Abstract
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) is an important tobacco-specific nitrosamine (TSNA) in the etiology of tobacco-related cancers, and N-glucuronidation is an important mechanism of NNAL detoxification. In the present study, an analysis of the UDP-glucuronosyltransferases (UGTs) responsible for the N-glucuronidation of the TSNAs N′-nitrosonornicotine, N′-nitrosoanabasine, and N′-nitrosoanatabine was performed. Using human embryonic kidney 293 cells overexpressing UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B15, and UGT2B17, only UGT1A4 and UGT2B10 exhibited N-glucuronidating activity against these TSNAs. The KMs for UGT2B10 were 15 to 22-fold lower than those of UGT1A4 against the three TSNAs and were similar to those observed for microsomes prepared from human liver specimens. The overall activity of UGT2B10 was 3.6 to 27-fold higher than UGT1A4 against the three TSNAs as determined by Vmax/KM after normalization by levels of UGT2B10 versus UGT1A4 mRNA. Similarly high levels of activity were also observed for UGT2B10 against a fourth TSNA, NNAL, exhibiting a 6.3-fold lower KM and 3-fold higher normalized Vmax/KM than that observed for UGT1A4. Real-time polymerase chain reaction analysis showed that UGT2B10 was expressed at a level that, on average, was 26% higher than that observed for UGT1A4 in a screening of normal liver tissue specimens from 20 individual subjects. These data suggest that UGT2B10 is likely the most active UGT isoform in human liver for the N-glucuronidation of TSNAs.
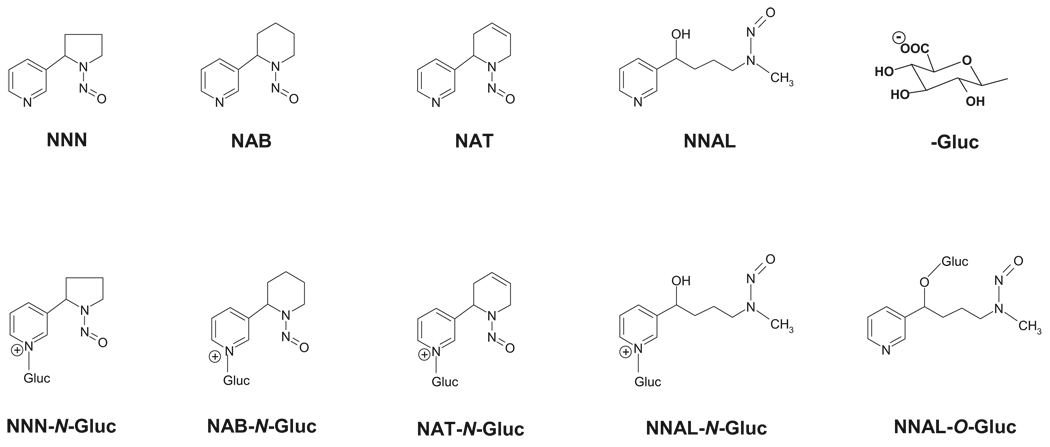
Tobacco-specific nitrosamines (TSNAs) (Fig. 1) are a family of carcinogens present in both tobacco smoke and smokeless tobacco products (Hecht and Hoffmann, 1989; Hecht, 1998; Nowell et al., 1999). The most abundant and potent TSNA is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). The major metabolic pathway for NNK is carbonyl reduction to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) (Carmella et al., 1993), which, like NNK, is a potent lung and pancreatic carcinogen in rodents (Rivenson et al., 1988; Hecht, 1998). It has been estimated that 39 to 100% of NNK is converted to NNAL in cigarette smokers (Carmella et al., 1993). Other abundant TSNAs include N′-nitrosonornicotine (NNN), a potent esophageal carcinogen, N′-nitrosoanabasine (NAB), a weak carcinogen, and N′-nitrosoanatabine (NAT), which is not considered to be carcinogenic (Hecht, 1998). The estimated daily exposure to TSNAs is 20 µg in smokers and 68 µg in smokeless tobacco users (Hoffmann et al., 1994).
FIG. 1.
Schematic of TSNA structures.
The UDP glucuronosyltransferase (UGT) superfamily of enzymes catalyze the glucuronidation of various compounds, including endogenous compounds such as bilirubin and steroid hormones, as well as xenobiotics, including drugs and environmental carcinogens (Tephly and Burchell, 1990; Gueraud and Paris, 1998; Nowell et al., 1999; Ren et al., 2000). Based on protein structure and sequence homology, UGTs are classified into several families and subfamilies, each containing several highly homologous UGT genes. Glucuronidation by the UGT superfamily of enzymes is a major mode of metabolism of NNAL in vivo. Glucuronide conjugates of NNAL and other TSNAs, including NNN, NAB, and NAT, have been identified in the urine of tobacco smokers and smokeless tobacco users, accounting for an average of 59 to 90% of the total NNAL, NNN, NAB, or NAT in urine (Carmella et al., 2002; Stepanov and Hecht, 2005). Unlike NNAL, which causes lung tumors in rats (Rivenson et al., 1988), the glucuronide conjugate of NNAL (NNAL-Gluc) is nontumorigenic after s.c. injection into A/J mice (Upadhyaya et al., 1999). It was also reported that skin fibroblasts from UGT family 1A-deficient rats were more sensitive to NNK-mediated cytotoxicity (Kim and Wells, 1996). These data strongly suggest that glucuronidation is a major mode of detoxification of NNAL and potentially other TSNAs.
