commonly found in the upstream promoters of human snRNA genes. SNAPC consists of five subunits: SNAP190, SNAP50, SNAP45, SNAP43, and SNAP19. Previous studies have shown that a partial SNAPC composed of SNAP190 (1–514), SNAP50, and SNAP43 expressed in baculovirus is capable of PSE-specific DNA binding and transcription of human snRNA genes by RNA polymerases II and III. Expression in a baculovirus system yields active complex but the concentration of such material is insufficient for many bio-analytical methods. Herein, we describe the co-expression in Escherichia coli of a partial SNAPC containing SNAP190 (1–505), SNAP50, SNAP43, and SNAP19. The co-expressed complex binds DNA specifically and recruits TBP to U6 promoter DNA. Importantly, this partial complex functions in reconstituted transcription of both human U1 and U6 snRNA genes by RNA polymerases II and III, respectively. This co-expression system will facilitate the functional characterization of this unusual multi-protein transcription factor that plays an important early role for transcription by two different polymerases.
Keywords: Transcription, SNAPC, Human snRNA, Co-expression, Escherichia coli
In eukaryotic organisms, transcription occurs by three different RNA polymerases I, II, and III that are unable to directly recognize promoter elements but instead require distinct assemblies of general transcription factors for efficient promoter recruitment. The initial step of promoter recognition by the general transcription machinery is a key step in transcription and is frequently targeted for intervention during gene regulation [1]. Interestingly, those components of the general transcription machinery that function directly in core promoter DNA binding are often composed of large multi-subunit complexes. For example, the TFIID complex is involved in pre-initiation complex assembly for RNA polymerase II transcription [reviewed in 2] and is composed of at least 13 subunits in humans [3]. While somewhat less complicated, other promoter recognition factors share this multi-subunit nature including SL1 for RNA polymerase I transcription, as well as TFIIIB and TFIIIC for RNA polymerase III transcription [4,5]. Potentially, multi-subunit complexes provide flexibility for modulating transcriptional responses to changing environmental conditions, in part, by providing many exposed surfaces for interactions with regulatory factors.
Typically, promoter recognition complexes are also specialized for transcription by a single class of polymerase; however, the SNAPC general transcription factor [6], also known as PTF [7], provides an interesting example of functional versatility through its role in snRNA gene transcription by both RNA polymerases II and III. Regardless of polymerase specificity, the promoters of human snRNA genes are very similar containing a proximal sequence element (PSE)1 within the core promoter region [reviewed in 8,9]. SNAPC binds to the PSE and depending upon the presence or absence of an adjacent TATA box participates in different pathways of pre-initiation complex assembly. The presence of a TATA box, such as in U6 snRNA genes, dictates a role for SNAPC in pre-initiation complex assembly for RNA polymerase III transcription along with TBP, as a component of TFIIIB [10,11]. In contrast, the absence of a TATA box, such as in U1 snRNA genes, prescribes SNAPC participation in pre-initiation complex assembly with other general transcription factors including TBP, TFIIA, TFIIB, TFIIE, and TFIIF, commonly used for RNA polymerase II transcription [12]. SNAPC participation in either pathway is stimulated by direct interactions with the transcriptional activator protein Oct-1 when bound to the distal sequence element (DSE) located upstream of most human snRNA genes [13–16]. Thus, the early steps of promoter recognition by SNAPC are common to both pathways, but subsequent steps involving unique interactions with other general transcription factors are specialized for transcription by a single polymerase. Presumably, components of SNAPC are differentially important for RNA polymerase II versus III transcription, although no evidence for subunit specialization has yet been uncovered.
SNAPC is composed of at least five subunits, SNAP19 [17], SNAP43 [18,19], SNAP45 [19,20], SNAP50 [21,22], and SNAP190 [23]. SNAP190, the largest of the subunits, provides the fundamental scaffold for assembly of the complex and directly interacts with all other SNAPC subunits [24] and with TBP [25,26]. SNAP190 also contains an unusual DNA binding domain in its N-terminal region consisting of four and a half myb domain repeats that is required for SNAPC DNA binding to the PSE [23] and for cooperative promoter recruitment of TBP to the TATA box during U6 transcription [26]. The central region of SNAP190 may negatively regulate SNAPC DNA binding, but interestingly, auto-inhibition of DNA binding can be overcome by interactions between the central region of SNAP190 and Oct-1 during activated transcription by RNA polymerases II and III [15]. The C-terminal region of SNAP190 interacts with SNAP45 [25], which may stabilize DNA binding of SNAPC in the context of the natural complex [15]. However, DNA binding by SNAPC does not require SNAP45 or SNAP19 as DNA binding activity can be reconstituted using a partial recombinant SNAPC (hereafter referred to as mini-SNAPC) that contains SNAP43, SNAP50, and the N-terminal region of SNAP190 encompassing amino acids 1–505 [15]. DNA binding by mini-SNAPC requires all three subunits [26] with SNAP50 possibly involved in direct DNA contacts, and SNAP43 acting as a bridge to coordinate DNA recognition by SNAP190 and SNAP50.
Recently, much has been learned about the composition and function of SNAPC. For example, both SNAPC and mini-SNAPC can be reconstituted from recombinant proteins produced in a baculovirus expression system or by in vitro translation [15,17], but the amounts of pure material are limiting. In bacterial expression systems, the recovery of individual subunits is problematic, resulting in complexes capable of DNA binding but not capable of transcription initiation. To better understand the function of SNAPC a co-expression system in Escherichia coli was employed, enabling the production of fully functional partial SNAPC at significantly increased amounts and purity. The partial complex is capable of specific DNA binding, promoter recruitment of TBP, and transcription initiation for both RNA polymerases II and III.
