Abstract
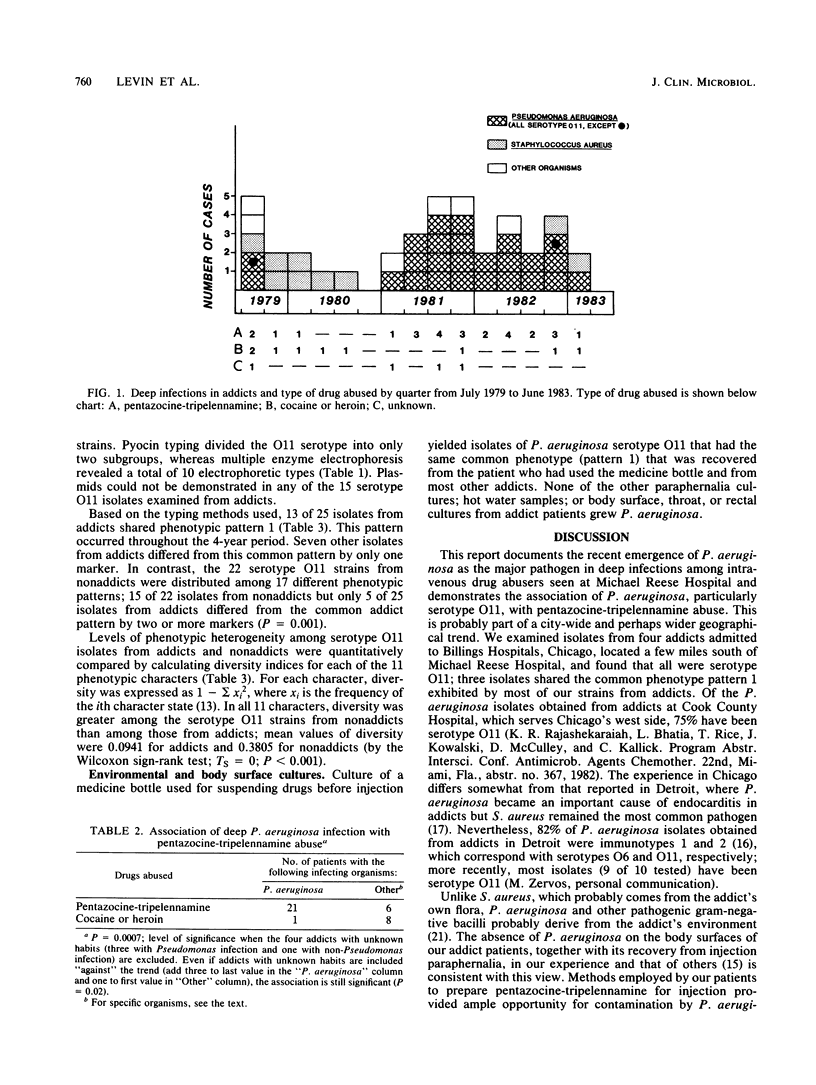
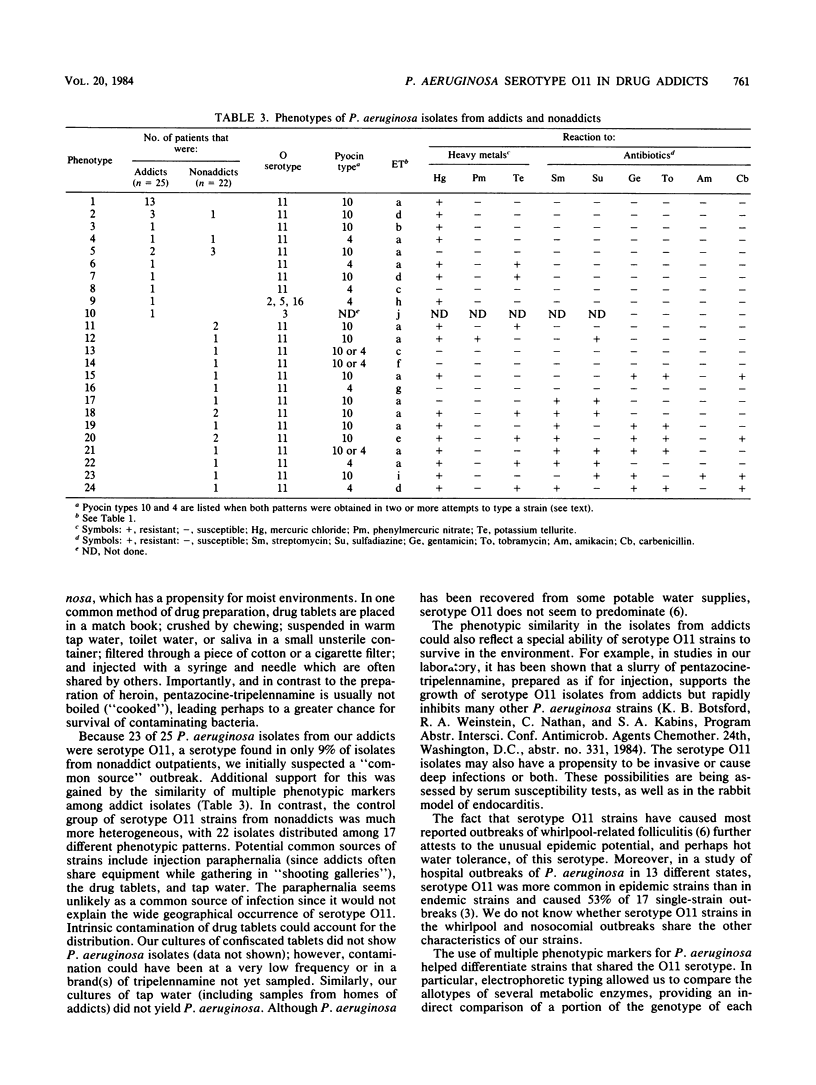
From July 1979 to June 1983, 25 of 40 intravenous drug addicts with systemic infections had Pseudomonas aeruginosa as the etiological agent; by 1982, P. aeruginosa had replaced Staphylococcus aureus as the most common pathogen. At least 21 of the 25 addicts with P. aeruginosa infection abused pentazocine mixed with tripelennamine (commonly known as T's and blues) compared with 6 of 15 addicts infected with other pathogens (P = 0.006). Of the 25 P. aeruginosa isolates, 23 were of serotype O11. Phenotypic patterns in isolates from addicts and in 22 serotype O11 control isolates from nonaddicts were determined by pyocin and electrophoretic enzyme typing, as well as by susceptibility to heavy metals and antibiotics. Of 25 isolates from addicts, 20 were identical or differed by only one marker, whereas the 22 nonaddict serotype O11 isolates were distributed among 17 distinct phenotypic patterns. We postulate that the emergence of P. aeruginosa as the major cause of deep infection in addicts is a consequence of contamination of their paraphernalia during preparation of pentazocine and tripelennamine for self-injection. The phenotypic similarity among isolates from addicts may reflect acquisition from related environmental sources and an unusual ability of certain serotype O11 strains to survive preparation of the drugs or to be invasive.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Selander R. K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics. 1981 Jul;98(3):467–490. doi: 10.1093/genetics/98.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Weinstein R. A., Zierdt C. H., Brokopp C. D. Hospital outbreaks caused by Pseudomonas aeruginosa: importance of serogroup O11. J Clin Microbiol. 1982 Aug;16(2):266–270. doi: 10.1128/jcm.16.2.266-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennewald M., Prevatt W., Meyer R., Shapiro J. Isolation of inc P-2 plasmid DNA from Pseudomonas aeruginosa. Plasmid. 1978 Feb;1(2):164–173. doi: 10.1016/0147-619x(78)90036-7. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Gillies R. R. Further studies in the pyocine typing of Pseudomonas pyocyanea. J Med Microbiol. 1969 Feb;2(1):17–25. doi: 10.1099/00222615-2-1-17. [DOI] [PubMed] [Google Scholar]
