Abstract
Falciparum malaria is initiated when Anopheles mosquitoes transmit the Plasmodium sporozoite stage during a blood meal. Irradiated sporozoites confer sterile protection against subsequent malaria infection in animal models and humans. This level of protection is unmatched by current recombinant malaria vaccines. However, the live-attenuated vaccine approach faces formidable obstacles, including development of accurate, reproducible attenuation techniques. We tested whether Plasmodium falciparum could be attenuated at the early liver stage by genetic engineering. The P. falciparum genetically attenuated parasites (GAPs) harbor individual deletions or simultaneous deletions of the sporozoite-expressed genes P52 and P36. Gene deletions were done by double-cross-over recombination to avoid genetic reversion of the knockout parasites. The gene deletions did not affect parasite replication throughout the erythrocytic cycle, gametocyte production, mosquito infections, and sporozoite production rates. However, the deletions caused parasite developmental arrest during hepatocyte infection. The double-gene deletion line exhibited a more severe intrahepatocytic growth defect compared with the single-gene deletion lines, and it did not persist. This defect was assessed in an in vitro liver-stage growth assay and in a chimeric mouse model harboring human hepatocytes. The strong phenotype of the double knockout GAP justifies its human testing as a whole-organism vaccine candidate using the established sporozoite challenge model. GAPs might provide a safe and reproducible platform to develop an efficacious whole-cell malaria vaccine that prevents infection at the preerythrocytic stage.
Keywords: genetically attenuated parasites, malaria vaccine, P36, P52, sporozoite
Malaria is a formidable global health problem, affecting 300 million to 500 million people worldwide annually (1). The resulting ≈1 million deaths per year are mainly caused by Plasmodium falciparum infections. Eradication of malaria will in large part depend on an effective vaccine that prevents infection by Plasmodium, but such a vaccine has remained elusive. The parasites' preerythrocytic stages, encompassing the mosquito-inoculated sporozoites and liver stages that develop from sporozoites after their invasion of hepatocytes, are attractive targets for antiinfection vaccines, because at this stage the number of infected host cells is low, and further transmission of the parasite is not yet possible. Occurrence of blood-stage infection after sporozoite challenge is completely preventable by immunization with radiation-attenuated sporozoites in mouse models of malaria (2). This was a landmark finding that set the standards for malaria preerythrocytic vaccine development. Radiation-attenuated sporozoites arrest in development during hepatocyte infection, but their safety and efficacy are dependent on a precise irradiation dose. Humans immunized with P. falciparum radiation-attenuated sporozoites have been effectively protected from subsequent challenge with homologous and heterologous wild type (WT) P. falciparum sporozoites (3–5). Therefore, the development of a widely deployable P. falciparum radiation-attenuated sporozoite vaccine has been proposed (6). Although genetic manipulation systems are available for P. falciparum, they have not been used to develop attenuated parasite strains. Recently, preerythrocytic stages of the rodent malaria parasites Plasmodium berghei and Plasmodium yoelii were attenuated by deletion of preerythrocytic-stage-expressed genes named Up-regulated in Infectious Sporozoites (UISs). UIS3 and UIS4 (7–9) are proteins of the liver-stage parasitophorous vacuole membrane, the principal host–parasite interface during liver infection (7, 10). Deletion of UIS3 and UIS4 led to complete arrest of early liver-stage development after hepatocyte infection (7–9). Deletion of another UIS gene, P52, encoding a putative GPI-anchored protein (11, 12), and P36, a gene encoding a putative secreted protein (12), also resulted in developmental arrest at the early stage of hepatocyte infection. Immunization of mice with uis3−, uis4−, or p52− parasites induced complete, long-lasting protection against infectious sporozoite challenge (7–9, 11), demonstrating that rodent malaria GAPs are highly effective vaccines. The GAP-induced protection was mediated mainly by CD8+ T cells (9, 13, 14), but antibodies also contributed to protection (9).
To assess the potential to create a GAP vaccine for human malaria, we deleted the P52 and P36 loci in P. falciparum. The deletions did not affect the parasites throughout most of the life cycle, including sporozoite production of the attenuated lines, but resulted in significant growth defects in a hepatocytic cell line. Furthermore, we report the successful genetic attenuation of P. falciparum preerythrocytic stages by simultaneous deletion of P52 and P36, creating a p52−/p36− double-gene knockout strain. Dual gene deletions might alleviate safety concerns for the use of GAPs as a vaccine in humans.