Glucuronidation of NNAL can occur at both the carbinol group (NNAL-O-Gluc) and the pyridine ring nitrogen (NNAL-N-Gluc), whereas glucuronidation of NNN, NAB, and NAT occurs exclusively on the nitrogen on the TSNA pyridine ring (Fig. 1). Both NNAL-N-Gluc and NNAL-O-Gluc have been observed in the urine of smokers, with an average of 1:1 in the ratio of these two metabolites observed in individuals (Carmella et al., 2002). A ratio of 1.7:1 in NNAL-N-Gluc/NNAL-O-Gluc formation was observed in vitro in human liver microsomal (HLM) specimens (Wiener et al., 2004a). Previous studies have shown that the hepatic UGT1A9 (Ren et al., 2000), UGT2B7 (Ren et al., 2000), and UGT2B17 (Lazarus et al., 2005) all specifically exhibit O-glucuronidating activity against NNAL, whereas UGT1A4 exclusively catalyzes NNAL-N-Gluc formation (Wiener et al., 2004a). Previous kinetic analysis showed that the KM of NNAL-N-Gluc formation catalyzed by UGT1A4 (15.5 mM) is much higher than that observed for HLM (0.31 mM) (Wiener et al., 2004a), suggesting that UGT isoform(s) other than UGT1A4 may be involved in catalyzing NNAL-N-Gluc formation.
No studies examining the UGTs responsible for the glucuronidation of NNN, NAB, and NAT have as yet been performed, and several UGTs have not been previously screened for activity against NNAL. The goal of the present study was to fully characterize the human UGT(s) responsible for the glucuronidation of these TSNAs. In this report, results are presented indicating that UGT2B10 is a major enzyme responsible for the glucuronidation and detoxification of TSNAs in humans.
Materials and Methods
Chemicals and Materials
14C-UDP-glucuronic acid (UDPGA) (200 mCi/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO). The high-performance liquid chromatography (HPLC) scintillation solution Ecoscint Flow was purchased from National Diagnostics (Atlanta, GA). TSNAs were purchased from Toronto Research Labs (Toronto, ON, Canada), and alamethicin, β-glucuronidase, and bovine serum albumin were from Sigma Aldrich (St. Louis, MO). Dulbecco’s modified Eagle’s medium, Dulbecco’s phosphate-buffered saline (minus calcium chloride and magnesium chloride), fetal bovine serum, penicillin-streptomycin, geneticin (G418), the Platinum Pfx DNA polymerase, and the pcDNA3.1/V5-His-TOPO mammalian expression vector were all obtained from Invitrogen (Carlsbad, CA). The Hotstar polymerase chain reaction (PCR) kit, the RNeasy Mini and Midi kits, and the QIAEX II gel extraction kit were all purchased from Qiagen (Valencia, CA). The bicinchoninic acid protein assay kit was purchased from Pierce (Rockford, IL). The UGT2B10, UGT2B11, UGT1A4, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression assay kits (TaqMan Gene Expression Assays, IDs HS02556282_s1, Hs01894900_gh, Hs0655285_s1, and Hs99999905_m1, respectively) were purchased from Applied Biosystems (Foster City, CA), and all the restriction enzymes were purchased from New England Biolabs (Beverly, MA). PCR primers were purchased from IDT (Coralville, IA). All the other chemicals were purchased from Fisher Scientific (Pittsburgh, PA) unless specified otherwise.
Tissues
The normal human liver tissue specimens used for these studies have been described previously (Wiener et al., 2004a). Bovine liver was purchased from a local supermarket. Liver microsomes were prepared through differential centrifugation as previously described (Coughtrie et al., 1986) and stored (10–20 mg protein/ml) at −80°C. Microsomal protein concentrations were measured using the bicinchoninic acid assay. Total RNA was extracted from cell lines using the RNeasy Midi kit from Qiagen as per manufacturer’s protocols.
Generation of UGT-Overexpressing Cell Lines and Cell Homogenate Preparation
Cells overexpressing wild-type UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UGT1A10, UGT2B4, UGT2B7, UGT2B15, and UGT2B17 have been described previously (Ren et al., 2000; Dellinger et al., 2006; Sun et al., 2006). Cell lines overexpressing UGT2B10 and UGT2B11 were generated as previously described (Dellinger et al., 2006) by reverse transcription-PCR using normal human liver total RNA as previously described. The sense and antisense primers used were 5′-AAGGATGGCTCTGAAATGGACTA-3′ (sense) and 5′-CCAGCTTCAAATCTCAGATATAACTAATCC-3′ (antisense), corresponding to nucleotides −4 to +19 and +1620 to +1591 relative to the UGT2B10 translation start site, and 5′-TGCACCAGGATGACTCTGAAA-3′ (sense) and 5′-CTTGCTGGAATAAACTGAAGTTGTCCT-3′ (antisense), corresponding to −9 to +21 and +1654 to +1628, respectively, relative to the UGT2B11 translation start site (GenBank accession numbers NM_001075 and NM_001073 for UGT2B10 and UGT2B11, respectively). The sequence of the cloned UGT coding region was compared with that described in GenBank for both UGTs, and they were confirmed to be 100% homologous to the respective wild-type UGT sequence. Human embryonic kidney (HEK) 293 cell lines overexpressing UGT2B10 or UGT2B11 were generated by electroporation as previously described (Dellinger et al., 2006). Cell homogenates were prepared by resuspending pelleted cells in Tris-buffered saline (25 mM Tris-HCl, pH 7.4, 138 mM NaCl, 2.7 mM KCl) and subjecting them to three rounds of freeze-thaw before gentle homogenization. Total RNA was extracted using the RNeasy Mini kit from Qiagen as per manufacturer’s protocols.
Glucuronidation Assays
Glucuronidation activities of HLM or cell homogenates from human UGT-overexpressing cell lines toward TSNAs were determined after an initial incubation of HLM (250 µg of protein) or cell homogenate (250 µg of protein) with alamethicin (50 µg/mg protein) for 15 min in an ice bath. Incubations (50 µl) were subsequently performed at 37°C in 50 mM Tris buffer, pH 7.5, 10 mM MgCl2, 4 mM 14C-UGPGA (1 µCi/50 µl reaction), and different concentration of substrate. Titrated stock concentrations of substrate (TSNAs) were used so that equivalent volumes of vehicle (100% methanol) were added to all the incubations (1:100 v/v). To screen for activity for individual UGT-overexpressing cell lines, incubations were performed for up to 18 h, whereas 2-h incubations were performed for initial rate kinetic analyses, a time which was found to be within the linear range of product formation for all the UGTs tested in this study (data not shown). Reactions were terminated by the addition of 50 µl of cold acetonitrile. Protein was then removed by centrifugation at 13,000g for 10 min at 4°C. The acetonitrile in the supernatant was evaporated in a SpeedVac (Thermo Electron Corporation, Waltham, MA) for 10 min.
