Abstract
As an alternative to functional magnetic resonance imaging (fMRI) with blood oxygenation level dependent (BOLD) contrast, cerebral blood volume (CBV)-weighted fMRI with intravascular contrast agents in animal models have become popular. In this study, dynamic measurements of CBV were performed by magnetic resonance imaging (MRI) and laser-Doppler flowmetry (LDF) in α-chloralose anesthetized rats during forepaw stimulation. All recordings were localized to the contralateral primary somatosensory cortex as revealed by BOLD at 11.7 T. Ultra-small superparamagnetic iron oxide (15 mg/kg)—a plasma-borne MRI contrast agent with a half-life of several hours in blood circulation—was used to quantify changes in magnetic field inhomogeneity in blood plasma. The LDF backscattered laser light (805 nm), which reflects the amount of red blood cells, was used to measure alterations in the non-plasma compartment. Dynamic and layer-specific comparisons of the two CBV signals during functional hyperemia revealed excellent correlations (>0.86). These results suggest that CBV measurements from either compartment may be used to reflect dynamic changes in total CBV. Furthermore, by assuming steady-state mass balance and negligible counter flow, these results indicate that volume hematocrit is not appreciably affected during functional activation.
Keywords: biosensor, blood flow, energetics, hematocrit, metabolism, nitric oxide
Introduction
Non-invasive mapping of brain activation by functional magnetic resonance imaging (fMRI) provides relatively high spatiotemporal resolution with fairly large coverage of the brain. Because of technological advances, fMRI can now be conducted with many different contrasts because the magnetic resonance imaging (MRI) signal can be made sensitive to changes in blood oxygenation (BOLD), blood flow (CBF), and blood volume (CBV). Although the BOLD contrast is the favored fMRI technique, CBF and CBV approaches are finding wider appeal because these methods are affected less by complicating factors that contribute to BOLD (Ogawa et al, 1993).
As the earliest fMRI maps of human brain function with CBV contrast using repeated bolus injections of a paramagnetic contrast agent that has a relatively short half-life in blood circulation (Belliveauet al, 1991), intravascular paramagnetic contrast agents that maintain a steady blood concentration over several hours have allowed CBV measurements in animals with much better spatiotemporal resolution (Kennanet al, 1998). Because these MRI contrast agents are solely plasma borne (Mandevilleet al, 1998), concerns remain about whether these signals reflect changes in total CBV.
Toward that end, in α-chloralose anesthetized rats during forepaw stimulation, we carried out dynamic CBV measurements by MRI using a superparamagnetic iron oxide contrast agent and compared these transients to CBV measured by laser-Doppler flowmetry (LDF). Using the LDF backscattered laser light to reflect the amount of red blood cells (Barfodet al, 1997), we assessed volume alterations in the cellular compartment. We found excellent correlations between CBV dynamics in plasma (CBVplasma) and red blood cell (CBVrbc) at different depths of the cortex in the contralateral somatosensory region (S1), suggesting that either of these measures may be used for transients in total CBV (CBVtotal). Issues of CBV quantification for understanding the BOLD contrast are discussed.
Materials and methods
Animal Preparation
Sprague–Dawley rats (n=18) were tracheotomized and artificially ventilated (70% N2O, 30% O2). The anesthesia was switched to α-chloralose (i.p., 80 mg/kg initial dose, then 40 mg/kg/h) from halothane (1% to 2%) after the surgery. A femoral arterial line was used for monitoring blood pressure, acid-base balance, and blood gases throughout the experiment. A femoral vein was cannulated for injecting the MRI contrast agent. A pair of copper wires was inserted below the skin of the forepaw. Each stimulus train lasted 30 secs with 3 Hz frequency using pulses of 2 mA in amplitude and 0.3 ms in duration. All stimulus presentation was controlled by a μ1401 analog-to-digital converter unit (CED, Cambridge, UK) running custom-written script for providing block design (off-on-off) electrical stimuli of 30 secs in duration with 900 secs in between consecutive stimuli presentations. MRI and LDF measurements were performed on two separate groups of animals.
