Abstract
A novel one-pot synthesis of N-substituted heterocycles via successive cyclization/annelation starting from primary sulfonamides is described. This process leads directly to N-sulfonyl pyrroles, indoles and carbazoles. The selection of appropriate reactant/triflic acid ratio successfully controls the formation of the desired product.
Keywords: Triflic acid, Annelation, Sulfonamides, N-heterocycles
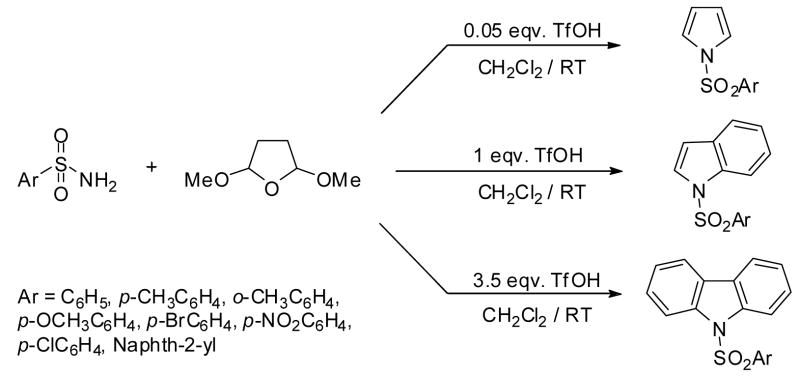
Nitrogen containing heterocycles, such as pyrroles, indoles and carbazoles have attracted considerable attention due to their numerous applications in pharmaceutical and synthetic chemistry.1 These heterocylic moieties are also found in a variety of biologically active synthetic and natural products.2 Many efficient processes had already been reported, however, the development of new methods is still in demand.3 Most methods involve two or more steps to synthesize these heterocycles resulting in 2,3-di or poly substituted products.4 Ideally the synthesis of these heterocycles would involve only one step, directly from simple, readily available substrates. Although, similar idea had been proposed earlier, it suffered with serious drawbacks such as low yields (up to 50%) and low selectivities.4 In the present study we report a convenient one-pot synthesis of N-sulfonyl-pyrroles, indoles and carbazoles from commercially available sulfonamides using trifluomethanesulfonic acid (TfOH) as an effective catalyst. This methodology provides the desired N-substituted products only, preserving other positions open for further functionalization. (Scheme 1)
Scheme 1.
Introduction of electron-withdrawing groups such as phenylsulfonyl group on the pyrrole nitrogen directs subsequent Friedel-Crafts electrophilic substitution predominantly to the 3-position. Similarly, it makes the 2-position of indole more facile for electrophilic substitution.5 This indicates that depending on the substituent on the nitrogen we can achieve unusual regioselective synthesis of pyrrole and indole derivatives. Traditional methods for synthesis of N-sulfonyl pyrroles involve strong base catalyzed nucleophilic substitution of pyrroles with sulfonyl chlorides.4, 5
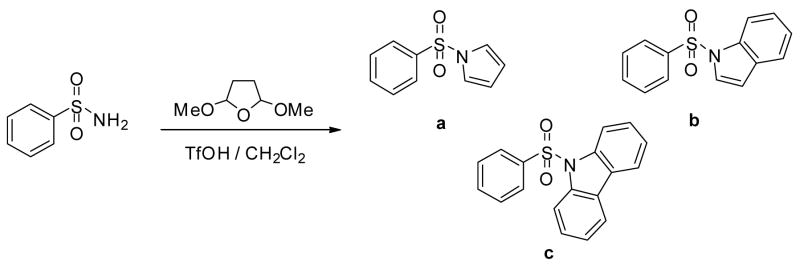
Paal-Knorr type cyclization reactions are often facilitated by strong acids.6 TfOH is a commonly used superacid (Ho = −14.1) and effective catalyst for many transformations. Its use is preferable to other acids with similar acid strength (e.g. H2SO4, ClSO3H, FSO3H) since it does not promote oxidative side reactions.7 We explored the effectiveness of triflic acid in cyclialkylations of sulfonamides to form N-sulfonyl pyrroles, which underwent successive annelation to form corresponding indoles and carbazoles depending upon the amount of triflic acid used. We have carried out several reactions using benzenesulfonamide as a probe and 2,5-dimethoxytetrahydrofuran as an alkylating agent to assess suitable reaction parameters. The results are summarized in Table 1.