Results
P. falciparum P52 and P36.
The P. falciparum genes PfP52 (GenBank, XP_001351357; PlasmoDB, PFD0215c) and PfP36 (GenBank, XP_001351356; PlasmoDB, PFD0210c) are paralogous, tandem-arranged genes on chromosome 4. Their products exhibit a predicted N-terminal cleavable signal peptide followed by two 6-Cys domains. In addition, P52 exhibits a C-terminal hydrophobic sequence predicted to be a putative GPI anchor attachment signal (15). PfP52 and PfP36 show 40% and 43% amino acid identity to their respective P. yoelii orthologs. To determine the subcellular localization of PfP52 and PfP36, we expressed each protein in a wheat germ cell-free expression system (16). Polyclonal antisera were raised in mice and rabbits, and reactivity was tested in immunofluorescence assays (IFAs) using P. falciparum sporozoites. A specific sporozoite-internal staining was observed for P52 that partially colocalized with the micronemal protein TRAP (Fig. S1). The fluorescence was only observed in sporozoites after permeabilization of membranes, indicating that P52 may localize to the secretory organelles of sporozoites. Preimmune sera did not show reactivity with sporozoites. Unfortunately, antisera raised against P36 did not react with sporozoites in IFAs, and therefore protein expression was not determined. However, a recent proteomic study of P. falciparum sporozoites detected P36 as well as P52 with multiple peptide hits, showing that both proteins are expressed in this life cycle stage (17).
Deletion of P. falciparum P52 and P36.
To delete PfP52 and PfP36 from the parasite genome, we used a positive–negative selection strategy (18). Double-cross-over homologous recombination between targeting sequences in transfection constructs and the endogenous genes resulted in replacement of PfP52 and PfP36 individually with the human dihydrofolate reductase (dhfr) selectable marker (19). Two independently transfected lines of the P. falciparum gametocyte-producing clone 3D7 were generated for each targeted locus. Transfectant parasites appeared between days 21 and 35 after transfection under positive selection with WR92210 (19). Parental transfectants were removed from positive selection for a 3-week period and then subjected to positive selection until a stable population was established after 2 weeks. This was followed by negative selection against cytosine deaminase-uracil phosphoribosyltransferase with 5-fluorocytosine. Transfectant lines were then analyzed by Southern blotting to detect the gene deletions. Clonal lines of recombinant parasites were derived from the parental population by limiting dilution and were analyzed for successful gene deletion and absence of WT by Southern blotting. The Southern blot analysis confirmed the genetic homogeneity of the knockouts (Fig. S2).
P52- and P36-Deficient P. falciparum Parasites Show Normal Infectivity and Development in the Mosquito.
Deletion of PfP52 and PfP36 in the erythrocytic stage did not result in any observable defect during blood-stage replication, indicating that these genes have no apparent critical function during this part of the parasite life cycle. Gametocyte cultures were used to infect Anopheles stephensi mosquitoes by membrane feeding. Evaluation of midgut oocyst infection in mosquitoes showed no discernible differences between WT, p52−, and p36− knockout lines. This indicated that gene deletions did not affect the sexual stages of the parasite. Furthermore, it provided evidence that prolonged culture of knockout parasite lines during drug selection did not significantly reduce knockout parasite transmissibility to mosquitoes. Importantly, invasion of the mosquito salivary glands appeared normal in the P52 and P36 knockout lines, because numbers of sporozoites isolated from the glands were comparable to WT sporozoite numbers (Table 1); no significant differences from WT were observed for p36− (P = 0.29) and p52− (P = 0.46).
Table 1.
Phenotypic analysis of Pf p52− and Pf p36−-deficient sporozoites and liver stages
| Parasite line | Salivary gland sporozoites per mosquito | Motility assay* | Invasion assay† | Liver-stage parasite abundance‡ |
|---|---|---|---|---|
| Pf WT | 45,233 ± 24,624 | 1.00 | 1.00 | 1.00 |
| Pf p52− | 57,162 ± 7,535 | 0.92 ± 0.11 | 1.04 ± 0.07 | 0.70 ± 0.11 |
| Pf p36− | 67,667 ± 29,365 | 0.92 ± 0.13 | 1.00 ± 0.13 | 0.65 ± 0.10 |
*Determined by counting CS protein sporozoite trails.
†Sporozoite invasion of HC-04 cells.
‡Determined at 72 h after infection.
P52- and P36-Deficient P. falciparum Sporozoites Are Biologically Active.
To ensure that gene deletions resulted in lack of expression of P52 or P36, we performed RT-PCR on sporozoite RNA isolated from WT and knockout parasite lines (Fig. S3A). Results indicated that p52− sporozoites did not express intact transcripts for P52 but expressed transcripts for P36; conversely, p36− knockout sporozoites expressed transcripts for P52 but not for P36. It has been shown that the biological activity of sporozoites is reflected in their motility on a solid substrate that can be assessed by detecting shed circumsporozoite (CS) protein, the main sporozoite surface protein (20). No significant differences in motility and CS protein shedding were observed between WT and p52− (P = 0.59) or p36− (P = 0.29) lines, as evidenced by quantification of trails stained with anti-CS protein antibodies (Fig. S3 B–D, Table 1, and Table S1).
P52- and P36-Deficient P. falciparum Parasites Invade Host Cells but Exhibit Developmental Arrest in a Hepatocytic Cell Line.