Materials and methods
Cloning of SNAPC expression plasmids
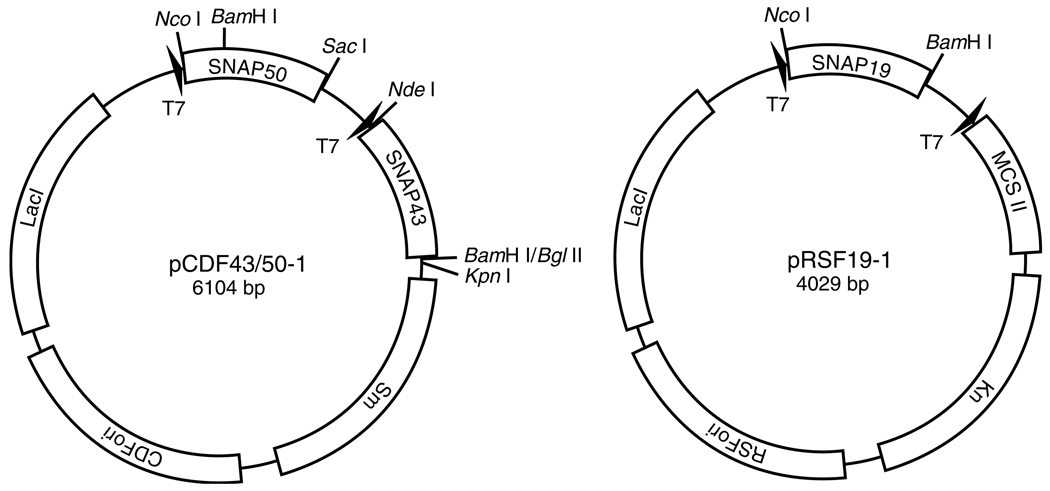
To generate an expression plasmid that permits co-expression in E. coli of the SNAP50 and SNAP43 subunits of human SNAPC, the full-length SNAP50 open reading frame was first amplified by polymerase chain reaction using Turbo Pfu polymerase with the primers 5′-TCAGCCATGGCTGAAGGAAGC-3′ and 5′-AGAGCTCTTAAT TAAAGGTTCCAGG-3′. The resultant amplified DNA fragment was inserted into the first multiple cloning site of the pCDFDuet-1 polycistronic expression vector (Novagen) via the NcoI and SacI recognition sites to generate the expression vector pCDF50-1. Next, the full-length SNAP43 open reading frame was amplified using the primers 5′-GGAATTCCATATGGGGACTCCTCCCGGCCTGCA-3′ and 5′-GAGGATCCTCAGTGTTTTCTCCTCTTCTTG GATGC-3′. The resultant PCR product was inserted into the pCDF50-1 expression vector via the NdeI and BglII recognition sites to generate the expression vector pCDF43/50-1 that permits co-expression of SNAP50 with SNAP43, and provides resistance to streptomycin. The full-length SNAP19 open reading frame was also amplified by PCR using the primers 5′-CTCACCATGGTGAGCCGGCTT C-3′ and 5′-AAGGATCCTTAGGAATCTGATTCTTC-3′. The resultant PCR product was inserted into pRSFDuet-1 (Novagen) via the NcoI and BamHI recognition sites to generate the expression vector pRSF19-1 that permits expression of full-length SNAP19, and provides resistance to kanamycin. Schematic representations of these plasmids are shown in Fig. 2.
Fig. 2.
The polycistronic plasmids used for co-expression of mSNAPCγ4. The pCDF43/50-1 expression plasmid contains the full-length SNAP50 ORF in the first multiple cloning site and the full-length SNAP43 ORF in the second multiple cloning site. This plasmid carries the CDF origin of replication and provides streptomycin resistance. The pRSF19-1 expression plasmid contains the SNAP19 ORF in the first multiple cloning site. The second multiple cloning site was not used in this strategy. This plasmid carries the RSF origin of replication and provides kanamycin resistance.
Expression of mSNAPCγ3 and mSNAPCγ4
To generate partial SNAPC complexes, the expression plasmid pGST190 (1–505) [26], which expresses SNAP190 (1–505) with a N-terminal GST-tag, was first transformed into the E. coli strain BL21(DE3) CodonPlus RIL (Strat-agene) and plated on LB plates supplemented with ampicillin (100 µg/mL) and chloramphenicol (50 µg/mL). Single colonies were then transferred to LB broth containing the same antibiotics and cells were made competent by CaCl2 treatment for subsequent transformation with pCDF43/50-1. Transformed cells harboring the pGST190 (1–505) and pCDF43/50-1 expression plasmids were incubated on LB plates supplemented with ampicillin (50 µg/ mL), chloramphenicol (50µg/mL), and streptomycin (20 µg/mL). These cells were then used for co-expression of GST-SNAP190 (1–505), SNAP50, and SNAP43 to generate the recombinant complex mSNAPCγ3 as described below. To co-express a partial SNAPC that additionally contains SNAP19, the previous strain was made competent for subsequent transformation with the expression plasmid pRSF19-1. Transformed cells harboring the three SNAPC-specific expression plasmids were plated on LB plates supplemented with ampicillin (50 µg/mL), chloramphenicol (50 g/mL), streptomycin (20 µg/mL), and kana-mycin (20 µg/mL). For these experiments, serial transformation of each expression plasmid was necessary as attempts to simultaneously transform all expression plasmids were unsuccessful. These cells were used for co-expression of GST-SNAP190 (1–505), SNAP50, SNAP43, and SNAP19 to generate the recombinant complex mSNAPCγ4, as described below.