Table 1.
Triflic acid catalyzed synthesis of N-phenylsulfonyl pyrrole, indole and carbazolea
 | ||||
|---|---|---|---|---|
| Entry | TfOH (mole%) |
Yieldb(%) |
||
| a | b | c | ||
| 1 | 3 | 85 | 0 | 0 |
| 2 | 5 | 98 | 0 | 0 |
| 3 | 50 | 40 | 60 | 0 |
| 4 | 100 | 5 | 95 | 0 |
| 5 | 200 | 0 | 30 | 70 |
| 6 | 300 | 0 | 15 | 85 |
| 7 | 325 | 0 | 11 | 89 |
| 8 | 350 | 0 | 8 | 92 |
Reaction conditions: sulfonamide (0.636 mmol), 2,5-dimethoxytetrahydrofuran (5 eqv.), RT, 2h.
Based on sulfonamide, determined by GCMS.
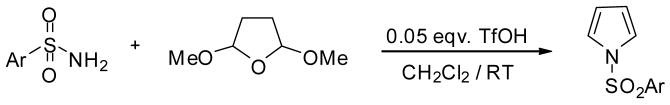
We optimized reaction conditions first by varying the amount of triflic acid from catalytic to quantitative. We have observed that the amount of triflic acid had a significant effect on the chemoselectivity of the reaction. Maximum yield of pyrrole was observed with 5 mol % TfOH, however, indole and carbazole syntheses required 1.0 eqv. and 3.5 eqv. respectively. To learn about the effects of time and temperature, the reaction was stirred for a longer time and elevated temperatures but no improvement was observed in yields. After proper optimization of reaction conditions, we were able to obtain the corresponding products in nearly quantitative yields and selectivities. With the optimized one-pot annelation reaction conditions, we explored the scope of the methodology using several commercially available substituted sulfonamides. We initially synthesized the sequence of various N-substituted pyrroles using 5 mol% TfOH and obtained excellent yields (90–95%) and almost exclusive selectivities. Representative examples are shown in Table 2.
Table 2.
Triflic acid catalyzed synthesis of N-sulfonyl pyrroles from aryl sulfonamides and 2,5-dimethoxytetrahydrofuran.a
 | ||||
|---|---|---|---|---|
| Entry | Ar | Time (h) |
Selectivityb (%) |
Yieldc (%) |
| 1 | C6H5 | 2 | 98 | 92 |
| 2 | p-CH3C6H4 | 2 | 95 | 90 |
| 3 | p-OCH3C6H4 | 2 | 98 | 89 |
| 4 | p-BrC6H4 | 2 | 92 | 86 |
| 5 | o-CH3C6H4 | 2 | 90 | 88 |
| 6 | p-ClC6H4 | 2 | 92 | 90 |
| 7 | p-NO2C6H4 | 2 | 88 | 80 |
| 8 | Naphth-2-yl | 2 | 90 | 85 |
Reaction conditions: sulfonamide (0.636 mmol), 2,5-dimethoxytetrahydrofuran (5 eqv.), TfOH (5 mol%), RT, 2h.
Determined by GCMS.
Isolated yields after flash chromatography.
As the data show, the corresponding substituted pyrroles are formed in good to excellent yields. The reaction can be carried out effectively with a wide variety of sulfonamides. In all cases the reaction occurred smoothly without showing any substitutent effect. Also, the formation of other products such as indole or carbazole was not observed. This cyclialkylation provides N-substituted pyrroles, which can be further functionalized as needed. We also tried to explore consistency with aliphatic sulfonamides, the reaction worked with poor yields.