MRI Measurements
All fMRI data (n=6) were obtained on a modified 11.7 T Bruker horizontal-bore spectrometer (Billerica, MA, USA) using a 1H surface coil radio-frequency probe (1.4 cm diameter). BOLD signal was acquired with sequentially sampled echo-planar imaging (EPI) using gradient echo contrast Hyderet al(1995) with repetition and echo times of 1000 and 15 ms, respectively. The field of view was 2.56 cm in both axes with in-plane matrix of 64×64 and slice thickness was 2 mm. CBV signal was measured by the same EPI parameters but in the presence of an intravenous injection of iron oxide nanocolloid particles (Combidex, 15 mg/kg, AMAG, Cambridge, MA, USA) that has a very long half-life in blood circulation of rodents (Kidaet al, 2000). Since with the MRI contrast agent on board stimulation-induced decrease in the EPI signal reflected an increase in volume, all CBV-weighted MRI data were multiplied by −1 to reflect positive changes in plasma during functional hyperemia (CBVplasma).
LDF Measurements
The rats (n=12) were placed in a stereotaxic holder (Kopf Instruments, Tujunga, CA, USA) on a vibration-free table inside a Faraday cage. Tiny burr holes above the S1 region (4.4 mm lateral and 1.0 mm anterior to bregma) were drilled and fiber optodes (interoptode distance of 200 μm; giving a sampling volume of ~0.1 μL) were inserted into three different depths (0.3±0.1, 1.0±0.1, and 1.5±0.1 mm, respectively, for upper, middle, and lower layers; n=4 for each depth) of the cortex with stereotaxic manipulators to measure two independent LDF signals (805 nm; Oxford Optronix, Oxford, UK) of red blood cell activity: the flux and backscattered light which are believed to reflect CBF and CBV dynamics, respectively (Barfodet al, 1997; Heet al, 2007). Data were digitized at a rate of 50 Hz. As stimulation-induced decrease in the backscattered signal reflected an increase in volume, all backscatter data were multiplied by −1 to reflect positive changes in red blood cell volume during functional hyperemia (CBVrbc).
Results
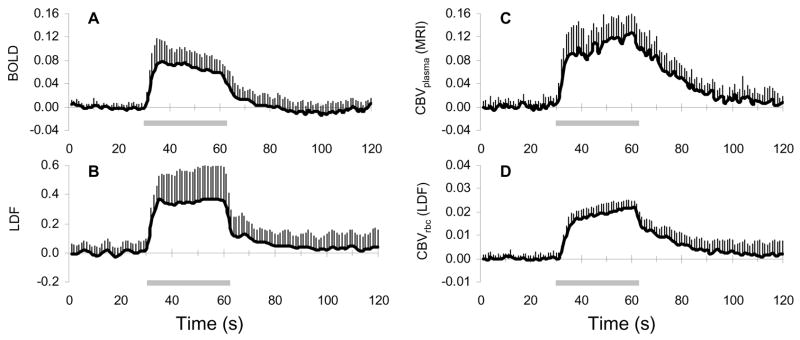
Forepaw stimulation in α-chloralose anesthetized rats, in agreement with prior results, reproducibly increased BOLD (Sanganahalliet al, 2008) and LDF (Anceset al, 1999) signals predominantly in the contralateral S1 (Figure 1A and 1B). The averaged BOLD and LDF responses from all layers of S1 were 6.1%±0.3% (n=6) and 36%±12% (n=12), respectively, where each averaged signal peaked within 6±1 and 4±1 secs, respectively. The magnitude of the relative CBF signals measured by LDF were lower than quantitative CBF changes measured by MRI methods (Hyderet al, 2001; Kidaet al, 2004; Maandaget al, 2007). In contrast, the CBV signals demonstrated different dynamics (Figure 1C and 1D).
Figure 1.
Functional changes in BOLD, CBF, and CBV signals during forepaw stimulation. Dynamics of (A) BOLD signal measured by fMRI at 11.7 T, (B) relative CBF measured by LDF flux, and CBV measured by (C) MRI intravascular contrast agent and (D) LDF backscattered light reflecting changes in blood plasma and red blood cell, respectively. Averaged data from the contralateral primary somatosensory cortex during forepaw stimulation (horizontal bar) from all experiments and layers (n=6 and 12, respectively, for MRI and LDF). Vertical bars represent the positive standard deviation from the mean. In each case, the CBV increase was actually represented by a decrease in the original signal.