Purification of mSNAPC and mSNAPCγ4
To generate the recombinant complexes mSNAPCγ3 and mSNAPCγ4, bacteria harboring the appropriate plasmids were grown at 37 °C in 1 L of LB broth containing ampicillin (50 µg/mL), chloramphenicol (50 g/mL), streptomycin (20 µg/mL), and if necessary, kanamycin (20 µg/mL) until a cell density of 0.8–1 OD600 was reached. Cultures were induced with 1mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 14–16 h at 16 °C. Typically, 6 × 1 L cultures were used for expression and purification of either mSNAPCγ3 or mSNAPCγ4. The concentration of recombinant proteins during purification was estimated by Bradford assay or by comparison to BSA standards after SDS–PAGE and Coomassie blue staining. To purify mSNAPCγ3 and mSNAPCγ4, cells were collected from 6 L cultures by centrifugation using a Komp spin KA-9 rotor (5000 rpm, 20min, 4 °C). The supernatant was decanted and the cell pellets were resuspended to a total volume of 160mL using HEMGT-250 (25mM Hepes, pH 7.9, 2mM EDTA, 12.5mM MgCl2, 10% glycerol, 0.1% Tween 20, 250mM KCl, and 3mM DTT) plus protease inhibitors (complete mini EDTA-free protease inhibitor cocktail tablets, Roche). The cells were lysed by ultrasonication using a Branson sonifier (3/8" tip, 3 × 1min pulses, 80% duty, power level 7), and the lysate was clarified by centrifugation using a GSA rotor (5000 rpm, 1 h, 4 °C). Recombinant mSNAPCγ3 or mSNAPCγ4 were affinity purified by mixing the clarified lysates with glutathione agarose beads (Amersham) at a ratio of 4:1 (v/v) for 2 h at 4 °C. After binding, the resin was collected by centrifugation and the beads were washed five times with 40mL HEMGT-250 buffer. The washed beads (~40mL) were transferred to a Kontes Flex-Column (2.5 cm ID 20 cm length 98mL maximum volume) and an additional 20mL of HEMGT-250 was added. The recombinant complexes were released from the beads by digestion with thrombin (50U, 12 h, 4 °C). Subsequently, digested proteins were eluted from the column with HEMGT-250 and 15-mL fractions were collected. Those fractions enriched for the recombinant complexes were then pooled. Approximately 9.6mg of mSNAPCγ3 (1.6 mg/L culture; average of six runs) and 13.5mg of mSNAPCγ4 (2.2mg/L culture; average of 17 runs) were obtained at this stage of purification. The partially purified complexes were then subjected to ion exchange column chromatography using 2mL of Source-S resin (Amersham). Bound proteins were eluted from the Source-S column using a 50mL linear elution gradient from buffer A (10mM Tris, pH 7.5, 50mM NaCl, 15.5mM MgCl2, 10% glycerol, 0.05% Tween 20, and 3mM DTT) to buffer A-500 (buffer A containing 500mM NaCl). Both mSNAPCγ3 and mSNAPCγ4 eluted from this column at salt concentrations between 150 and 250mM NaCl in a volume of ~15mL. The average recovery was 5mg for mSNAPCγ3 (52% average yield) and 10mg for mSNAPCγ4 (74% average yield). The recombinant complexes were diluted to an appropriate concentration in HEMGT-80 for functional studies, as described below.
Electrophoretic mobility shift assay
EMSA was performed using either recombinant mSNAPCγ4 or mini-SNAPC containing SNAP190 (1–505), SNAP43, and SNAP50 that were expressed individually in E. coli and assembled into a complex, as previously described [26]. The individually expressed mini-SNAPC used for the experiments shown in Fig. 1 was assembled and purified by binding each subunit to glutathione aga-rose beads followed by thrombin digestion. Briefly, mini-SNAPC was assembled by mixing approximately 60 ng of each purified subunit together for 2 h. The complex was then further purified by mono-Q HR 5/5 column chromatography (~5 ng mini-SNAPC/µL). The amounts of mini-SNAPC and mSNAPCγ4 used for the DNA binding reactions are indicated in the figure legends. To test mSNAPCγ4 for ability to recruit TBP, an additional 50 ng of full-length human recombinant TBP was included in DNA binding reactions, as indicated. The radiolabeled DNA probes used contained a high affinity wild-type mouse U6 PSE and human U6 TATA box. Additional reactions were performed with probes that contain debilitating mutations in each element. Resultant protein–DNA complexes were separated on a 5% Tris–glycine–EDTA–Mg2+ (TGEM) polyacrylamide gel at 150V and protein–DNA complexes were visualized by autoradiography.
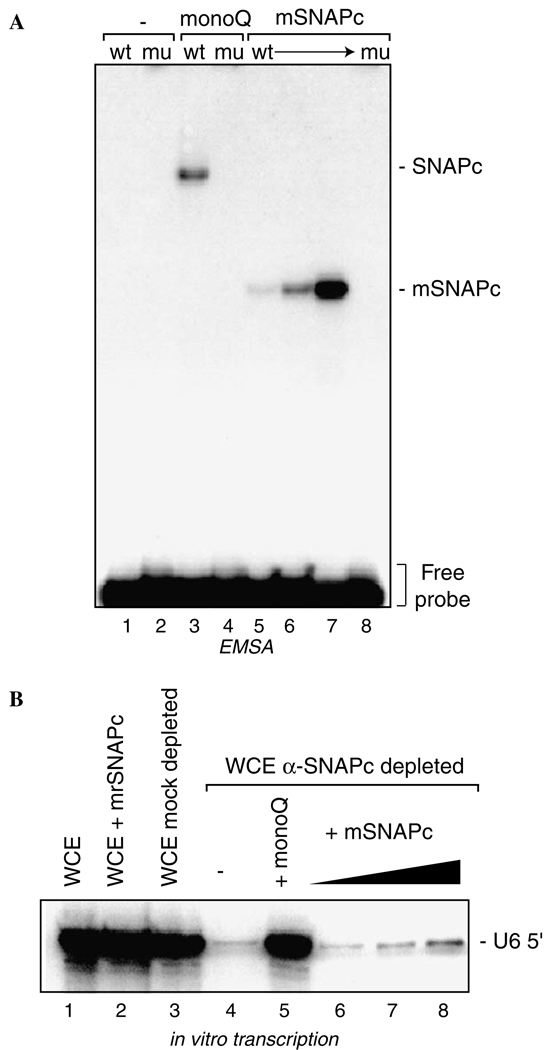
Fig. 1.
A partial SNAPC assembled from individually expressed subunits is functional for DNA binding but not for transcription of a human U6 snRNA gene by RNA polymerase III. (A) Increasing amounts of mini-SNAPC that was assembled from subunits individually expressed in E. coli (~1, 2.5, and 7.5 ng; lanes 5–7, respectively) were added to EMSA reactions containing dsDNA probes containing a wild-type (wt) or mutant (mu) PSE, as indicated. Reactions loaded in lanes 3 and 4 contained 7.5 µL of partially purified endogenous SNAPC (~0.3 ng SNAPC/mL). Reactions containing only the probe DNAs are shown in lanes 1 and 2. (B) Increasing amounts of mini-SNAPC were added to human U6 in vitro transcription reactions for which the HeLa whole cell extract (WCE) was treated with α-SNAP43 antisera to remove endogenous SNAPC, as shown in lanes 6–8. Lane 4 shows the decreased signal for the correctly initiated U6 transcription upon removal of endogenous SNAPC. Lane 5 shows the U6 signal dependent upon addition of endogenous SNAPC obtained from biochemical fractionation of a HeLa cell nuclear extract. Note, this signal is comparable to the mock depleted WCE (lane 3) or WCE alone (lane 1).