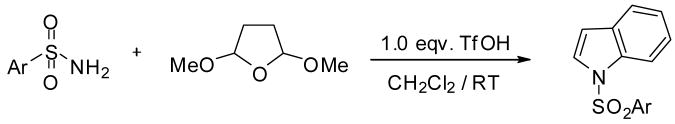
As shown above (Table 1) the amount of TfOH is crucial in these systems. The TfOH/reactant ratio will determine the major product and its actual selectivity. Table 1 indicates that using stoichiometric amount of TfOH, indole derivatives will exclusively form as a major product. Accordingly, in this case a two-step sequence occurs in the reaction; first the already studied Paal-Knorr cyclization takes place, which is followed by a successive annelation on the pyrrole ring. Variety of sulfonamides has been targeted to the above one-pot/two step reaction sequence, using stoichiometric amount of TfOH. The results are summarized in Table 3.
Table 3.
Triflic acid catalyzed synthesis of N-sulfonyl indoles from aryl sulfonamides and 2,5-dimethoxytetrahydrofuran.a
 | ||||
|---|---|---|---|---|
| Entry | Ar | Time (h) |
Selectivityb (%) |
Yieldc (%) |
| 1 | C6H5 | 2 | 95 | 90 |
| 2 | p-CH3C6H4 | 2 | 91 | 85 |
| 3 | p-OCH3C6H4 | 2 | 90 | 87 |
| 4 | p-BrC6H4 | 2 | 95 | 91 |
| 5 | o-CH3C6H4 | 2 | 90 | 82 |
| 6 | p-ClC6H4 | 2 | 89 | 85 |
| 7 | p-NO2C6H4 | 2 | 80 | 75 |
| 8 | Naphth-2-yl | 2 | 82 | 88 |
Reaction conditions: sulfonamide (0.636 mmol), 2,5-dimethoxytetrahydrofuran (5 eqv.), TfOH (100 mol%), RT, 2h.
Determined by GCMS.
Isolated yields after flash chromatography.
As the data show sulfonamides readily undergo cyclization and annelation. The corresponding indole derivatives have been formed with high selectivities and in good to excellent yields.
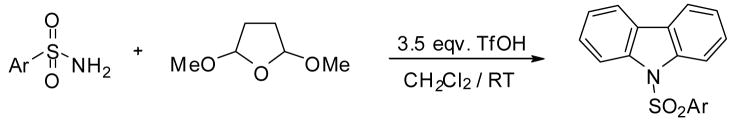
To explore the further extension of this method we carried out a third sequence of reactions with even higher amount of TfOH as determined in Table 1. For this step we used 3.5 eqv. excess of TfOH. Representive results are shown in Table 4. The results clearly show that in this case the reaction sequence is even further expanded. After cyclialkylation and annelation, a second annelation takes place, providing the corresponding carbazole derivatives in high selectivities and good isolated yields
Table 4.
Triflic acid catalyzed synthesis of N-sulfonyl carbazoles from aryl sulfonamides and 2,5-dimethoxytetrahydrofuran.a
 | ||||
|---|---|---|---|---|
| Entry | Ar | Time (h) |
Selectivityb (%) |
Yieldc (%) |
| 1 | C6H5 | 2 | 92 | 79 |
| 2 | p-CH3C6H4 | 2 | 91 | 81 |
| 3 | p-OCH3C6H4 | 2 | 88 | 75 |
| 4 | p-BrC6H4 | 2 | 85 | 77 |
| 5 | o-CH3C6H4 | 2 | 91 | 82 |
| 6 | p-ClC6H4 | 2 | 93 | 86 |
| 7 | p-NO2C6H4 | 2 | 85 | 75 |
| 8 | Naphth-2-yl | 2 | 91 | 82 |
Reaction conditions: sulfonamide (0.636 mmol), 2,5-dimethoxytetrahydrofuran (5 eqv.), TfOH (3.5 eqv.), RT, 2h.
Determined by GCMS.
Isolated yields after flash chromatography.