The averaged CBVplasma and CBVrbc changes from all layers of S1 were 9.5%±3.6% (n=6) and 1.6%±0.1% (n=12), respectively. Despite the magnitude difference between these signals, the dynamics of these CBV-weighted transients appeared to be quite similar. Both normalized CBV signals demonstrated a rapid increase (~16% per second) within the first 5 secs (i.e., onset phase), then reached a pseudo steady-state with the signals increasing very slowly (~0.8% per second) until the end of stimulation (i.e., plateau phase), and after stimulation offset the signals began to decrease (i.e., offset phase) but much more slowly (~1.3% per second) than during the initial rise.
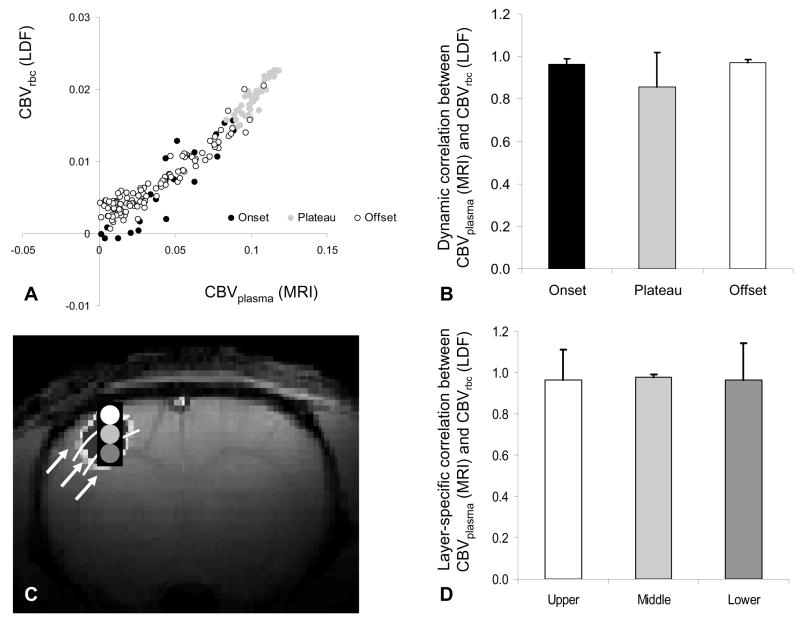
The dynamic CBVplasma and CBVrbc signals from all layers of S1 were compared (Figure 2A) to reveal a strong linear regression fit between the individual data points of the non-normalized signals (R2=0.97). Excellent correlation between the CBVplasma and CBVrbc signals for the onset (0.96±0.02), activation plateau (0.86±0.16), and offset (0.97±0.02) phases of the functional hyperemic response was observed (Figure 2B). Time courses of CBVplasma and CBVrbc signals from different cortical depths of S1 were compared (Figure 2C) to reveal excellent regional correlations (from upper to lower; 0.97±0.15, 0.98±0.01, 0.97±0.17) between these signals (Figure 2D). Together these results suggest good dynamic and layer-specific correlations between MRI and LDF signals.
Figure 2.
Correlations between CBV signals during forepaw stimulation. (A) CBV of red blood cell measured by LDF backscattered light (ordinate) as a function of CBV of plasma measured by MRI intravascular contrast (abscissa) for onset (black), plateau (gray), and offset (white) phases of the hyperemic response. See Figure 1 for details of transients. (B) Dynamic correlation between normalized CBV signals from MRI and LDF for onset, plateau, and offset phases of the hyperemic response. Vertical bars represent the positive standard deviation from the mean data from all experiments (n=6 and 12, respectively, for MRI and LDF). (C) A cross-correlation BOLD activation map reflecting the approximate location of the LDF probe (black rectangle) and the upper (white), middle (light gray), and lower (dark gray) regions of interest analyzed (for MRI and LDF signals). (D) Layer-specific correlation between normalized CBV signals from MRI and LDF for upper (white), middle (light gray), and lower (dark gray) regions of interest. Vertical bars represent the positive standard deviation from the mean data from all experiments (n=6 and 12, respectively, for MRI and LDF). Good dynamic (A, B) and layer-specific (C, D) correlations between MRI and LDF signals were observed.