In vitro transcription assay
Antibody immunodepletion of HeLa cell nuclear extract was performed with either rabbit α-SNAP43 antiserum or preimmune sera as described previously [17,23]. In vitro transcription assays of human U1 and U6 snRNA genes were performed for 1 h at 30 °C using the depleted extracts as described previously [6,27–29]. For the U6 transcription experiment shown in Fig. 1, approximately 1, 2.5, and 7.5 µL of recombinant mini-SNAPC (~1ng/µL) and 7.5 µL of a mono-Q fraction enriched for endogenous SNAPC (~0.3ng SNAPC/µL) was used to reconstitute transcription. For the experiment shown in Fig. 4, approximately 0.08, 0.25, 0.75, 2.5, 7.5, 25, and 75 ng of mSNAPCγ4 was used for the reconstitution of both U1 and U6 transcription.
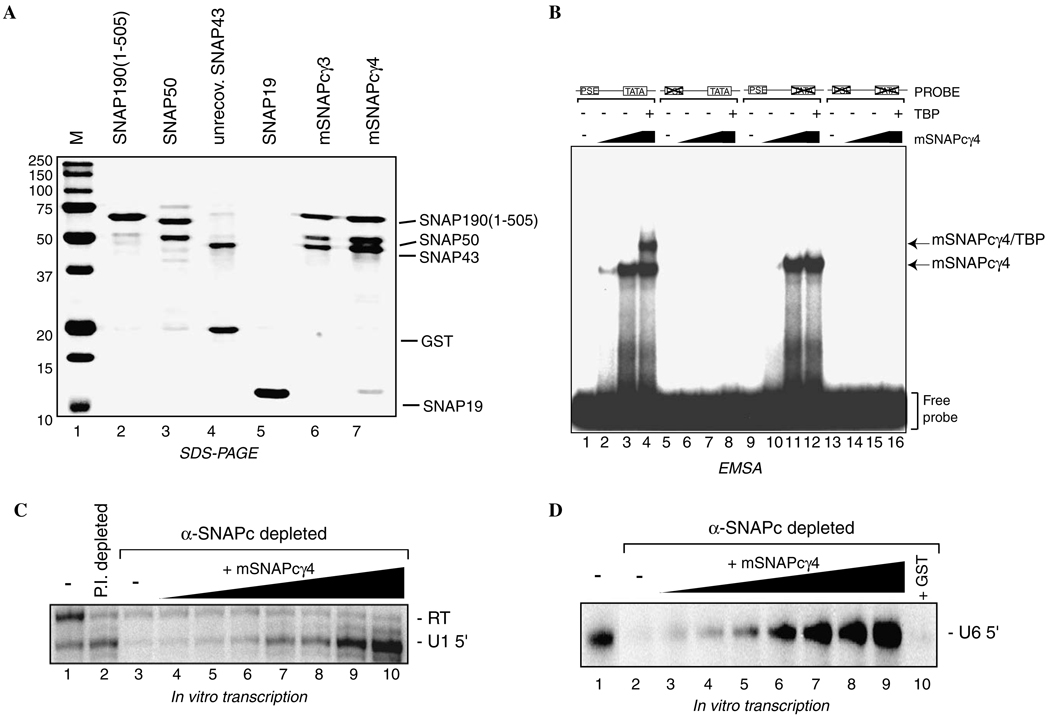
Fig. 4.
mSNAPCγ4 functions for DNA binding, TBP recruitment, and in vitro transcription by RNA polymerases II and III. (A) SNAP19 improves complex stability and subunit recovery. Subunit recovery for partial SNAPC assembled without and with co-expressed SNAP19 (mSNAPCγ3 and mSNAPCγ4, respectively) was estimated by SDS–PAGE and Coomassie blue staining. Note the increased recovery of SNAP43 and SNAP50 in mSNAPCγ4 (lane 7) compared to mSNAPCγ3 (lane 6). Lanes 2–5 contain aliquots of the individually expressed SNAPC subunits for reference. (B) mSNAPCγ4 facilitates TBP promoter recruitment. Increasing amounts of mSNAPCγ4 (3 and 10 ng) were added to DNA binding reactions containing radiolabeled probes that harbor a wt PSE and wt TATA box (lanes 1–4), mu PSE and wt TATA box (lanes 5–8), wt PSE and mu TATA box (lanes 9–12), or mu PSE and mu TATA box (lanes 13–16). Lanes 4, 8, 12, and 16 contain 50 ng of recombinant full-length human TBP in addition to 10 ng of mSNAPCγ4. Reactions containing only the DNA probes are shown in lanes 1, 5, 9, and 13. Positions of the mSNAPCγ4 and mSNAPCγ4 plus TBP complexes are indicated. (C) mSNAPCγ4 functions for U1 snRNA transcription by RNA polymerase II. HeLa cell nuclear extract was either mock depleted with a preimmune rabbit sera (lane 2) or α-SNAP43 antisera (lanes 3–10) to deplete endogenous SNAPC. Extracts were then used for human U1 in vitro transcription assays. The U1-specific signal was diminished upon removal of endogenous SNAPC, as shown in lane 3. Increasing amounts of mSNAPCγ4 (0.08, 0.25, 0.75, 2.5, 7.5, 25, and 75 ng) reconstituted the correctly initiated transcription from a human U1 reporter, as shown in lanes 4–10. Lanes 1 and 2 show the U1 signal obtained from either untreated or mock depleted reactions. (D) mSNAPCγ4 functions for U6 snRNA transcription by RNA polymerase III. In vitro transcription of human U6 snRNA was carried out using HeLa cell nuclear extract that was treated as in (C). Lane 2 shows the reduced U6 signal upon removal of endogenous SNAPC. Increasing amounts of mSNAPCγ4 (0.08, 0.25, 0.75, 2.5, 7.5, 25, and 75 ng) reconstituted the correctly initiated transcription from a human U6 reporter as shown in lanes 3–9. Approximately 75 ng of GST was added to the transcription reaction shown in lane 10.