Samples (50 µl) were analyzed for TSNA glucuronides by HPLC using a Beckman Coulter System Gold 126 Solvent Module HPLC system (Fullerton, CA) equipped with an automatic injector (model 508), a UV detector operated at 254 nm (model 166), and a radioactive flow detector with 1000-µl flow cell (INUS Systems, Tampa, FL). HPLC was performed using a Synergi-Fusion-RP-80 4-µm column (4.6 × 250 mm) (Phenomenex, Torrance, CA) and an Aquasil 5-µm C18 analytical column (4.6 × 250 mm) (Thermo, Bellefonte, PA) in series. The gradient elution conditions were as follows: starting with 100% buffer A (100 mM NH4Ac, pH 5.0) for 5 min, a subsequent linear gradient to 78% buffer B (90% acetonitrile) over 20 min was performed and then maintained at 78% buffer B for 10 min. The elution flow rate was 1 ml/min, and the scintillation solution flow rate was 3 ml/min. The amount of N-glucuronide formed was calculated based on the ratio of the radioactivity of the N-glucuronide versus total radioactivity. TSNA-N-glucuronides were confirmed by 1 M NaOH hydrolysis and sensitivity to β-glucuronidase as previously described (Upadhyaya et al., 1999). As controls, glucuronidation assays were regularly performed using HLM as a positive control for glucuronidation activity and untransfected HEK293 cell homogenate protein as a negative control for glucuronidation activity. Experiments were always performed in triplicate in independent assays.
Analysis of TSNA Glucuronides Obtained from Bovine Liver Microsomes
To obtain pure NNN, NAB, and NAT glucuronides for NMR analysis, bovine liver microsomes (5 mg protein/1 ml reaction) were used to catalyze TSNA glucuronidation reactions. The predicted glucuronide conjugates of NNN, NAB, and NAT were purified by HPLC using the same system as described above with a modified gradient program. For NNN, a gradient of 2% acetonitrile/98% buffer A was used for 10 min, increased to 78% buffer B in 20 min, and then maintained at 78% buffer B for 10 min. For NAB and NAT, 4% buffer B/96% buffer A was used for 15 min, increased to 78% B in 10 min, and then maintained in 78% for 10 min. TSNA collections were desalinated by a second purification using the gradient programs described above except that water was used instead of buffer A. Purity of the collected fraction was confirmed by HPLC analysis. Collections were dried in a SpeedVac (Thermo Electron Corporation), and the NMR spectra of unmodified and modified NNN, NAB, and NAT were acquired with a 300-MHz Bruker (Newark, DE) spectrometer.
NAB (300 MHz, D2O), 8.35 (d, 1H, 2-E), 8.34 (m, 1H, 6-E), 8.28 (m, 1H 6-Z), 8.12 (d, 1H, 2-Z), 7.68 (dt, 1H, 4-E), 7.33 (dd, 1H, 5-E), 7.44 (dt, 1H, 4-Z), 7.28 (dd, 1H, 5-Z), 6.12 (m, 1H, 2′-E), 5.60 (m, 1H, 2′-Z), 4.58 (m, 1H, 6′-E), 4.0 (m, 1H, 6′-E), 3.66 (m, 1H, 6′-Z), 3.42 (m, 1H, 6′-Z), 2.4 to 2.2 (m, 4H, 3′-E and -Z), 2.05 (m, 1H, 5′-E or -Z), 1.8 to 1.4 (m, 7H 5′-E or -Z, 4′-E and -Z).
NAB-Gluc (300 MHz, D2O), 8.92 (m, 2H, 2-E and 6-E), 8.65 (m, 1H, 6-Z), 8.54 (m, 1H, 2-Z), 8.22 (m, 2H, 4-E and -Z), 8.03 (m, 2H, 5-E and -Z), 6.18 (m, 1H, 2′-E or -Z), 5.7 (m, 1H, 2′-E or -Z), 5.65 (m, 2H, 1″), 3.94 (m, 1H, 6′-E), 3.8 to 3.4 (m, 8H, 2″, 3″, 4″, and 5″), 3 (m, 1H,), 2.25 (m, 1H, 3′-E or -Z), 2.2 (m, 1H, 3′-E or -Z), 2 to 1.5 (m, 8H, 4′, 5′).
NAT (300 MHz, D2O), 8.36 (d, 1H, 2-E), 8.32 (dd, 1H, 6-E), 8.28 (dd, 0.2H, 5, 6-Z), 8.24 (d, 0.25H, 2-Z), 7.69 (m, 1H, 4-E), 7.59 (m, 0.25H, 4-Z), 7.30 (dd, 1H, 5-E), 7.25 (dd, 0.25H, 5-Z), 6.31 (m, 0.25H, 5′-Z), 6.0 (t, 1H, 5′-E), 5.9 (m, 1H, 2′-E), 5.75 (m, 0.25H, 2′-Z), 5.58 (m, 1.25H, 4′-E and -Z), 4.2 (m, 0.5H, 6′-Z), 3.49 (m, 2H, 6′-E), 2.84 (m, 1.25H, 3′-E and -Z).
NAT-Gluc (300 MHz, D2O), 8.9 (m, 2H, 2 and 6), 8.5 (m, 1H, 4), 8.01 (m, 1H, 5), 6.21 (m, 1H, 5′), 5.9 (m, 2H, 2′ and 4′), 5.64 (m, 1H, 1″), 3.9 to 3.4 (m, 4H, 2″, 3″, 4″, and 5″), 3.05 to 2.85 (m, 2H, 3′).
NNN (300 MHz, D2O), 8.35 (m, 1.6H, 2-E), 8.33 (m, 1H, 6-E), 8.24 (m, 1H, 6-Z), 8.16 (m, 1.6H, 2-Z), 7.64 (dt, 1.6H, 4-E), 7.43 (dt, 1H, 4-Z), 7.32 (q, 1.6H, 5-E), 7.24 (q, 1H, 5-Z), 5.52 (t, 1.6H, 2′-E), 5.13 (t, 1H, 2′-Z), 4.5 to 4.3 (m, 2H, 5′-Z), 3.8 to 3.55 (m, 3.2H, 5′-E), 2.5 to 2.38 (m, 2H, 3′-E and -Z), 2.1 to 2.0 (m, 3.2H, 4′-E and -Z).