We investigated the ability of p52− and p36− sporozoites to invade host cells in vitro by using the HC-04 cell line, which supports invasion and complete liver-stage development of P. falciparum (21). Invasion was assessed microscopically by counting the number of cells invaded by sporozoites. Invasion rates were comparable among WT and knockout parasite lines, indicating that the lack of P52 or P36 did not impact sporozoite host cell entry (Table 1 and Table S2), because no significant differences between hepatocyte invasion rates of WT and the p52− (P = 0.29) or p36− (P = 0.59) were observed.
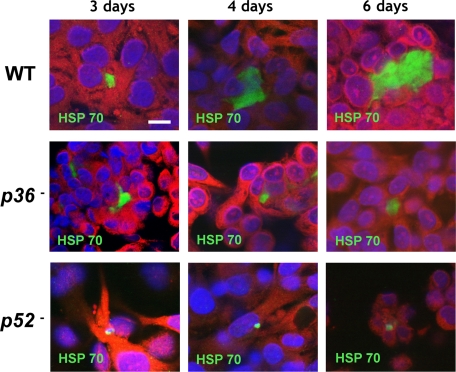
Next, intracellular development of the knockout parasite liver stages was compared to WT parasite development in HC-04 cells at 3, 4, and 6 days after sporozoite infection (Fig. 1, Table 1, and Table S3). Overall numbers of intrahepatocytic parasites observed in vitro at 72 h after invasion appeared lower in knockout parasites when compared to WT (Table 1), but the difference was not significant for p52− (P > 0.99) or p36− (P = 0.11). However, at day 4, knockout parasite liver stages exhibited smaller sizes in infected HC-04 cultures when compared to WT parasites, and at day 6 knockout parasite liver stages showed severe growth arrest when compared to well-developed WT parasite liver stages (Fig. 1).
Fig. 1.
The p52- and p36-deficient P. falciparum parasites show defective liver-stage development. Development of mutant and WT liver stages was assessed in vitro by using cultured cells of the HC-04 human hepatocytic line. Parasite growth was monitored over 6 days, and liver stages were visualized by immunofluorescence microscopy at 400× magnification with anti-HSP70 at 3, 4, and 6 days after infection. P. falciparum p52− and p36− parasites exhibited abnormal, arrested development that is most apparent at the 6-day developmental time point. (Scale bar: 10 μm.)
Production and Evaluation of P. falciparum p52−/p36− Double-Deficient Parasite Lines.
We next simultaneously deleted P52 and P36 from the parasite genome. Because PfP52 and PfP36 are tandem-arranged in the parasite genome, we deleted both genes in a single double-cross-over homologous recombination event (Fig. S4). Similarly to single deletion of P52 or P36 knockout lines, the double-deletion knockout lines showed no observable defects in blood-stage replication, the morphology of gametocytes, male gamete exflagellation, and oocyst development in the mosquito midgut. RT-PCR performed on p52−/p36− sporozoites confirmed a lack of intact transcripts for both genes (Fig. S5A). We were also able to isolate p52−/p36− mosquito salivary gland sporozoites in numbers comparable to WT salivary gland sporozoites (P = 0.89) (Fig. 2A). Similarly to individual deletions of P52 or P36, the simultaneous deletion of P52 and P36 did not result in a major decrease of sporozoite biological activity, as determined by evaluation of gliding motility [p52−/p36− showed slightly lower gliding motility activity compared with WT parasites, but the effect was not statistically significant at the 95% confidence level (P = 0.11)]. Neither did the p52−/p36− parasite lines show a difference in their ability to enter hepatocytes compared with WT (P = 0.11) (Fig. 2 B and C, Fig. S5 B and C and Tables S4 and S5). Importantly, however, the p52−/p36− parasites showed a more severe developmental arrest in hepatocytes when compared to the P52- or P36-deficient parasites. Intrahepatocytic p52−/p36− parasites were only rarely detectable 3 days after HC-04 infection (Fig. S5D) and were not detectable 4–6 days after infection of the HC-04 cell line (Fig. 2D, Table 2, and Table S6). We further evaluated the degree of attenuation of p36−, p52− single-knockout and p52−/p36− double-knockout liver stages at 72 h (Table 3) and at 144 h after infection in the HC-04 cell line (Table 2) by using antibodies to known liver-stage-expressed antigens. At 72 h after infection, the expression profile of p36− and p52− single knockouts and p52−/p36− double knockout resembled the staining profile of WT liver stages, including expression of LSA-1 (Table 3). At 144 h after infection, p36− and p52− continued to show expression of LSA-1, but we could not detect expression of EBA175 (a late liver-stage/blood-stage marker) (22). Staining of p52−/p36− liver stages at 144 h after infection could not be evaluated because no parasites were detectable at this time point with any of the tested antibodies (Table 2). Our data show that individual gene deletions of P36 and P52 result in a liver-stage growth defect. The p36− and p52− lines express liver-stage antigen but fail to continue development and do not initiate expression of blood-stage antigen. However, they survive inside HC-04 cells, at least until day 6 after infection. In contrast, the p52−/p36− double knockout not only shows a severe growth defect but also cannot survive beyond day 3 after infection.
Fig. 2.