Results and discussion
As a first step towards dissecting the function of SNAPC, a partial complex containing SNAP190 (1–505), SNAP50, and SNAP43 was assembled using recombinant factors individually expressed in E. coli as previously described [26]. This bacterially expressed mini-SNAPC was compared to partially purified endogenous SNAPC [18] for its ability to bind to DNA in an electrophoretic mobility shift assay (EMSA) using radiolabeled probes that contain either a wild-type (wt) PSE or a mutant (mu) PSE sequence. As shown in Fig. 1A, the endogenous SNAPC binds specifically to the wtPSE but not a muPSE, as expected, and the bacterially expressed mini-SNAPC exhibited similar DNA binding properties (compare lanes 5–7 with lane 3). Thus, recombinant mini-SNAPC assembled from individually expressed subunits is functional for DNA binding. This result is consistent with those previously described for this complex when it was expressed and assembled in a similar manner [26] or by co-expression of the recombinant SNAPC subunits using a baculovirus expression system [30].
Next, these same amounts of endogenous SNAPC and recombinant mini-SNAPC were tested for ability to reconstitute U6 snRNA gene transcription in vitro using a HeLa cell whole cell extract that was depleted of endogenous SNAPC. As shown in Fig. 1B, addition of recombinant mini-SNAPC to the extract did not substantially influenced RNA polymerase III transcription from this reporter plasmid (lane 2) nor did mock depletion of the extract using an irrelevant preimmune serum (lane 3). However, transcription was debilitated by removal of endogenous SNAPC by anti-SNAP43 immunodepletion (lane 4). As expected, transcription was efficiently restored by addition of the bio-chemically purified SNAPC (lane 5), whereas recombinant mini-SNAPC was essentially inactive in these assays (lanes 6–8). Thus, the recombinant mini-SNAPC assembled from individually expressed subunits is crippled for U6 transcription even though it is capable of efficient DNA binding and U6 promoter recognition.
One explanation for the inability of the recombinant mini-SNAPC to function for U6 transcription is that a post-translational modification of SNAPC is crucial for its activity and that this event is not successfully recapitulated in E. coli cells. Indeed, the SNAP190 (1–505) subunit is a substrate for phosphorylation by the protein kinase CK2 [31]. Another possibility is that mini-SNAPC does not obtain its fully active, native conformation when its subunits are expressed separately and reassembled in vitro. Both possibilities are consistent with the observation that a functional mini-SNAPC can be obtained by co-expression of these mini-SNAPC subunits in a baculovirus expression system [15].
To address the latter hypothesis, a co-expression system was devised wherein the individual subunits of mini-SNAPC were simultaneously expressed in E. coli. This approach has been used in other systems for high level expression soluble protein complexes [reviewed in 32]. The open reading frames encoding full-length SNAP50 and SNAP43 were sub-cloned into a pCDFDuet-1 plasmid for co-expression from the same promoter, while full-length SNAP19 was sub-cloned into a pRSFDuet-1 plasmid. Both pCDFDuet-1 and pRSFDuet-1 are polycistronic expression plasmids, and although in the current example SNAP19 was expressed from its own plasmid, the use of the pRSFDuet-1 plasmid allows, if necessary, for the co-expression of SNAP19 and SNAP190 (1–505) that together form a tight complex. In the expression system presented herein, GST-SNAP190 (1–505) was also expressed from its own plasmid. Schematic representations of the newly generated pCDF43/50-1 and pRSF19-1 plasmids are shown in Fig. 2. As different antibiotic selection can be used for each vector, we were able to sequentially transform pGST190 (1–505) and pCDF43/50-1 into E. coli cells thus enabling the expression of the three subunits SNAP190 (1–505), SNAP50, and SNAP43 to generate the recombinant complex hereafter referred to as mSNAPCγ3. The co-expression of an additional fourth subunit SNAP19 from the pRSF19-1 plasmid was also performed to generate the recombinant complex hereafter referred to as mSNAPCγ4.
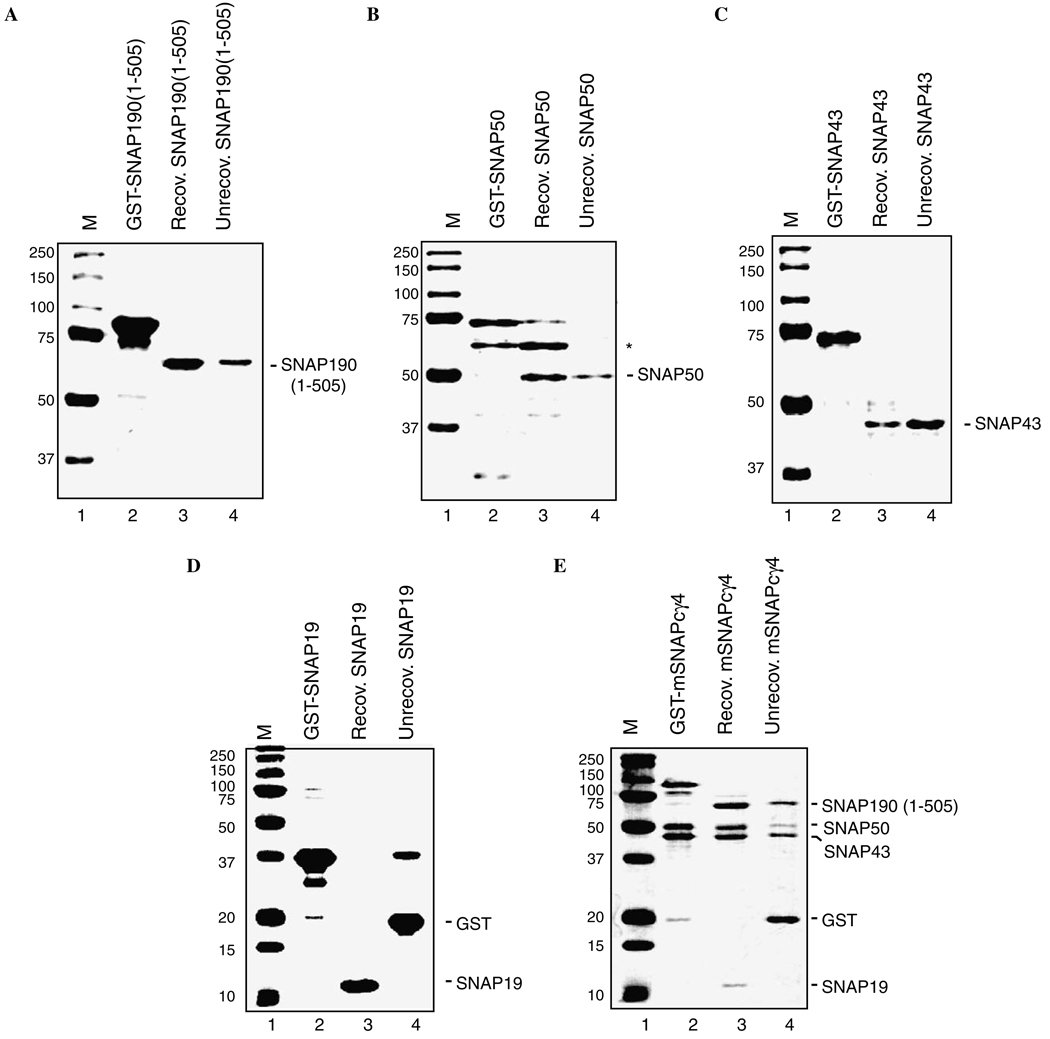