NNN-Gluc (300 MHz, D2O), 9.04 (m, 1H, 2-E), 8.92 (m, 1H, 6-E), 8.83 (m, 1H, 6-Z), 8.81 (m, 1H, 2-Z), 8.50 (m, 1H, 4-E), 8.27 (m, 1H, 4-Z), 8.04 (m, 1H, 5-E), 7.95 (m, 1H, 5-Z), 5.7 (m, 1H, 2′-E), 5.65 (m, 2H, 1″), 5.28 (m, 1H, 2′-Z), 4.55 (m, 1H, 5′-Z), 4 to 3.4 (m, 9H, 5′-E, 2″, 3″, 4″, and 5″), 2.6 (m, 2H, 3′), 2.0 (m, 6H, 3′ and 4′).
Analysis of UGT2B10 versus UGT1A4 Expression Levels
To examine the relative expression of UGT2B10, UGT2B11, and UGT1A4 in newly developed HEK293-overexpressing cell lines or in 20 human liver specimens, real-time PCR was performed using the TaqMan Gene Expression Assay kit from Applied Biosystems (IDs Hs02556282_s1, Hs01894900_gh, and Hs0655285_s1 for UGT2B10, UGT2B11, and UGT1A4, respectively, and Hs99999905_m1 for GAPDH as a control) as per manufacturer’s protocols. Real-time PCR was performed using a 10-µl total reaction volume containing 40 ng of reverse transcription-PCR cDNA using GAPDH as the normalizing “housekeeping” gene for expression, with triplicates performed for each sample.
Statistical Analysis
Kinetic constants were determined using Prism version 4.01 software (GraphPad Software, San Diego, CA).
Results
Characterization of NNN, NAB, and NAT Glucuronides in HLM
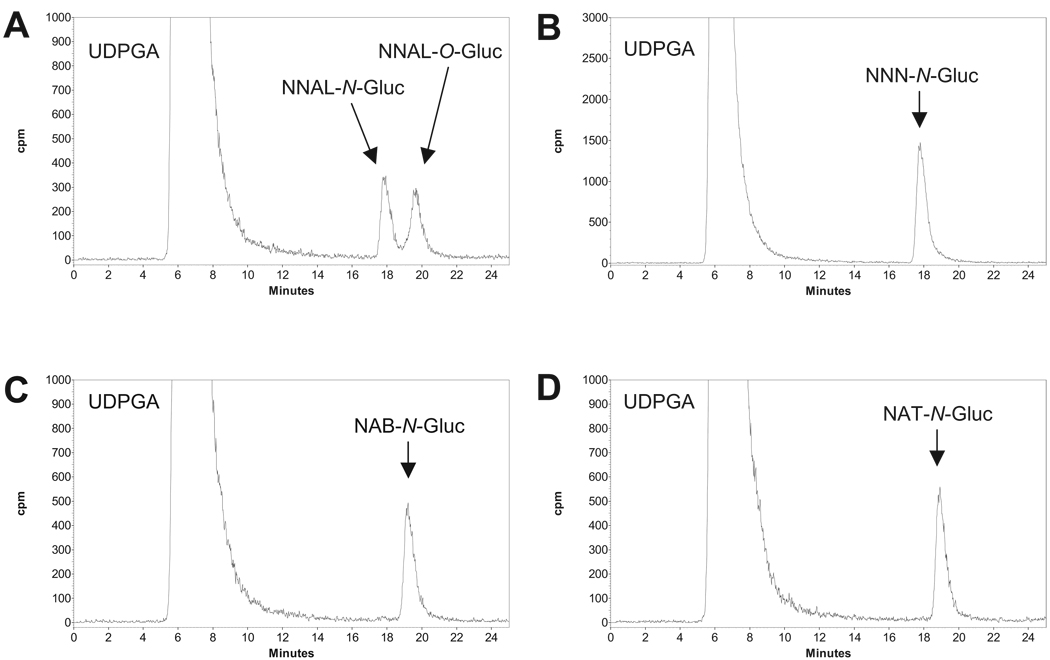
As shown previously for NNAL (Ren et al., 2000; Wiener et al., 2004b), TSNA-glucuronide formation was observed for NNN, NAB, and NAT in HLM as determined by HPLC analysis, with postulated glucuronide peaks observed at retention times of 17.8, 19.2, and 18.9 min, respectively (Fig. 2). Collection of HPLC peaks predicted to correspond to the glucuronide conjugates of NNN, NAB, and NAT was sensitive to treatment with either β-glucuronidase and 1 M NaOH (data not shown), suggesting that these glucuronides corresponded to N-glucuronides as observed previously for NNAL (Wiener et al., 2004a). To better characterize the HPLC peaks corresponding to predicted TSNA glucuronides, the 1H NMR spectra were obtained for the putative glucuronides. For these analyses, large microsomal preparations were made from bovine liver (30 g of tissue) and used in assays identical to those performed for HLM as described above, with the HPLC peaks predicted to contain each of the TSNA glucuronides collected and analyzed by NMR spectroscopy. Bovine liver was used because there was insufficient human liver available for synthesis of the large quantities (microgram amounts) of TSNA glucuronides required for NMR analysis. Predicted TSNA-glucuronide HPLC peaks were observed for bovine liver microsomes with retention times and sensitivities to β-glucuronidase or 1 M NaOH that were identical to that observed for HLM (data not shown). The NMR spectra of the nitrosamines show variable amounts of the E- and Z-isomers of the nitroso group; NNN had a 3:2 ratio, whereas its corresponding glucuronide had a 1:1 ratio of the E- and Z-isomers. Both NAB and NAB-Gluc showed equal amounts of the E- and Z-isomers, whereas NAT had a 4:1 ratio of the E- and Z-isomers. The small amount of NAT-Gluc that was isolated did not allow the observation of the Z-isomer. The 1H NMR spectra of NNN, NAB, and NAT were compared with the isolated glucuronides to determine the site of glucuronidation. The key changes in the NMR spectra resulting from the glucuronidation were in the chemical shifts of the pyridyl group. The NNN, NAB, and NAT pyridyl protons were shifted downfield 0.4 to 0.7 ppm in the glucuronides. This chemical shift difference is consistent with glucuronidation of the pyridyl nitrogen as previously shown for NNK and NNN (Upadhyaya et al., 2001).