Phenotypic analysis of P. falciparum p52−/p36−-deficient sporozoites and liver stages reveals a severe defect in liver-stage development. (A) The p52−/p36− parasites show normal invasion of the mosquito salivary glands; no significant differences were observed between WT and the double-knockout clones (P = 0.89). (B) The p52−/p36− parasites showed slightly lower gliding activity compared with WT parasites; the effect was not statistically significant at the 95% confidence level (P = 0.11). (C) WT and p52−/p36− parasites have comparable ability to enter hepatocytes; no significant difference was seen between p52−/p36− and WT parasites (P = 0.11). (D) The double-knockout parasites show a severe developmental arrest and do not persist, because no p52−/p36− liver stages are detected at 4 days after HC-04 cell line invasion. Statistical differences between the mutant and WT parasite lines were evaluated by the Wilcoxon matched-pairs, signed-rank test. nd, not detected.
Table 2.
Staining profile of liver stages at 144 h after infection
| Marker | Pf p36− | Pf p52− | Pf p52−/p36− | WT |
|---|---|---|---|---|
| CSP | Positive (faint) | Positive (faint) | ND | Positive (faint) |
| HSP70 | Positive | Positive | ND | Positive |
| LSA 1 | Positive | Positive | ND | Positive |
| EBA175 | Negative | Negative | ND | Positive |
ND indicates liver stages not detected.
Table 3.
Staining profile of liver stages at 72 h after infection
| Marker | Pf p36− | Pf p52− | Pf p52−/p36− | WT |
|---|---|---|---|---|
| CSP | Positive | Positive | Positive | Positive |
| HSP70 | Positive | Positive | Positive | Positive |
| LSA 1 | Positive | Positive | Positive | Positive |
| EBA175 | Negative | Negative | Negative | Negative |
P. falciparum p52−/p36− Double-Deficient Sporozoites Fail to Productively Infect the Livers of Humanized SCID Alb-uPA Mice.
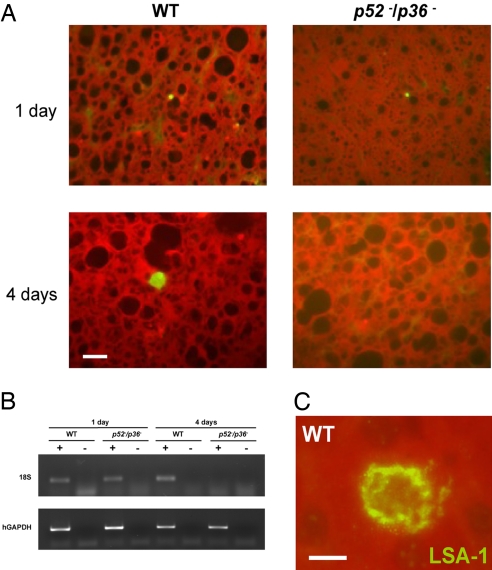
The liver phase of P. falciparum develops only in human hepatocytes, and it is not adequately assessed in conventional animal models. Therefore, we used a humanized mouse model (23) to study infectivity of the double-knockout parasite p52−/p36−. Immunodeficient mice homozygous for the urokinase-type plasminogen activator transgene (SCID Alb-uPA) were inoculated with 106 primary human hepatocytes to create chimeric human–mouse livers (24). After 6 weeks, successful engraftment was evaluated (Table S7). Positive engrafted chimeric mice were infected intravenously with 106 sporozoites of either p52−/p36− (n = 3) or WT (n = 3) P. falciparum, and livers were harvested 1 day or 4 days after infection. Tissue sections of livers were labeled with antisera against parasite HSP70 (Fig. 3A), LSA-1 (Fig. 3C), and CS protein and were examined by immunofluorescence microscopy. The p52−/p36− and WT liver stages were easily detected at 1 day after infection by microscopy and by amplification of parasite 18S rDNA (Fig. 3 A and B). Enumeration of intrahepatic parasites using microscopic counting showed no significant difference between WT (22 ± 5.5 per square centimeter of tissue) and p52−/p36− knockout (20 ± 3.2 per square centimeter of tissue) 1 day after infection. However, no p52−/p36− parasites were detected at 4 days after infection by using microscopy and RT-PCR, whereas WT parasites were easily detected in both assays (Fig. 3 A and B).
Fig. 3.