The purification properties of mSNAPCγ4 were next compared to the properties of the same SNAPC components that were expressed individually in E. coli (Fig. 3). This analysis revealed two important advantages for the co-expression system. First, the recovery of full-length SNAP43 is typically very low (10–30%) when it is expressed individually even though expression of the parent GST-SNAP43 protein is relatively robust (Fig. 3C). After thrombin cleavage of GST-SNAP43 the majority of the cleaved SNAP43 typically remains irreversibly bound to the glutathione agarose beads despite the lack of a covalently attached GST-tag. In contrast, Fig. 3E shows that the proportion of SNAP43 recovered is improved when it is expressed together with the rest of the SNAPC proteins. Presumably, the recovery is improved because SNAP43 can form a tight complex with SNAP50 or SNAP190 (1–505) to prevent inappropriate aggregation on the glutathione agarose beads. Second, when SNAP50 is expressed individually, stoichiometric amounts of a 60kDa contaminating protein associate with SNAP50 (Fig. 3B). Although the identity of this contaminating protein from E. coli is unknown, the association between this factor and SNAP50 is stable to a least 1M KCl (data not shown). Furthermore, after assembly of mini-SNAPC using individually expressed subunits, this factor also remains associated during a variety of affinity purification and column chromatography steps making the purification of mini-SNAPC problematic (data not shown). In contrast, no trace of this contaminating factor was observed during the one-step affinity purification of the mSNAPCγ4 complex. Therefore, the overall purity of the complex is markedly improved by the co-expression of the SNAPC components.
Fig. 3.
Co-expression of mSNAPCγ4 improves subunit recovery and purity. (A–D) Each SNAPC subunit was individually expressed in E. coli as a GST-fusion protein for subsequent affinity purification using glutathione agarose beads. The amounts of each GST-fusion protein originally bound to the beads are shown in lane 2. After binding, proteins were released by digestion with thrombin, which cuts between the GST-tag and each SNAPC protein to release the soluble untagged protein (lane 3). Laemmli buffer was then added to the washed beads and any proteins that remained associated with the beads were released by boiling (lane 4). Equivalent amounts of the recovered and unrecovered samples were loaded to estimate the proportion of each recombinant protein that remains associated with the beads after digestion. Proteins were separated by SDS–PAGE and were visualized by staining with Coomassie blue. Note the association of stoichiometric amounts of an E. coli protein with SNAP50 [(B), labeled *] and the significant proportion of SNAP43 that remains bound to the beads after thrombin digestion (C) when these proteins are expressed individually. (E) The multi-subunit complex mSNAPCγ4 was co-expressed in E. coli from the expression plasmids pCDF43/50-1, pRSF19-1, and pGST-190 (1–505). In this case, the complex was affinity purified through GST-SNAP190 (1–505) and the recovery of each component was estimated as above.
Previous experiments demonstrated that even though SNAP19 is not essential for transcription [15], it facilitates the association between SNAP43 and SNAP190 [17], and the inclusion of SNAP19 in assembly reactions of mini- SNAPC dramatically improved the DNA binding performance of mini-SNAPC [26]. Therefore, whether SNAP19 was necessary to facilitate the assembly of a complex in this co-expression system was examined by comparing the partial complexes with and without SNAP19 (Fig. 4A). Samples of the individually expressed subunits are shown in lanes 2–5, and served to identify these factors within the co-expressed complex. In the presence of co-expressed SNAP19, there is a substantial increase in the amounts of SNAP43 and SNAP50 recovered during affinity purification of the complex through GST-SNAP190 (1–505) as compared to the complex lacking SNAP19 (compare lane 6 to lane 7). Nonetheless, the partially purified mSNAPCγ3 and mSNAPCγ4 obtained by binding complexes to glutathione agarose beads subsequently eluted as single complexes of apparent molecular weight of 150kDa during size exclusion chromatography (data not shown), suggesting that differences in SNAP43 and SNAP50 recovery after ion exchange chromatography are due to differences in complex stability rather than complexes with altered subunit stoichiometry during complex expression and assembly. This result suggests that co-expression of SNAP19 stimulates complex assembly and is consistent with the previously described role for SNAP19 in stimulating association between SNAP43 and SNAP190. Furthermore, while SNAP19 is not essential for function of recombinant SNAPC, the amount of complex recovered was increased using the co-expression system that includes SNAP19 and therefore mSNAPCγ4 was chosen for further study.