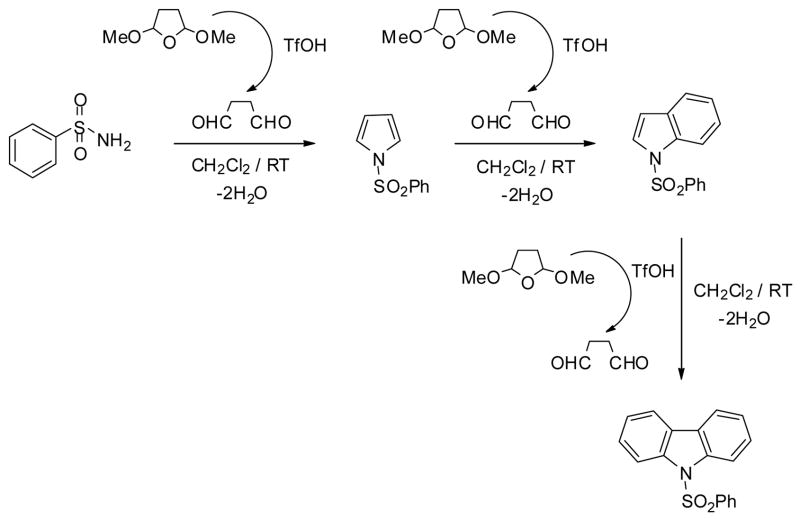
Based on the earlier literature data7 and our own experimental results, Scheme 2 summarizes the most probable reaction sequence. Reactant 2 undergoes rearrangement under acidic conditions to form 1,4-butanedial, which immediately reacts with the sulfonamides and undergoes Paal-Knorr cyclization to form pyrrole derivatives after eliminating two water molecules. It is known that the acid strength of TfOH is significantly modified by H2O.8 Due to the substantial amount of H2O formed (2 moles of H2O/1mol of 2,5-dimethoxytetrahydrofuran) in the cyclialkylation; the acidity of the system significantly drops. This low acidity is not able to catalyze further reactions.
Scheme 2.
The acidity drop is still significant even after increasing the amount of TfOH to 100 mol%. The higher amount of TfOH, however, is able to maintain the necessary acid strength of the reaction mixture, and initiates the annelation on the pyrrole ring. The additional two moles of H2O formed in the annelation have a similar effect to that mentioned above. A further increase in TfOH concentration enables the system to catalyze the second annelation as well, to give the corresponding carbazoles virtually in one step. This analysis indicates that although the TfOH amount exceeds the 1:1 stoichiometric ratio, it is only needed to maintain the necessary acid strength of the reaction mixture. As such the reaction is still catalytic. Based on our earlier studies,9 we suggest that both cyclialkylation and subsequent annelation occur in stepwise manner. Under the highly polar experimental conditions the occurrence of the concerted process is improbable.
In conclusion, a one-pot triflic acid controlled cyclization/annelation provides efficient protocol for preparing a wide variety of N-sulfonyl pyrroles, indoles and carbazoles from commercially available sulfonamides. This attractive method provides the products in excellent yields and selectivities in short reaction times. The simplicity and wide variability of the method makes it a novel alternative to current synthetic processes, which produce these products in multistep reactions.
A general experimental procedure for the synthesis of N-sulfonyl pyrroles, indoles and carbazoles
Benzenesulfonamide (100 mg, 0.636 mmol) and 2,5-dimethoxytetrahydrofuran (420 mg, 3.18 mmol) were placed in a round bottom flask with 2 ml of CH2Cl2. This mixture was cooled to 0°C for 10–15 min and TfOH (0.05 eqv. for pyrroles, 1.0 eqv. for indoles and 3.5 eqv. for carbazoles) was added slowly dropwise to the reactants. After addition the mixture was stirred at room temperature for an additional 2 h. Acid was quenched with water and the product was extracted with CH2Cl2. Combined organic layers were dried over sodium sulfate. Solvent was evaporated in vacuo and the residue was subjected to flash chromatography. The pure products were characterized by GCMS and NMR as shown below.
Supplementary Material
Acknowledgments
The financial support provided by University of Massachusetts Boston and NIH (R-15 AG025777-02) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson B. The Fischer Indole Synthesis. John Wiley & Sons; Chichester: 1982. Gribble GW. J Chem Soc Perkin Trans 1. 2000:1045.and references cited therein. Humphrey GR, Kuethe JT. Chem Rev. 2006;106:2875. doi: 10.1021/cr0505270.Török B, Abid M, London G, Esquibel J, Török M, Mhadgut SC, Yan P, Prakash GKS. Angew Chem Int Ed. 2005;44:3086. doi: 10.1002/anie.200462877.