P. falciparum p52−/p36− parasites do not grow and persist in a humanized chimeric liver mouse model. SCID Alb-uPA mice were inoculated with 106 primary human hepatocytes to create chimeric human–mouse livers. Mice positive for engraftment were infected intravenously with 1 × 106 sporozoites of either p52−/p36− or WT P. falciparum. (A) Immunofluorescent micrographs of chimeric SCID/Alb-uPA liver cryosections after WT P. falciparum infection, stained with parasite-specific antibodies. Tissue was harvested at 1 day or 4 days after mice were inoculated with P. falciparum sporozoites. Sections in panels were stained with an anti-PfHSP70 monoclonal antibody. The data show that p52−/p36− liver stages are detected 1 day after infection but are not detected 4 days after infection. (Scale bar: 10 μm.) (B) RT-PCR analysis was performed with RNA isolated from individual livers of chimeric mice 1 day or 4 days after infection. Total RNA was extracted, and reverse-transcription reactions were done with (+) or without (−) the reverse transcriptase. Primers specific for the 18S (small-subunit) ribosomal RNA of P. falciparum and human glyceraldehyde phosphate dehydrogenase (hGAPDH) were then used to amplify the reverse-transcribed message. The GAPDH control reaction was positive for samples from all of the mice. The parasite-specific 18S reaction was positive for the mice infected with the WT parasites at day 1 and day 4 after infection. For the double-knockout parasite-infected mice, the 18S reaction was only positive for tissues harvested 1 day after infection and negative for tissue harvested at 4 days after infection, indicating that p52−/p36− parasites were no longer present in the liver at the later time point. RT-PCR was performed on all mice used in the experiment, and results were consistent with the results shown here. (C) An example of WT P. falciparum liver stage from chimeric SCID/Alb-uPA liver cryosection stained with polyclonal rabbit antisera against the repeat region of LSA-1 at 4 days after infection. (Scale bar: 5 μm.)
Together, the data obtained with HC-04 assays and chimeric mouse infections indicate that the P. falciparum p52−/p36− double-knockout GAP infects human hepatocytes but is more severely attenuated then the single-knockout GAPs, cannot successfully develop in human hepatocytes, and does not persist beyond day 4 of infection.
Discussion
Malaria remains a major public health threat in the developing world, with most deaths attributed to P. falciparum infection (1, 25). Radiation-attenuated, sporozoite-mediated sterile protection has served as a paradigm to guide the search for a recombinant preerythrocytic vaccine candidate but was not considered a viable solution to malaria vaccine development until recently (6). Yet, a vaccine against the parasite is not available, and the most advanced recombinant vaccine, RTS/S, which is based on the CS protein, conferred significant but limited protection against infection (26, 27). A significant breakthrough toward feasibility of a live-attenuated malaria vaccine was the demonstration of genetic attenuation by gene deletions in rodent malaria models. GAP vaccinations confer sterile, long-lasting protection against high-dose WT sporozoite challenge in mice (7–9, 11). However, it has remained unknown whether precise genetic attenuation, and thus the development of an engineered live-attenuated candidate vaccine strain of P. falciparum, is feasible.
Here, we targeted two sporozoite-expressed loci in P. falciparum—P52 and P36—for deletion. Deletion of the orthologous genes in the rodent malaria models had indicated that they are important for productive liver infection (11, 12, 28). Transmission to the mosquito vector and passage through the mosquito stages were not affected by deletion of either P52 or P36. Furthermore, although loss of gametocyte production and transmissibility has been reported after prolonged culture of P. falciparum (29, 30), the selection procedure for cytoadherence we used to minimize loss of chromosome ends (31) appeared to have circumvented this problem.
Analysis of the p52− and p36− lines showed normal mosquito salivary gland infection rates, and knockout salivary gland sporozoites exhibited normal gliding motility, demonstrating that the viability of knockout sporozoites was not affected up to this point in the parasites' developmental progression. Further analysis of the human parasite preerythrocytic stages has been challenging because no practical animal models for in vivo evaluation of liver infection exist. Nonetheless, liver infection can be modeled in cell culture, and we made use of the HC-04 continuous hepatocytic cell line that was shown to support complete liver-stage development of P. falciparum (21). Cell infection rates of the p52− and p36− lines were comparable with those of WT P. falciparum, indicating that the corresponding protein functions did not support host cell entry. However, the knockouts showed clear defects in liver-stage development. Recently, in an independent study, disruption of the P52 gene in P. falciparum has been achieved by single-cross-over integration (32). The observed phenotype was in agreement with the p52− phenotype presented here. However, single-cross-over recombination insertions are prone to reversion by plasmid excision, and indeed, the study by van Schaijk et al. (32) observed reverting parasites that had restored WT growth. Our strategy for gene deletions was to use double-cross-over recombination gene replacement, which makes it impossible for the parasite to genetically restore the WT locus, and the parasite is haploid in blood stage, during which the genes are deleted. Additional genes that encode members of the 6-Cys family are encoded in the Plasmodium genome, but so far there is no evidence that they could compensate for the function of P52 and P36. Yet, we cannot completely rule out that the attenuated strains might revert in extremely rare instances. It is noteworthy that P52 and P36 are each apparently important for liver infection. Although the genes are paralogues and share a similar expression profile, our results show that neither compensates functionally for the loss of the other. The P. falciparum p52− and p36− phenotypes are therefore similar to the phenotypes observed for the corresponding rodent malaria knockouts (11, 12). Such experimental concurrence of phenotypes between rodent malaria parasite mutants and P. falciparum mutants is important because it has been observed that knockout of orthologous genes in rodent and human malaria parasites can result in distinct phenotypes (33, 34).