To determine whether the mSNAPCγ4 obtained using this co-expression system was functional, the ability of mSNAPCγ4 to bind DNA in a PSE-specific fashion and recruit TBP was tested using EMSA. As shown in Fig. 4B, mSNAPCγ4 bound efficiently to both the wtPSE/wtTATA (lanes 2 and 3) and wtPSE/muTATA probes (lanes 10 and 11), but failed to bind to any probes in which the PSE was mutated (lanes 6 and 7, and lanes 14 and 15). The mSNAPCγ4 also functioned to recruit TBP to the wtPSE/wtTATA DNA probe (lane 5) but not to a wtPSE/muTATA probe lacking a functional TATA box (lane 12). These results show that the mSNAPCγ4 obtained using the co-expression system does indeed behave as previously described for both endogenous SNAPC and all other previously described versions of recombinant SNAPC for DNA binding and TBP recruitment.
Next, the ability of mSNAPCγ4 to function for transcription by both RNA polymerases II and III was examined using reconstituted in vitro transcription initiated from plasmids containing either a human U1 snRNA promoter (Fig. 4C) or human U6 snRNA promoter (Fig. 4D). In these experiments, HeLa cell nuclear extract was either mock depleted with rabbit preimmune sera or with α-SNAP43 antiserum to remove endogenous SNAPC. In the absence of endogenous SNAPC, production of the U1-specific transcript is markedly reduced relative to the mock depleted extract (compare lanes 1 and 2 with lane 3), whereas the non-specific read-through transcript (labeled RT) is unaffected by SNAPC depletion. U1-speciWc transcription was restored by addition of increasing amounts of mSNAPCγ4 (lanes 4–10), again with no obvious effect on read-through transcription. Thus, mSNAPCγ4 functions specifically to reconstitute RNA polymerase II transcription from the U1 promoter with no effect on RNA polymerase II transcription driven by a cryptic mRNA-like promoter located upstream from the U1 start site. Similarly, U6 snRNA transcription by RNA polymerase III was diminished by removal of endogenous SNAPC (Fig. 4D, lane 2), and transcription was restored by increasing amounts of mSNAPCγ4 (lanes 3–9), whereas transcription was not restored by addition of a non-specific protein GST (lane 10). For both U1 and U6 transcription, the amounts of recombinant mSNAPCγ4 required to restore transcription levels equivalent to the untreated extract was similar (~2.5ng), although U1 transcription continued to be stimulated by further increasing the amounts of mSNAPCγ4 (Fig. 4C, lanes 8–10), perhaps indicating that SNAPC activity is limiting for U1 transcription in this in vitro system. Importantly, robust U6 transcriptional activity was achieved with mSNAPCγ4 using the amounts of recombinant protein that were completely ineffective when these factors were combined from individually expressed factors (see Fig. 1B, lane 7). These results show that mSNAPCγ4 is indeed functional for both RNA polymerases II and III transcription. In these experiments, reconstitution was achieved using a similar mass of recombinant complex as that achieved with endogenous SNAPC, indicating that a priori modification of the complex, such as by phosphorylation by CK2, is not essential for activity. This study does not examine whether the complex could be targeted for modification during transcription. Overall, this report describes an important advance in the production of a functional multi-protein complex by co-expression of its recombinant subunits, which will accelerate further structure and functional studies of this interesting general transcription factor.
Acknowledgments
We thank Justin Earl Bammer for technical assistance. This research was supported by the NIH Grants R01-GM59805 and R01-GM063894 to R.W.H. and J.H.G., respectively.
Footnotes
Abbreviations used: sn, small nuclear RNA genes; PSE, proximal sequence element; DSE, distal sequence element; IPTG, isopropyl-β-d-thiogalactopyranoside; TGEM, 5% Tris–glycine–EDTA–Mg2+; EMSA, electrophoretic mobility shift assay; wt, wild-type.
References
- 1.Roeder RG. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 2005;579:909–915. doi: 10.1016/j.febslet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Albright SR, Tjian R. TAFs revisited: more data reveal new twists and conWrm old ideas. Gene. 2000;242:1–13. doi: 10.1016/s0378-1119(99)00495-3. [DOI] [PubMed] [Google Scholar]
- 3.Tora L. A uniWed nomenclature for TATA box binding protein (TBP)-associated factors (TAFs) involved in RNA polymerase II transcription. Genes Dev. 2002;16:673–675. doi: 10.1101/gad.976402. [DOI] [PubMed] [Google Scholar]
- 4.Geiduschek EP, Kassavetis GA. Comparing transcriptional initiation by RNA polymerases I and III. Curr. Opin. Cell Biol. 1995;7:344–351. doi: 10.1016/0955-0674(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 5.Paule MR, White RJ. Survey and summary: transcription by RNA polymerases I and III. Nucleic Acids Res. 2000;28:1283–1298. doi: 10.1093/nar/28.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadowski CL, Henry RW, Lobo SM, Hernandez N. Targeting TBP to a non-TATA box cis-regulatory element: a TBP-containing complex activates transcription from snRNA promoters through the PSE. Genes Dev. 1993;7:1535–1548. doi: 10.1101/gad.7.8.1535. [DOI] [PubMed] [Google Scholar]
- 7.Murphy S, Yoon JB, Gerster T, Roeder RG. Oct-1 and Oct-2 potentiate functional interactions of a transcription factor with the proximal sequence element of small nuclear RNA genes. Mol. Cell. Biol. 1992;12:3247–3261. doi: 10.1128/mcb.12.7.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henry RW, Ford E, Mital R, Mittal V, Hernandez N. Crossing the line between RNA polymerases: transcription of human snRNA genes by RNA polymerases II and III. Cold Spring Harb. Symp. Quant. Biol. 1998;63:111–120. doi: 10.1101/sqb.1998.63.111. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez N. Small nuclear RNA genes: a model system to study fundamental mechanisms of transcription. J. Biol. Chem. 2001;276:26733–26736. doi: 10.1074/jbc.R100032200. [DOI] [PubMed] [Google Scholar]
- 10.Mittal V, Hernandez N. Role for the amino-terminal region of human TBP in U6 snRNA transcription. Science. 1997;275:1136–1140. doi: 10.1126/science.275.5303.1136. [DOI] [PubMed] [Google Scholar]