Discussion
CBV-weighted fMRI, using long half-life and plasma-borne intravascular contrast agents, has become an attractive alternative to BOLD-weighted fMRI in animal models (Keilholzet al, 2004; Kidaet al, 2007; Luet al, 2007). A central question that remains, however, is whether the MRI signal changes from such experiments demonstrate changes in total CBV (CBVtotal) because the technique is sensitive to plasma volume (CBVplasma). To measure CBV changes associated with red blood cells (CBVrbc), we used LDF that can be used to determine both red blood cell flux and backscatter simultaneously where the latter signal is related to volume changes (Barfodet al, 1997;Heet al, 2007). Dynamic and layer-specific comparisons of CBVplasma and CBVrbc revealed excellent correlations during functional activation, suggesting that CBV measurements from either method could be used to reflect dynamic changes in CBVtotal. A limitation of our study is the exclusion of hypercapnia or hypocapnia challenges. CO2 inhalation has been used to study the dynamic range of vascular reactivity (Kannurpattiet al, 2003). However, we chose not to include this systemic perturbation because of concerns about the effects of oxygen binding on hemoglobin with CO2 (Popel, 1989).
MRI Measurements of Plasma Volume
The MRI-based CBVplasma measurement is based on a first-order assumption (Kennanet al, 1998) that changes in the transverse relaxation rate (i.e., T2 or T2* for spin-echo or gradient-echo MRI, respectively) caused by stimulation is separable in terms of blood volume (caused by the exogenous contrast agent) and blood oxygenation (caused by the endogenous hemoglobin) changes. The contrast agent causes the MRI signal to decrease with stimulation, whereas the BOLD effect causes the MRI signal to increase. At high dose of the contrast agent, the magnetic susceptibility effect of the contrast agent can essentially overpower the BOLD effect, thereby leaving the remaining MRI signal to be primarily CBVplasma weighted (Luet al, 2007). Since an underlying premise for interpreting magnetic susceptibility weighted MRI signal change is that cerebral hematocrit is unchanged (Kennanet al, 1998), CBVplasma changes are assumed to reflect changes in CBVtotal (Mandevilleet al, 1998).
There are several studies in α-chloralose anesthetized rats that have demonstrated time courses of CBVplasma during forepaw stimulation at field strengths of 2.0/4.7 T (Mandevilleet al, 1999), 7.0 T (Kidaet al, 2007), and 9.4 T (Luet al, 2007) that are nearly identical to our observations at 11.7 T (Figure 1C). Furthermore studies in isoflurane-anesthetized rats during forepaw stimulation (Shenet al, 2008), isoflurane-anesthetized cats during visual stimulation (Jin and Kim, 2008), and awake monkeys during visual stimulation (Leiteet al, 2002) all showed similar time courses as in α-chloralose anesthetized rats during forepaw stimulation (i.e., a rapid onset phase followed by a very slowly increasing plateau phase and then a slow offset phase). These studies used different types of iron oxide superparamagnetic contrast agents with nm range particles that have partiality for blood plasma albumin (Jung and Jacobs, 1995). These results suggest that the time courses of CBVplasma are not significantly affected by sensory modality, anesthetic agent, nanocolloid agent, and/or magnetic field dependent factors.
Optical Studies of Red Blood Cell Volume
The LDF-based CBVrbc measurement depends on the modified Lambert–Beer law (Kocsiset al, 2006) that accounts for light intensity changes in a scattering environment. Changes in the backscattered signal depends on concentration of the chromophores (i.e., hemoglobin in red blood cells) as well as the scattering loss within tissue. As the LDF technique uses light-source of only one wavelength, it is important to measure the backscattered signal close to an isosbestic point of hemoglobin (e.g., 805 nm) where the absorption coefficients of oxygenated and deoxygenated hemoglobin are approximately the same, and therefore, the signal is only influenced by the changes in concentration, not the oxygenated state.
The LDF-based CBVrbc recording cannot be used for absolute measurements because tissue scattering coefficients are difficult to measure (Duncanet al, 1996; Nishimura and Tamura, 2005), and moreover, the backscattered signal intensity depends on characterization of the LDF device (Barfodet al, 1997). A limitation of our study, however, could be that small vessels may have been damaged as a consequence of inserting the LDF probe into brain tissue for the layer-specific measurements. Because good agreement was found between the averaged LDF signals from the different layers and data from surface LDF probe over a thinned skull (PH unpublished results), we believe that normal flow responses were observed by the LDF probes.