Single-gene deletions of P52 and P36 in rodent parasites led to occasional breakthrough blood-stage infections when high numbers of knockout sporozoites were injected (11, 12). Clearly, breakthrough blood-stage infections in human GAP vaccinations will not be acceptable. In the P. yoelii model, a p52−/p36− double-knockout GAP did not show any breakthrough infection when inoculated at high doses of 105 sporozoites (28). Furthermore, this model of a GAP vaccine induced sterile protection against high-dose i.v. challenge with 104 WT sporozoites and against challenge by infectious mosquito bite (28).
We have here produced P. falciparum p52−/p36− parasite lines carrying deletions of both P52 and P36. The phenotype of the created double-gene knockout exhibited a more profound intrahepatocytic growth arrest in vitro compared with the single-gene knockout lines. Importantly, intrahepatocytic parasites did not survive beyond the third day of infection and are therefore unable to persist in a growth-arrested state. To further assess the developmental phenotype of the double-knockout parasites in vivo, we tested p52−/p36− in an immunodeficient chimeric mouse model that carries human hepatocyte transplants (23, 24). The p52−/p36− parasites were able to infect chimeric mouse livers and were clearly detected one day after sporozoite inoculation. However, they were not detected 4 days after infection by either immunohistochemical methods with parasite-specific antibodies or RT-PCR for the 18S ribosomal RNA of P. falciparum. In contrast, WT parasites were detected in the chimeric livers 4 days after infection by RT-PCR as well as immunohistochemical methods. The findings in conjunction with the in vitro observations demonstrate that the knockout parasites remain infectious but do not develop and do not persist in the host cells. Previous experience with irradiation-attenuated rodent malaria parasites indicated that persistence of liver stages might be a prerequisite for protection (35). However, more recent data obtained for the p52−/p36− GAP rodent malaria line showed clearly that persistence of parasites is not necessary to induce and maintain protection against sporozoite challenge (28).
Together, our results demonstrate the critical role of P52 and P36 in P. falciparum hepatocyte infection. The phenotype of arrested liver-stage development exhibited by the p52−/p36− P. falciparum justifies its testing as a live-attenuated vaccine candidate. Based on the promising preclinical data gathered, the P. falciparum p52−/p36− GAP line could be selected for advancing into proof-of-concept (POC) clinical development with administration to human volunteers via bite of A. stephensi mosquitoes (36). To support the POC investigational new drug program, it will be critical to produce a p52−/p36− GAP Master Cell Bank under phase-appropriate current Good Manufacturing Practice conditions and characterize it per respective Food and Drug Administration and International Conference of Harmonization guidance. Next, it would be important to conduct a phase 1/2a study of the p52−/p36− GAP vaccine candidate with administration via mosquito bite to healthy malaria-naive adults. Phase 1 would involve a dose-escalation step to assess safety and tolerability. If safety criteria are met, the phase 2a study would commence with p52−/p36− GAP vaccination followed by challenge with P. falciparum WT-infected mosquitoes to assess safety, preliminary efficacy, and immunogenicity of the GAP malaria vaccine candidate. With demonstration of protective efficacy, subjects would be rechallenged 6 months later to assess longevity of protection.
Clinical development of a parenteral formulation of genetically attenuated sporozoites is essential. A demonstration that the p52−/p36− GAP is safe, well-tolerated, and offers protection against malaria challenge, would allow for further clinical investigation in areas that are endemic for malaria by using a second-generation parenteral GAP vaccine product.
A designed P. falciparum live vaccine candidate that has been attenuated by gene deletion as presented here offers the advantages of genetic homogeneity, standardization, batch-to-batch consistency, testable genetic identity, and possibly improved safety of the vaccine with regard to breakthrough infections. These are critical factors on the path to development of a live-attenuated human malaria vaccine.
Materials and Methods
Additional materials and methods are included in the SI Text.
Design and Production of Gene-Targeting Constructs.
Targeting sequences for P. falciparum P52 and P36 were cloned into plasmid pCC1 to facilitate positive–negative selection (18). Restriction sites in the multiple-cloning site (MCS) were SacII/SpeI for the 5′ flank and AvrII/SfoI for the 3′ flank. Sequencing was performed to confirm inserts. Primer sequences can be found in the SI Text.
Transfection of P. falciparum with Targeting Constructs.
Transfection of P. falciparum with targeting constructs was preformed as described previously (37, 38). This was followed by negative selection against the cytosine deaminase/uracil phosphoribosyl transferase gene product with 5-fluorocytosine to obtain a parental line with double-cross-over homologous recombination, which results in specific gene deletion. More information about transfection can be found in SI Text.
Design and Generation of p52−/p36− Double-Gene Deletion Parasite Lines.
To produce p52−/p36− double-gene knockout parasites, we followed the methods described above for P52 or P36 single-gene disruption, except that the NF54 line was used as recipient parasite because the NF54 line is a more stable gametocyte producer then 3D7 under continuous culture. We considered this important because this line might move forward into production as a vaccine candidate. NF54 and 3D7 showed no differences in sporozoite cell invasion and liver-stage development (Tables S8 and S9), and thus direct comparison between knockouts is possible.
RT-PCR and Southern Blotting.