- 11.Schramm L, Pendergrast PS, Sun Y, Hernandez N. DiVerent human TFIIIB activities direct RNA polymerase III transcription from TATA-containing and TATA-less promoters. Genes Dev. 2000;14:2650–2663. doi: 10.1101/gad.836400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhlman TC, Cho H, Reinberg D, Hernandez N. The general transcription factors IIA, IIB, IIF, and IIE are required for RNA polymerase II transcription from the human U1 small nuclear RNA promoter. Mol. Cell. Biol. 1999;19:2130–2141. doi: 10.1128/mcb.19.3.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford E, Hernandez N. Characterization of a trimeric complex containing Oct-1, SNAPC, and DNA. J. Biol. Chem. 1997;272:16048–16055. doi: 10.1074/jbc.272.25.16048. [DOI] [PubMed] [Google Scholar]
- 14.Ford E, Strubin M, Hernandez N. The Oct-1 POU domain activates snRNA gene transcription by contacting a region in the SNAPC largest subunit that bears sequence similarities to the Oct-1 coactivator OBF-1. Genes Dev. 1998;12:3528–3540. doi: 10.1101/gad.12.22.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittal V, Ma B, Hernandez N. SNAP(c): a core promoter factor with a built-in DNA-binding damper that is deactivated by the Oct-1 POU domain. Genes Dev. 1999;13:1807–1821. doi: 10.1101/gad.13.14.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovde S, Hinkley CS, Strong K, Brooks A, Gu L, Henry RW, Geiger J. Activator recruitment by the general transcription machinery: X-ray structural analysis of the Oct-1 POU domain/human U1 octamer/SNAP190 peptide ternary complex. Genes Dev. 2002;16:2772–2777. doi: 10.1101/gad.1021002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry RW, Mittal V, Ma B, Kobayashi R, Hernandez N. SNAP19 mediates the assembly of a functional core promoter complex (SNAPC) shared by RNA polymerases II and III. Genes Dev. 1998;12:2664–2672. doi: 10.1101/gad.12.17.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry RW, Sadowski CL, Kobayashi R, Hernandez N. A TBPTAF complex required for transcription of human snRNA genes by RNA polymerase II and III. Nature. 1995;374:653–656. doi: 10.1038/374653a0. [DOI] [PubMed] [Google Scholar]
- 19.Yoon JB, Roeder RG. Cloning of two proximal sequence element-binding transcription factor subunits (gamma and delta) that are required for transcription of small nuclear RNA genes by RNA polymerases II and III and interact with the TATA-binding protein. Mol. Cell. Biol. 1996;16:1–9. doi: 10.1128/mcb.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadowski CL, Henry RW, Kobayashi R, Hernandez N. The SNAP45 subunit of the small nuclear RNA (snRNA) activating protein complex is required for RNA polymerase II and III snRNA gene transcription and interacts with the TATA box binding protein. Proc. Natl. Acad. Sci.USA. 1996;93:4289–4293. doi: 10.1073/pnas.93.9.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry RW, Ma B, Sadowski CL, Kobayashi R, Hernandez N. Cloning and characterization of SNAP50, a subunit of the snRNA-activating protein complex SNAPC. EMBO J. 1996;15:7129–7136. [PMC free article] [PubMed] [Google Scholar]
- 22.Bai L, Wang Z, Yoon JB, Roeder RG. Cloning and characterization of the beta subunit of human proximal sequence element-binding transcription factor and its involvement in transcription of small nuclear RNA genes by RNA polymerases II and III. Mol. Cell. Biol. 1996;16:5419–5426. doi: 10.1128/mcb.16.10.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong MW, Henry RW, Ma B, Kobayashi R, Klages N, Matthias P, Strubin M, Hernandez N. The large subunit of basal transcription factor SNAPC is a Myb domain protein that interacts with Oct-1. Mol. Cell. Biol. 1998;18:368–377. doi: 10.1128/mcb.18.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma B, Hernandez N. A map of protein-protein contacts within the small nuclear RNA-activating protein complex SNAPC. J. Biol. Chem. 2001;276:5027–5035. doi: 10.1074/jbc.M009301200. [DOI] [PubMed] [Google Scholar]
- 25.Ma B, Hernandez N. Redundant cooperative interactions for assembly of a human U6 transcription initiation complex. Mol. Cell. Biol. 2002;22:8067–8078. doi: 10.1128/MCB.22.22.8067-8078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinkley CS, Hirsch HA, Gu L, LaMere B, Henry RW. The SNAP190 Myb DNA binding domain stimulates TBP-TATA box recognition. J. Biol. Chem. 2003;278:18649–18657. doi: 10.1074/jbc.M204247200. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch HA, Gu L, Henry RW. The retinoblastoma tumor suppressor protein targets distinct general transcription factors to regulate RNA polymerase III gene expression. Mol. Cell. Biol. 2000;20:9182–9191. doi: 10.1128/mcb.20.24.9182-9191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch HA, Jawdekar GW, Lee KA, Gu L, Henry RW. Distinct mechanisms for repression of RNA polymerase III transcription by the Retinoblastoma tumor suppressor protein. Mol. Cell. Biol. 2004;24:5989–5999. doi: 10.1128/MCB.24.13.5989-5999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gridasova AA, Henry RW. The p53 tumor suppressor protein represses human snRNA gene transcription by RNA polymerases II and III independently of sequence-speciWc DNA binding. Mol. Cell. Biol. 2005;25:3247–3260. doi: 10.1128/MCB.25.8.3247-3260.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittal V, Cleary MA, Herr W, Hernandez N. The Oct-1 POU-spe-ciWc domain can stimulate small nuclear RNA gene transcription by stabilizing the basal transcription complex SNAPC. Mol. Cell. Biol. 1996;16:1955–1965. doi: 10.1128/mcb.16.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu L, Esselman WJ, Henry RW. Cooperation between small nuclear RNA-activating protein complex (SNAPC) and TATA-box-binding protein antagonizes protein kinase CK2 inhibition of DNA binding by SNAPC. J. Biol. Chem. 2005;280:27697–27704. doi: 10.1074/jbc.M503206200. [DOI] [PubMed] [Google Scholar]
- 32.Tolia NH, Joshua-Tor L. Strategies for protein coexpression in Escherichia coli. Nat. Methods. 2006;3:55–64. doi: 10.1038/nmeth0106-55. [DOI] [PubMed] [Google Scholar]