Optical studies have examined dynamics of CBVrbc in α-chloralose anesthetized rats. Matsuuraet al(1999) used LDF (780 nm) during 5 Hz hindpaw stimulation to reveal CBVrbc dynamics that exhibited a rapid onset after start of stimulation, a pseudo plateau state during stimulation, and slow offset after stimulation. The continuous wavelength near infrared spectroscopy method (Kocsiset al, 2006) can measure the relative change of oxygenated and deoxygenated hemoglobin concentration non-invasively in the brain, where the total hemoglobin concentration is related to CBVrbc. Recently Culveret al(2005) applied near infrared spectroscopy in a topographic format during 3 Hz forepaw stimulation to obtain relative changes in CBVrbc. Both of these optical results are in good accord with our LDF observations (Figure 1D).
Microcirculation and Volume Hematocrit
Dynamic vascular behavior is heterogeneous, spanning from arterioles or venules to capillaries. Therefore spatial resolution of the methods used to measure different vascular parameters may affect dynamic behavior of the signals. However with quite different spatial resolutions of MRI and LDF signals, where neither technique had appropriate spatial resolution to resolve the dynamic behavior at the level of individual blood vessels, we observed comparable transients of blood plasma and red blood cell compartments, thereby suggesting that the total blood volume follows similar kinetics. If the microvasculature network experiences negligible counter flow with steady-state mass balance during the span of a typical experimental run, then these results suggest that volume hematocrit does not vary significantly during prolonged activation. The studies discussed above show that our results are generally consistent with prior observations using both MRI and optical methods. Nevertheless there is potential that with the spatial resolutions of MRI and LDF measurements used in this study, and those mentioned above, could have averaged out heterogeneous microscopic mismatch, if any, between blood plasma and red blood cell compartments. Much higher spatial resolution may be needed for differentiating between the different branches in the microvasculature (Herman and Eke, 2006; Parket al, 2008). But data comparison across large spatial dimensions could provide a more inclusive perception about the gradation of blood circulation in vivo.
There are several methods that measure blood plasma and red blood cell compartments at the level of arterioles, venules, and capillaries. Classical autoradiographic volume measurements rely on radioactively labeled red blood cells and serum albumin (Bereczkiet al, 1992; Hanset al, 1993; Weiet al, 1993). However the results reflect the state of blood circulation just moments before tissue freezing. Microscopy-based optical techniques have been used to measure transient behavior of microvasculature for a range of perturbations (Rovainenet al, 1993;Coxet al, 1993; Kleinfeldet al, 1998). But these techniques are limited to detection just below the pia mater. Future studies, utilizing these or some combinations of these techniques, may reveal the interplay of volumes as well as velocities between blood plasma and red blood cell compartments during functional activation, specifically given that velocity of red blood cell changes are quite dominant during activation-flow coupling (Detreet al, 1998;Matsuuraet al, 1999). An important consideration, however, in these future studies would be the involvement of the Fahraeus (1929) effect itself on BOLD contrast as the spatiotemporal resolution of the fMRI techniques improve (Kidaet al, 2007;Shenet al, 2008).
Acknowledgments
The authors thank scientists and engineers at MRRC (mrrc.yale.edu), and QNMR (qnmr.yale.edu). This work was supported by grants from National Institutes of Health (R01 MH-067528, R01 DC-003710, P30 NS-52519).