A total of 2.4 million P. falciparum sporozoites per parasite line were used for RNA extraction with TRIzol (Invitrogen) and were treated with amplification-grade DNAseI (Invitrogen). cDNA was synthesized with SuperScript III Platinum RT-PCR kit (Invitrogen). Amplification with P52 and P36 gene-specific primers was done for 35 cycles at 94 °C −30 sec, 55 °C −30 sec, and 60 °C −2 min. Primers used for P52 were: forward 5′-CCAGAAAATTGCCCTTCTAGAGCCTTTGTT-3′, reverse 5′- GCCCAATACATCATTTGAATAAGCATG-3′; and for P36 were: forward 5′-TGTTTACACTCGAATGTGGGATGGCATCCT-3′, reverse 5′-GAATGGCATGTAAATTCCCACATTATATCT-3′. Southern blotting methodology is described in detail in SI Text.
Mosquito Infections.
Gametocyte cultures of WT P. falciparum and knockout lines were cultured in vitro by using pooled human A+ sera (Interstate Blood Bank), RPMI-Hepes (Life Technologies/GIBCO), hypoxanthine (Sigma), and washed, type O+ erythrocytes. Media were changed daily, and exflagellation was observed at room temperature by phase-contrast microscopy at 200× magnification beginning 12 to 13 days after the cultures were initiated. Parasites from the cultures were fed to the mosquitoes when the majority of the gametocytes were morphologically mature and vigorous exflagellation was observed. A. stephensi aged 4–7 days were prestarved for 2–4 h and then fed for a minimum of 30 min on a 37 °C culture by using a membrane feeder apparatus with bandruche membrane (Joseph Long Inc.). One cage of 250–300 mosquitoes was exposed to concentrated erythrocytes from a 30-mL gametocyte culture mixed with an equal volume of fresh erythrocytes and 2 volumes of serum. Mosquitoes were incubated at 27 °C, 80% humidity, and sporozoites were harvested at 16–22 days after infection.
Sporozoite Counts and Motility Assays.
A total of 20,000 sporozoites were seeded per well on 12-well glass slides previously coated with 3% BSA in RPMI-1640. The slides were incubated at 37 °C for 1 h. They were fixed for 10 min with 4% paraformaldehyde at room temperature and washed with 1% FBS in 1× PBS. Slides were blocked with 10% FCS/PBS overnight at 4 °C. Sporozoite trails were immunostained by incubation with anti-PfCSP monoclonal antibody for 45 min at 37 °C and were washed with 1% FCS/PBS. Slides were incubated with anti-mouse IgG AlexaFluor 488 (1:200; Molecular Probes) for 45 min at 37 °C and washed with 1% FCS/PBS. Slides were mounted by using Vectashield mounting medium (Vector Laboratories) and were evaluated at 400× magnification by epifluorescence microscopy (Olympus BX 50 microscope). Quantification was performed by direct microscopic counting of triplicate wells. For p52−/p36− parasite lines, the experiment was performed in 3 independent experiments.
In Vitro Invasion and Development Assays.
Invasion assay was preformed as described previously (39). For more details, please refer to SI Text. Development assays were performed by adding 60,000 sporozoites per well to HC-04 cell monolayers in 8-well Permanox Labtek chamber slides (Thermo Fisher Scientific). Excess sporozoites were removed, and cells were washed after 3-h incubation at 37 °C and 5% CO2. Cultures were maintained with daily medium changes for 72, 96, and 144 h. Chamber slides were methanol-fixed and stained by using an mAb against HSP70 (mAb 4C9) (40), CSP (mAb 2A10) (41), LSA-1 (42), and EBA175 (MRA-2; MR4; American Type Culture Collection) as the primary antibody and AlexaFluor 488 anti-mouse IgG (Molecular Probes) as the secondary antibody diluted in 0.1% Evans blue/PBS in a similar manner as described above. Slides were mounted by using Vectashield plus DAPI (Vector Laboratories). The total number of liver stages per well were counted in triplicate wells in 3 independent experiments by using an Olympus BX 50 epifluorescent microscope. Parasites were observed by epifluorescence microscopy at 400× magnification. Photographs were taken by using a BioRad Radiance 2100 Confocal microscope.
In Vivo Assessment of Infection in a Hepatic Chimera Murine Model.
To assess defects in the liver-stage development for p52−, p36−, and p52−/p36− knockout parasites, we used a human hepatic chimera murine model developed by Mercer et al. (24). Methodology to evaluate P. falciparum infection and liver-stage development in the chimera mice was described by Sacci et al. (23). For a more detailed description, please refer to the SI Text.
Statistical Analysis.
Quantitative differences in salivary gland sporozoites, gliding motility activity, hepatocyte invasion, and liver-stage development between WT and mutant parasite lines were evaluated statistically by using the Wilcoxon matched-pairs signed-rank test at the 95% confidence level with STATA version 10.1 (StataCorp). Paired tests were performed to account for temporal variation in assay conditions.
Supplementary Material
Acknowledgments.