FIG. 2.
HPLC analysis of NNAL-Gluc, NNN-Gluc, NAB-Gluc, and NAT-Gluc formation by human liver microsomes. Human liver microsomes (0.5 mg of protein) were incubated at 37°C for 2 h with 4 mM 14C-UDPGA and 5 mM TSNA. HPLC was performed as described under Materials and Methods. A, NNAL; B, NNN; C, NAB; D, NAT. TSNA-glucuronide and UDPGA peaks are indicated.
TSNA Glucuronidation in UGT-Overexpressing Cells
Real-time PCR showed that UGT2B10 and UGT2B11 mRNA were expressed at 0.075 ± 0.006- and 0.179 ± 0.028-fold the levels of GAPDH mRNA in the newly constructed UGT2B10- and UGT2B11-overexpressing cells, respectively. Homogenates of these and previously described UGT-overexpressing cell lines were used in assays to identify the specific UGTs that exhibited N-glucuronidation activity against TSNAs in vitro (Table 1). As described previously for NNAL (Wiener et al., 2004a), UGT1A4 was active against NNN, NAB, and NAT in the present study. In addition to UGT1A4, UGT2B10 was also found to be active against NNN, NAB, and NAT. No other UGT screened in this study (including UGT2B11) exhibited detectable N-glucuronidating activity against any of these TSNAs. All the HPLC peaks identified for UGT1A4 and UGT2B10 against these TSNAs exhibited the same retention times as that observed for the respective TSNA-N-glucuronide formed with HLM (or bovine liver microsomes) and exhibited sensitivity to β-glucuronidase and 1 M NaOH (data not shown), suggesting N-glucuronide formation for these enzymes. Because NNAL is N-glucuronidated like other TSNAs, homogenates from UGT2B10-overexpressing cells were also tested for activity against this TSNA. Similar to that observed previously for UGT1A4, UGT2B10 also exhibited N-glucuronidation activity against NNAL (Table 1). The retention time for the NNAL glucuronide peak observed for UGT2B10 homogenates was identical to that observed for UGT1A4 and exhibited sensitivity to β-glucuronidase and 1 M NaOH (data not shown). No activity was observed for homogenates of UGT2B11-overexpressing cells against NNAL.
TABLE 1.
N-Glucuronide formation activity of individual UGT-overexpressing HEK293 cell lines against TSNAs
| UGT | TSNA | |||
|---|---|---|---|---|
| NNN | NAB | NAT | NNAL | |
| UGT1A1 | −a | − | − | −b |
| UGT1A3 | − | − | − | −b |
| UGT1A4 | + | + | + | +c |
| UGT1A6 | − | − | − | −b |
| UGT1A7 | − | − | − | −b |
| UGT1A8 | − | − | − | −b |
| UGT1A9 | − | − | − | −b |
| UGT1A10 | − | − | − | −b |
| UGT2B4 | − | − | − | −b |
| UGT2B7 | − | − | − | −b |
| UGT2B10 | + | + | + | + |
| UGT2B11 | − | − | − | − |
| UGT2B15 | − | − | − | −b |
| UGT2B17 | − | − | − | −d |
Not detected.
No N-glucuronidation activity was observed against NNAL for the indicated UGTs in previous studies by Wiener et al. (2004a) and
UGT1A4 exhibited N-glucuronidation activity against NNAL in previous studies by Wiener et al. (2004a) and in the present study.
Kinetic Analysis of NNAL, NNN, NAB, and NAT Glucuronidation by UGT1A4, UGT2B10, and HLM
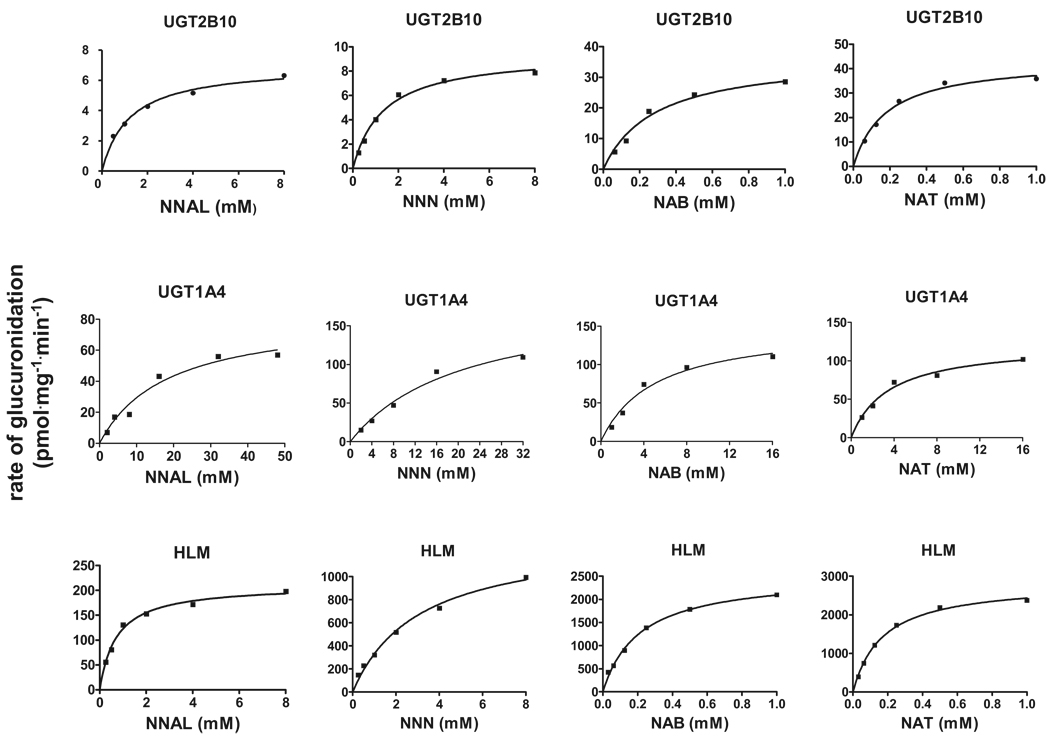
Kinetic analysis was performed for UGT1A4, UGT2B10, and HLM against NNAL, NNN, NAB, and NAT. Representative plots of glucuronidation rate versus substrate concentration are shown in Fig. 3. As summarized in Table 2, UGT2B10 exhibited between 6- and 22-fold lower KMs against NNN, NAB, and NAT than UGT1A4. The KMs of HLM were comparable with those observed with UGT2B10 against NAB and NAT and 3.7-fold lower than HLM against NNN and approximately 4-fold higher than HLM against NNAL.