References
- 1.Ances BM, Greenberg JH, Detre JA. Activation-flow coupling with forepaw stimulation in female and male rats. Neurosci Res. 1999;35:37–41. doi: 10.1016/s0168-0102(99)00065-6. [DOI] [PubMed] [Google Scholar]
- 2.Barfod C, Akgören N, Fabricius M, Dirnagl U, Lauritzen M. Laser-Doppler measurements of concentration and velocity of moving blood cells in rat cerebral circulation. Acta Physiol Scand. 1997;160:123–132. doi: 10.1046/j.1365-201X.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 3.Belliveau JW, Kennedy DN, McKinstry RC, Buchbinder BR, Weiskoff RM, Cohen MS, Vevea JM, Brady TJ, Rosen BR. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 4.Bereczki D, Wei L, Acuff V, Gruber K, Tajima A, Patlak C, Fenstermacher J. Technique-dependent variations in cerebral microvessel blood volumes and hematocrits in the rat. J Appl Physiol. 1992;73:918–924. doi: 10.1152/jappl.1992.73.3.918. [DOI] [PubMed] [Google Scholar]
- 5.Cox SB, Woolsey TA, Rovainen CM. Localized dynamic changes in cortical blood flow with whisker stimulation corresponds to matched vascular and neuronal architecture of rat barrels. J Cereb Blood Flow Metab. 1993;13:899–913. doi: 10.1038/jcbfm.1993.113. [DOI] [PubMed] [Google Scholar]
- 6.Culver JP, Siegel AM, Franceschini MA, Mandeville JB, Boas D. Evidence that cerebral blood volume can provide brain activation maps with better spatial resolution than deoxygenated hemoglobin. Neuroimage. 2005;27:947–959. doi: 10.1016/j.neuroimage.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 7.Detre JA, Ances BM, Takahashi K, Greenberg JH. Signal averaged laser Doppler measurements of activation-flow coupling in the rat forepaw somatosensory cortex. Brain Res. 1998;796:91–98. doi: 10.1016/s0006-8993(98)00322-9. [DOI] [PubMed] [Google Scholar]
- 8.Duncan A, Meek JH, Clemence M, Elwell CE, Fallon P, Tyszczuk L, Cope M, Delpy DT. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res. 1996;39:889–894. doi: 10.1203/00006450-199605000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Fahraeus R. The suspension stability of the blood. Physiol Rev. 1929;9:241–274. [Google Scholar]
- 10.Hans FJ, Wei L, Bereczki D, Acuff V, Demaro J, Chen JL, Otsuka T, Patlak C, Fenstermacher J. Nicotine increases microvascular blood flow and flow velocity in three groups of brain areas. Am J Physiol. 1993;265:H2142–H2150. doi: 10.1152/ajpheart.1993.265.6.H2142. [DOI] [PubMed] [Google Scholar]
- 11.He J, Devonshire IM, Mayhew JE, Papadakis NG. Simultaneous laser Doppler flowmetry and arterial spin labeling MRI for measurement of functional perfusion changes in the cortex. Neuroimage. 2007;34:1391–1404. doi: 10.1016/j.neuroimage.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Herman P, Eke A. Nonlinear analysis of blood cell flux fluctuations in the rat brain cortex during stepwise hypotension challenge. J Cereb Blood Flow Metab. 2006;26:1189–1197. doi: 10.1038/sj.jcbfm.9600266. [DOI] [PubMed] [Google Scholar]
- 13.Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- 14.Hyder F, Rothman DL, Blamire AM. Image reconstruction of sequentially sampled echo-planar data. Magn Reson Imaging. 1995;13:97–103. doi: 10.1016/0730-725x(94)00068-e. [DOI] [PubMed] [Google Scholar]
- 15.Jin T, Kim SG. Improved cortical-layer specificity of vascular space occupancy fMRI with slab inversion relative to spin-echo BOLD at 9.4 T. Neuroimage. 2008;40:59–67. doi: 10.1016/j.neuroimage.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn Reson Imaging. 1995;13:661–674. doi: 10.1016/0730-725x(95)00024-b. [DOI] [PubMed] [Google Scholar]
- 17.Kannurpatti SS, Biswal BB, Hudetz AG. Regional dynamics of the fMRI-BOLD signal response to hypoxia-hypercapnia in the rat brain. J Magn Reson Imaging. 2003;17:641–647. doi: 10.1002/jmri.10311. [DOI] [PubMed] [Google Scholar]
- 18.Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP. BOLD and CBV-weighted functional magnetic resonance imaging of the rat somatosensory system. Magn Reson Med. 2004;55:316–324. doi: 10.1002/mrm.20744. [DOI] [PubMed] [Google Scholar]
- 19.Kennan RP, Scanley BE, Innis RB, Gore JC. Physiological basis for BOLD MR signal changes due to neuronal stimulation: separation of blood volume and magnetic susceptibility effects. Magn Reson Med. 1998;40:840–846. doi: 10.1002/mrm.1910400609. [DOI] [PubMed] [Google Scholar]