- 2.(a) Somei M, Yamada F. Nat Prod Rep. 2004;21:278. doi: 10.1039/b212257j. [DOI] [PubMed] [Google Scholar]; (b) Furstner A, Szillat H, Gabor B, Mynott R. J Am Chem Soc. 1998;120:8305. [Google Scholar]; (c) Somei M, Yamada F. Nat Prod Rep. 2005;22:73. doi: 10.1039/b316241a. [DOI] [PubMed] [Google Scholar]; (d) Török M, Abid M, Mhadgut SC, Török B. Biochemistry. 2006;45:5377. doi: 10.1021/bi0601104. [DOI] [PubMed] [Google Scholar]; (e) Abid M, Török B. Tetrahedron: Asymmetry. 2005;16:1547. [Google Scholar]
- 3.(a) Hodges ML, Spera ML, Moody MW, Harman WD. J Am Chem Soc. 1996;118:7117. [Google Scholar]; (b) Katritzky AR, Fali CN, Li J. J Org Chem. 1997;62:4148. doi: 10.1021/jo9710846. [DOI] [PubMed] [Google Scholar]
- 4.(e) Bur SK, Padwa A. Chem Rev. 2004;104:2401. doi: 10.1021/cr020090l. [DOI] [PubMed] [Google Scholar]; (b) Shimada T, Nakamura I, Yamamoto Y. J Am Chem Soc. 2004;126:10546. doi: 10.1021/ja047542r. [DOI] [PubMed] [Google Scholar]; (c) Nishibayashi Y, Yoshikawa M, Inada Y, Milton MD, Hidai M, Uemura S. Angew Chem, Int Ed. 2003;42:2681. doi: 10.1002/anie.200351170. [DOI] [PubMed] [Google Scholar]; (d) Gorin DJ, Davis NR, Toste FD. J Am Chem Soc. 2005;127:11260. doi: 10.1021/ja053804t. [DOI] [PubMed] [Google Scholar]; (e) Larionov OV, de Meijere A. Angew Chem, Int Ed. 2005;44:5664. doi: 10.1002/anie.200502140. [DOI] [PubMed] [Google Scholar]; (f) Fang YQ, Lautens M. Org Lett. 2005;7:3549. doi: 10.1021/ol051286l. [DOI] [PubMed] [Google Scholar]
- 5.(a) Zelikin A, Shastri VR, Langer R. J Org Chem. 1999;64:3379. doi: 10.1021/jo9823339. [DOI] [PubMed] [Google Scholar]; (b) Roy S, Gribble GW. Tetrahedron Lett. 2005;46:1325. [Google Scholar]
- 6.(a) Ekkati AR, Bates DK. Synthesis. 2003:1959. [Google Scholar]; (b) Banik BK, Samajdar S, Banik I. J Org Chem. 2004;69:213. doi: 10.1021/jo035200i. [DOI] [PubMed] [Google Scholar]; (c) Abid M, Landge S, Török B. Org Prep Proc Int. 2006;38:495. [Google Scholar]; (d) Abid M, Spaeth A, Török B. Adv Synth Catal. 2006;348:2191. [Google Scholar]
- 7.(a) Olah GA, Prakash GKS, Sommer J. Superacids. John Wiley & Sons; New York: 1985. [Google Scholar]; (b) Puglici A, Lee AL, Schrock RR, Hoveyda AH. Org Lett. 2006;8:1871. doi: 10.1021/ol060430f. [DOI] [PubMed] [Google Scholar]; (c) Bennasar ML, Zulaica E, Tummers S. Tetrahedron Lett. 2004;45:6283. [Google Scholar]
- 8.(a) Saito S, Sato Y, Ohwada T, Shudo K. J Am Chem Soc. 1994;116:2312. [Google Scholar]; (b) Olah GA, Batamack P, Deffieux D, Török B, Wang Q, Molnár Á, Prakash GKS. Applied Catal A. 1996;146:107. [Google Scholar]
- 9.(a) Molnár Á, Török B, Bucsi I, Földvári A. Top Catal. 1998;6:9. [Google Scholar]; (b) Prakash GKS, Yan P, Török B, Olah GA. Catal y>Lett. 2003:87, 109. [Google Scholar]; (c) Abid M, Török B. Adv Synth Catal. 2005;347:1797. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.