FIG. 3.
Concentration curves for NNAL-, NNN-, NAB-, and NAT-N-glucuronide formation with homogenates from UGT1A4- and UGT2B10-overexpressing cells and HLM from one individual. Glucuronide formation assays were performed at 37°C for 2 h using 250 µg of cell homogenate or HLM protein as described under Materials and Methods. Representative curves are shown, and kinetic analysis was performed in three independent experiments.
TABLE 2.
Kinetic analysis of N-glucuronidation in UGT1A4- and UGT2B10-overexpressing cell homogenates and HLMs of TSNAs
| TSNA | Vmax | KM | Vmax/KM | Normalized Vmax/KM UGT2B10/UGT1A4 Ratioa |
|
|---|---|---|---|---|---|
| pmol/min/mg total protein | mM | nl/min/mg total protein | |||
| UGT1A4 | NNAL | 99 ± 27b | 22.79 ± 3.83 | 4.35 ± 0.96 | Referent |
| NNN | 182 ± 43 | 26.24 ± 14.27 | 7.70 ± 2.13 | Referent | |
| NAB | 166 ± 22 | 5.37 ± 1.61 | 32.1 ± 6.63 | Referent | |
| NAT | 124 ± 4 | 3.31 ± 0.23 | 37.6 ± 2.31 | Referent | |
| UGT2B10 | NNAL | 7.55 ± 0.37 | 3.62 ± 2.31 | 3.07 ± 2.13 | 3.0 |
| NNN | 7.66 ± 1.63 | 1.18 ± 0.27 | 6.60 ± 1.08 | 3.6 | |
| NAB | 41.09 ± 11.28 | 0.28 ± 0.00 | 147 ± 42.0 | 19 | |
| NAT | 52.65 ± 14.99 | 0.22 ± 0.06 | 242 ± 2.86 | 27 | |
| HLMc | NNAL | 259 ± 42 | 0.85 ± 0.42 | 348 ± 163 | |
| NNN | 1400 ± 60 | 3.17 ± 0.37 | 446 ± 61 | ||
| NAB | 2476 ± 621 | 0.20 ± 0.03 | 12,806 ± 5069 | ||
| NAT | 2593 ± 457 | 0.16 ± 0.01 | 16,227 ± 2852 |
Vmax/KM normalized by mRNA expression levels in UGT1A4- and UGT2B10-overexpressing HEK293 cell lines using GAPDH as an expression control.
Data are expressed as the mean ± S.D. of three independent experiments.
HLM from one individual.
As the Vmax data reported in Table 2 were calculated based on total cell homogenate or HLM protein and were not normalized for UGT protein, direct comparisons of Vmax were not performed between UGT-overexpressing cell lines or with HLM. However, real-time PCR of UGT2B10 versus UGT1A4 expression analysis was performed for their respective HEK293 UGT-overexpressing lines, showing that UGT1A4 was expressed at the level of mRNA at a relative level that was 4 times higher than that observed for UGT2B10 in their respective overexpressing cell lines. Using this ratio as a normalization factor, UGT2B10 exhibited 3 to 3.6-fold higher overall activity against NNAL and NNN than UGT1A4, and a 19 to 27-fold higher level of overall activity than UGT1A4 against NAB and NAT.
Assessment of UGT1A4 and UGT2B10 Expression in Human Liver
To better assess the relative importance of UGT2B10 versus UGT1A4 in the N-glucuronidation of TSNAs in human liver, real-time PCR was performed using total RNA purified from 20 human liver specimens obtained from individual subjects. Relative to the expression of a housekeeping gene (GAPDH), the expression of UGT2B10 was 1.3-fold that observed for UGT1A4 for the 20 specimens. The range of expression of UGT2B10 and UGT1A4 between liver specimens was 14- and 13-fold, respectively. The relative expression of UGT2B10 versus UGT1A4 ranged from 0.16 to 5.8 for the 20 specimens analyzed, with 85% (n = 17) of the samples analyzed exhibiting a UGT2B10/UGT1A4 ratio of >0.50 and 55% (n = 11) exhibiting a ratio of >1.0 (results not shown).
Discussion
Glucuronidation is a major pathway for the elimination of NNAL and other TSNAs. Both the O- and N-glucuronide forms of NNAL-Gluc have been observed in the urine of smokers (Carmella et al., 1995, 2002; Richie et al., 1997), never-smokers exposed to environmental tobacco smoke (Carmella et al., 1993; Parsons et al., 1998), and tobacco chewers (Murphy et al., 1994; Carmella et al., 2002), and both glucuronides were shown to be formed in HLM (Kim and Wells, 1996; Ren et al., 2000; Wiener et al., 2004a,b; Lazarus et al., 2005). Glucuronide conjugates comprise between 59 and 90% of the total NNAL, NNN, NAB, and NAT found in the urine of smokers (Carmella et al., 2002; Stepanov and Hecht, 2005). The importance of glucuronidation as a detoxification mechanism for NNAL and potentially other TSNAs is suggested by the fact that, in contrast to the relatively high tumorigenicity exhibited by NNAL, NNAL-Gluc is nontumorigenic after s.c. injection into A/J mice (Upadhyaya et al., 1999), and skin fibroblasts from UGT family 1-deficient rats with decreased glucuronidation capacity were more sensitive to NNK-mediated cytotoxicity (Kim and Wells, 1996).