- 20.Kida I, Kennan RP, Rothman DL, Behar KL, Hyder F. High-resolution CMRO2 mapping in rat cortex: a multi-parametric approach to calibration of BOLD image contrast at 7 Tesla. J Cereb Blood Flow Metab. 2000;20:847–860. doi: 10.1097/00004647-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Kida I, Maciejewski PK, Hyder F. Dynamic imaging of perfusion and oxygenation by functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2004;24:1369–1381. doi: 10.1097/01.WCB.0000141501.12558.9B. [DOI] [PubMed] [Google Scholar]
- 22.Kida I, Rothman DL, Hyder F. Dynamics of changes in blood flow, volume, and oxygenation: Implications for dynamic fMRI calibration. J Cereb Blood Flow Metab. 2007;27:690–696. doi: 10.1038/sj.jcbfm.9600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinfeld D, Mitra PP, Helmchen F, Denk W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci USA. 1998;95:15741–15746. doi: 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocsis L, Herman P, Eke A. Mathematical model for the estimation of hemodynamic and oxygenation variables by tissue spectroscopy. J Theor Biol. 2006;241:262–275. doi: 10.1016/j.jtbi.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Leite FP, Tsao D, Vanduffel W, Fize D, Sasaki Y, Wald LL, Dale AM, Kwong KK, Orban GA, Rosen BR, Tootell RB, Mandeville JB. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- 26.Lu H, Scholl CA, Zuo Y, Stein EA, Yang Y. Quantifying the blood oxygenation level dependent effect in cerebral blood volume-weighted functional MRI at 9.4T. Magn Reson Med. 2007;58:616–621. doi: 10.1002/mrm.21354. [DOI] [PubMed] [Google Scholar]
- 27.Maandag NJ, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, Shulman RG, Hyder F. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci USA. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO2 during rat forepaw stimulation. Magn Reson Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Mandeville JB, Marota JJ, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med. 1998;39:615–624. doi: 10.1002/mrm.1910390415. [DOI] [PubMed] [Google Scholar]
- 30.Matsuura T, Fujita H, Seki C, Kashikura K, Yamada K, Kanno I. CBF change evoked by somatosensory activation measured by laser-Doppler flowmetry: independent evaluation of RBC velocity and RBC concentration. Jpn J Physiol. 1999;49:289–296. doi: 10.2170/jjphysiol.49.289. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura G, Tamura M. Simple peak shift analysis of time-of-flight data with a slow instrumental response function. J Biomed Opt. 2005;10:14016. doi: 10.1117/1.1854684. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SH, Masamoto K, Hendrich K, Kanno I, Kim SG. Imaging brain vasculature with BOLD microscopy: MR detection limits determined by in vivo two-photon microscopy. Magn Reson Med. 2008;59:855–865. doi: 10.1002/mrm.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popel AS. Theory of oxygen transport to tissue. Crit Rev Biomed Eng. 1989;17:257–321. [PMC free article] [PubMed] [Google Scholar]
- 35.Rovainen CM, Woolsey TA, Blocher NC, Wang DB, Robinson OF. Blood flow in single surface arterioles and venules on the mouse somatosensory cortex measured with videomicroscopy, fluorescent dextrans, nonoccluding fluorescent beads, and computer-assisted image analysis. J Cereb Blood Flow Metab. 1993;13:359–371. doi: 10.1038/jcbfm.1993.49. [DOI] [PubMed] [Google Scholar]
- 36.Sanganahalli BG, Herman P, Hyder F. Frequency-dependent tactile responses in rat brain measured by functional MRI. NMR Biomed. 2008;21:410–416. doi: 10.1002/nbm.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Q, Ren H, Duong TQ. CBF, BOLD, CBV, and CMRO2 fMRI signal temporal dynamics at 500-msec resolution. J Magn Reson Imaging. 2008;27:599–606. doi: 10.1002/jmri.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei L, Otsuka T, Acuff V, Bereczki D, Pettigrew K, Patlak C, Fenstermacher J. The velocities of red cell and plasma flows through parenchymal microvessels of rat brain are decreased by pentobarbital. J Cereb Blood Flow Metab. 1993;13:487–497. doi: 10.1038/jcbfm.1993.63. [DOI] [PubMed] [Google Scholar]