- Gustafson T. L., Band J. D., Hutcheson R. H., Jr, Schaffner W. Pseudomonas folliculitis: an outbreak and review. Rev Infect Dis. 1983 Jan-Feb;5(1):1–8. doi: 10.1093/clinids/5.1.1. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Properties of R plasmids determining gentamicin resistance by acetylation in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Sep;6(3):239–252. doi: 10.1128/aac.6.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahmeyer H. W., Steingold R. G. Medical and psychiatric complications of pentazocine and tripelennamine abuse. J Clin Psychiatry. 1980 Aug;41(8):275–278. [PubMed] [Google Scholar]
- Mills J., Drew D. Serratia marcescens endocarditis: a regional illness associated with intravenous drug abuse. Ann Intern Med. 1976 Jan;84(1):29–35. doi: 10.7326/0003-4819-84-1-29. [DOI] [PubMed] [Google Scholar]
- Ochman H., Selander R. K. Evidence for clonal population structure in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(1):198–201. doi: 10.1073/pnas.81.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekaraiah K. R., Rice T. W., Kallick C. A. Recovery of Pseudomonas aeruginosa from syringes of drug addicts with endocarditis. J Infect Dis. 1981 Nov;144(5):482–482. doi: 10.1093/infdis/144.5.482. [DOI] [PubMed] [Google Scholar]
- Reyes M. P., Lerner A. M. Current problems in the treatment of infective endocarditis due to Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):314–321. doi: 10.1093/clinids/5.2.314. [DOI] [PubMed] [Google Scholar]
- Saravolatz L. D., Markowitz N., Arking L., Pohlod D., Fisher E. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann Intern Med. 1982 Jan;96(1):11–16. doi: 10.7326/0003-4819-96-1-11. [DOI] [PubMed] [Google Scholar]
- Savage D., Brown J. Endocarditis due to group A streptococcus. Am J Med Sci. 1981 Nov-Dec;282(3):98–103. doi: 10.1097/00000441-198111000-00001. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Levin B. R. Genetic diversity and structure in Escherichia coli populations. Science. 1980 Oct 31;210(4469):545–547. doi: 10.1126/science.6999623. [DOI] [PubMed] [Google Scholar]
- Stickler D. J., Thomas B., Chawla J. C. Antiseptic and antibiotic resistance in gram-negative bacteria causing urinary tract infection in spinal cord injured patients. Paraplegia. 1981;19(1):50–58. doi: 10.1038/sc.1981.13. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to tellurium compounds. J Bacteriol. 1977 Jan;129(1):276–281. doi: 10.1128/jb.129.1.276-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C. Changing epidemiology and newer aspects of infective endocarditis. Adv Intern Med. 1977;22:21–47. [PubMed] [Google Scholar]