The present study is the first to examine the activity of specific UGT enzymes against the TSNAs NNN, NAT, and NAB, with both UGT1A4 and UGT2B10 exhibiting glucuronidation activity against all the TSNAs tested. Whereas UGT1A4 was previously shown to exhibit N-glucuronidation activity against NNAL (Wiener et al., 2004a), the present study is also the first to show N-glucuronidation activity against NNAL for UGT2B10. The N-glucuronide formation activity exhibited by UGT2B10 against TSNAs is consistent with the recent finding that UGT2B10 exhibits glucuronidation activity against both nicotine and cotinine (Chen et al., 2007). However, the glucuronidation activity observed for UGT2B10 against NNAL in the present study was not observed in a previous study (Wiener et al., 2004a). This may be because of differences in glucuronidation assay sensitivities because several differences in the glucuronidation assay methodology used between the two studies were identified, including the use of alamethicin, which increased glucuronidation activity 2 to 3-fold in in vitro assay systems (Fisher et al., 2000), in the current study.
The KM exhibited by homogenates of UGT2B10-overexpressing cells was significantly lower than that observed for homogenates from UGT1A4-overexpressing cells against all the TSNAs tested in the present study. This is similar to the differential kinetic pattern observed previously for UGT2B10 versus UGT1A4 against nicotine and cotinine (Chen et al., 2007; Kaivosaari et al., 2007). The mean relative mRNA expression level of UGT2B10 versus UGT1A4 was 1.3 in a series of normal human liver specimens, with 85% of the samples analyzed exhibiting a UGT2B10/UGT1A4 ratio of >0.50 and 55% exhibiting a ratio of >1.0. Because the overall activity of UGT2B10 in cell homogenate as determined by Vmax/KM after normalization for UGT expression levels was 3 to 27-fold higher for UGT2B10 than UGT1A4 against all the TSNAs tested, these data suggest that UGT2B10 is the major N-glucuronidating enzyme for TSNAs in human liver and are consistent with the fact that the KM of UGT2B10-overexpressing cell homogenates was similar to that observed for HLM against the same TSNAs.
The KM observed for UGT2B10 against NNAL (3.6 mM) was comparable with that observed previously for the most active NNAL-O-glucuronidating enzyme, UGT2B17 (1.76 mM) (Lazarus et al., 2005). Previous studies have shown that UGT2B10 is expressed at a 4-fold higher level than UGT2B17 in human liver (Nishimura and Naito, 2006). UGT2B10 is also well expressed in target sites for tobacco-related cancers including lung (Strassburg et al., 1997; Turgeon et al., 2001; Nishimura and Naito, 2006). Together, these data suggest that UGT2B10 is a major enzyme responsible for the glucuronidation and detoxification of NNAL and potentially other TSNAs in humans.
Acknowledgments
We thank the Tissue Procurement Facility at the H. Lee Moffitt Cancer Center for the human tissues examined in these studies. We also thank the Functional Genomics Core Facility at the Penn State College of Medicine for real-time PCR services.
These studies were supported by Public Health Service Grants P01-CA68384 (P.L.), R01-DE13158 (P.L.), and R01-CA75074 (T.E.S.) from the National Institutes of Health and two formula grants under the Pennsylvania Department of Health’s Health Research Formula Funding Program (State of PA, Act 2001-77, part of the PA Tobacco Settlement Legislation; P.L., T.E.S.).
Abbreviations
- TSNA
tobacco-specific nitrosamine
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- NNN
N′-nitrosonornicotine
- NAB
N′-nitrosoanabasine
- NAT
N′-nitrosoanatabine
- UGT
UDP glucuronosyltransferase
- NNAL-Gluc
glucuronide conjugate of NNAL
- HLM
human liver microsome
- UDPGA
UDP-glucuronic acid
- HPLC
high-performance liquid chromatography
- PCR
polymerase chain reaction
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HEK
human embryonic kidney
References
- Carmella SG, Akerkar S, Hecht SS. Metabolites of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers’ urine. Cancer Res. 1993;53:721–724. [PubMed] [Google Scholar]
- Carmella SG, Akerkar SA, Richie JP, Jr, Hecht SS. Intraindividual and interindividual differences in metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers’ urine. Cancer Epidemiol Biomarkers Prev. 1995;4:635–642. [PubMed] [Google Scholar]
- Carmella SG, Le Ka KA, Upadhyaya P, Hecht SS. Analysis of N- and O-glucuronides of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Chem Res Toxicol. 2002;15:545–550. doi: 10.1021/tx015584c. [DOI] [PubMed] [Google Scholar]
- Chen G, Blevins-Primeau AS, Dellinger RW, Muscat JE, Lazarus P. Glucuronidation of nicotine and cotinine by UGT2B10: loss of function by the UGT2B10 codon 67 (Asp>Tyr) polymorphism. Cancer Res. 2007;67:9024–9029. doi: 10.1158/0008-5472.CAN-07-2245. [DOI] [PubMed] [Google Scholar]
- Coughtrie MW, Burchell B, Bend JR. A general assay for UDP glucuronosyltransferase activity using polar amino-cyano stationary phase HPLC and UDP[U-14C]glucuronic acid. Anal Biochem. 1986;159:198–205. doi: 10.1016/0003-2697(86)90328-3. [DOI] [PubMed] [Google Scholar]
- Dellinger RW, Fang JL, Chen G, Weinberg R, Lazarus P. Importance of UDP-glucuronosyltransferase 1A10 (UGT1A10) in the detoxification of polycyclic aromatic hydrocarbons: decreased glucuronidative activity of the UGT1A10139Lys isoform. Drug Metab Dispos. 2006;34:943–949. doi: 10.1124/dmd.105.009100. [DOI] [PubMed] [Google Scholar]
- Fisher MB, Campanale K, Ackermann BL, VandenBranden M, Wrighton SA. In vitro glucuronidation using human liver microsomes and the pore-forming peptide alamethicin. Drug Metab Dispos. 2000;28:560–566. [PubMed] [Google Scholar]
- Guéraud F, Paris A. Glucuronidation: a dual control. Gen Pharmacol. 1998;31:683–688. doi: 10.1016/s0306-3623(98)00114-1. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Hoffmann D. The relevance of tobacco-specific nitrosamines to human cancer. Cancer Surv. 1989;8:273–294. [PubMed] [Google Scholar]
- Hoffmann D, Brunnemann KD, Prokopczyk B, Djordjevic MV. Tobacco-specific N-nitrosamines and Areca-derived N-nitrosamines: chemistry, biochemistry, carcinogenicity, and relevance to humans. J Toxicol Environ Health. 1994;41:1–52. doi: 10.1080/15287399409531825. [DOI] [PubMed] [Google Scholar]
- Kaivosaari S, Toivonen P, Hesse LM, Koskinen M, Court MH, Finel M. Nicotine glucuronidation and the human UDP-glucuronosyltransferase UGT2B10. Mol Pharmacol. 2007;72:761–768. doi: 10.1124/mol.107.037093. [DOI] [PubMed] [Google Scholar]
- Kim PM, Wells PG. Genoprotection by UDP-glucuronosyltransferases in peroxidase-dependent, reactive oxygen species-mediated micronucleus initiation by the carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyreneq. Cancer Res. 1996;56:1526–1532. [PubMed] [Google Scholar]
- Lazarus P, Zheng Y, Aaron RunkleE, Muscat JE, Wiener D. Genotype-phenotype correlation between the polymorphic UGT2B17 gene deletion and NNAL glucuronidation activities in human liver microsomes. Pharmacogenet Genomics. 2005;15:769–778. doi: 10.1097/01.fpc.0000175596.52443.ef. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Carmella SG, Idris AM, Hoffmann D. Uptake and metabolism of carcinogenic levels of tobacco-specific nitrosamines by Sudanese snuff dippers. Cancer Epidemiol Biomarkers Prev. 1994;3:423–428. [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet. 2006;21:357–374. doi: 10.2133/dmpk.21.357. [DOI] [PubMed] [Google Scholar]
- Nowell SA, Massengill JS, Williams S, Radominska-Pandya A, Tephly TR, Cheng Z, Strassburg CP, Tukey RH, MacLeod SL, Lang NP, et al. Glucuronidation of 2-hydroxyamino-1-methyl-6-phenylimidazo[4, 5-b]pyridine by human microsomal UDP-glucuronosyltransferases: identification of specific UGT1A family isoforms involved. Carcinogenesis. 1999;20:1107–1114. doi: 10.1093/carcin/20.6.1107. [DOI] [PubMed] [Google Scholar]
- Parsons WD, Carmella SG, Akerkar S, Bonilla LE, Hecht SS. A metabolite of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the urine of hospital workers exposed to environmental tobacco smoke. Cancer Epidemiol Biomarkers Prev. 1998;7:257–260. [PubMed] [Google Scholar]
- Ren Q, Murphy SE, Zheng Z, Lazarus P. O-Glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) by human UDP-glucuronosyltransferases 2B7 and 1A9. Drug Metab Dispos. 2000;28:1352–1360. [PubMed] [Google Scholar]
- Richie JP, Jr, Carmella SG, Muscat JE, Scott DG, Akerkar SA, Hecht SS. Differences in the urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in black and white smokers. Cancer Epidemiol Biomarkers Prev. 1997;6:783–790. [PubMed] [Google Scholar]
- Rivenson A, Hoffmann D, Prokopczyk B, Amin S, Hecht SS. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res. 1988;48:6912–6917. [PubMed] [Google Scholar]
- Stepanov I, Hecht SS. Tobacco-specific nitrosamines and their pyridine-Nglucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers Prev. 2005;14:885–891. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Oldhafer K, Manns MP, Tukey RH. Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol Pharmacol. 1997;52:212–220. doi: 10.1124/mol.52.2.212. [DOI] [PubMed] [Google Scholar]
- Sun D, Chen G, Dellinger RW, Duncan K, Fang JL, Lazarus P. Characterization of tamoxifen and 4-hydroxytamoxifen glucuronidation by human UGT1A4 variants. Breast Cancer Res. 2006;8:R50. doi: 10.1186/bcr1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly TR, Burchell B. UDP-glucuronosyltransferases: a family of detoxifying enzymes. Trends Pharmacol Sci. 1990;11:276–279. doi: 10.1016/0165-6147(90)90008-v. [DOI] [PubMed] [Google Scholar]
- Turgeon D, Carrier JS, Levesque E, Hum DW, Belanger A. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology. 2001;142:778–787. doi: 10.1210/endo.142.2.7958. [DOI] [PubMed] [Google Scholar]
- Upadhyaya P, Kenney PM, Hochalter JB, Wang M, Hecht SS. Tumorigenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol enantiomers and metabolites in the A/J mouse. Carcinogenesis. 1999;20:1577–1582. doi: 10.1093/carcin/20.8.1577. [DOI] [PubMed] [Google Scholar]
- Upadhyaya P, McIntee EJ, Hecht SS. Preparation of pyridine-N-glucuronides of tobacco-specific nitrosamines. Chem Res Toxicol. 2001;14:555–561. doi: 10.1021/tx000262e. [DOI] [PubMed] [Google Scholar]
- Wiener D, Doerge DR, Fang JL, Upadhyaya P, Lazarus P. Characterization of N-glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human liver: importance of UDP-glucuronosyltransferase 1A4. Drug Metab Dispos. 2004a;32:72–79. doi: 10.1124/dmd.32.1.72. [DOI] [PubMed] [Google Scholar]
- Wiener D, Fang JL, Dossett N, Lazarus P. Correlation between UDP-glucuronosyltransferase genotypes and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone glucuronidation phenotype in human liver microsomes. Cancer Res. 2004b;64:1190–1196. doi: 10.1158/0008-5472.can-03-3219. [DOI] [PubMed] [Google Scholar]