This work was funded by a grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative. Development of the SCID Alb-uPA model for P. falciparum was funded by National Institutes of Health Grant AI067980 (to J.B.S.).
Footnotes
Conflict of interest statement: S.H.I.K. is an inventor listed on U.S. Patent No. 7,22,179, U.S. Patent No. 7,261,884, and international patent application PCT/US2004/043023, each titled “Live Genetically Attenuated Malaria Vaccine.”
This article contains supporting information online at www.pnas.org/cgi/content/full/0906387106/DCSupplemental.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 3.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973;266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rieckmann KH, Carson PE, Beaudoin RL, Cassells JS, Sell KW. Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1974;68:258–259. doi: 10.1016/0035-9203(74)90129-1. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman SL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 6.Luke TC, Hoffman SL. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J Exp Biol. 2003;206:3803–3808. doi: 10.1242/jeb.00644. [DOI] [PubMed] [Google Scholar]
- 7.Mueller AK, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci USA. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 9.Tarun AS, et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J Infect Dis. 2007;196:608–616. doi: 10.1086/519742. [DOI] [PubMed] [Google Scholar]
- 10.Mikolajczak SA, Jacobs-Lorena V, MacKellar DC, Camargo N, Kappe SH. L-FABP is a critical host factor for successful malaria liver stage development. Int J Parasitol. 2007;37:483–489. doi: 10.1016/j.ijpara.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 11.van Dijk MR, et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci USA. 2005;102:12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005;58:1264–1275. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- 13.Jobe O, et al. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex Class I-dependent interferon-gamma-producing CD8+ T cells. J Infect Dis. 2007;196:599–607. doi: 10.1086/519743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller AK, et al. Genetically attenuated Plasmodium berghei liver stages persist and elicit sterile protection primarily via CD8 T cells. Am J Pathol. 2007;171:107–115. doi: 10.2353/ajpath.2007.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kappe SH, et al. Exploring the transcriptome of the malaria sporozoite stage. Proc Natl Acad Sci USA. 2001;98:9895–9900. doi: 10.1073/pnas.171185198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuboi T, et al. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect Immun. 2008;76:1702–1708. doi: 10.1128/IAI.01539-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasonder E, et al. Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog. 2008;4:e1000195. doi: 10.1371/journal.ppat.1000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maier AG, Braks JA, Waters AP, Cowman AF. Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol Biochem Parasitol. 2006;150:118–121. doi: 10.1016/j.molbiopara.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart MJ, Vanderberg JP. Malaria sporozoites release circumsporozoite protein from their apical end and translocate it along their surface. J Protozool. 1991;38:411–421. doi: 10.1111/j.1550-7408.1991.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 21.Sattabongkot J, et al. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg. 2006;74:708–715. [PubMed] [Google Scholar]
- 22.Sim BK, et al. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol. 1990;111:1877–1884. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacci JB, Jr, et al. Plasmodium falciparum infection and exoerythrocytic development in mice with chimeric human livers. Int J Parasitol. 2006;36:353–360. doi: 10.1016/j.ijpara.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Mercer DF, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 25.Greenwood BM, et al. Malaria: Progress, perils, and prospects for eradication. J Clin Invest. 2008;118:1266–1276. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alonso PL, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: Single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 27.Aponte JJ, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: A double blind randomised controlled phase I/IIb trial. Lancet. 2007;370:1543–1551. doi: 10.1016/S0140-6736(07)61542-6. [DOI] [PubMed] [Google Scholar]
- 28.Labaied M, et al. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun. 2007;75:3758–3768. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corcoran LM, Forsyth KP, Bianco AE, Brown GV, Kemp DJ. Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell. 1986;44:87–95. doi: 10.1016/0092-8674(86)90487-3. [DOI] [PubMed] [Google Scholar]
- 30.Day KP, et al. Genes necessary for expression of a virulence determinant and for transmission of Plasmodium falciparum are located on a 0.3-megabase region of chromosome 9. Proc Natl Acad Sci USA. 1993;90:8292–8296. doi: 10.1073/pnas.90.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodyer ID, Johnson J, Eisenthal R, Hayes DJ. Purification of mature-stage Plasmodium falciparum by gelatine flotation. Ann Trop Med Parasitol. 1994;88:209–211. doi: 10.1080/00034983.1994.11812859. [DOI] [PubMed] [Google Scholar]
- 32.van Schaijk BC, et al. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS ONE. 2008;3:e3549. doi: 10.1371/journal.pone.0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tewari R, Dorin D, Moon R, Doerig C, Billker O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol. 2005;58:1253–1263. doi: 10.1111/j.1365-2958.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- 34.Dorin-Semblat D, et al. Functional characterization of both MAP kinases of the human malaria parasite Plasmodium falciparum by reverse genetics. Mol Microbiol. 2007;65:1170–1180. doi: 10.1111/j.1365-2958.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 35.Scheller LF, Azad AF. Maintenance of protective immunity against malaria by persistent hepatic parasites derived from irradiated sporozoites. Proc Natl Acad Sci USA. 1995;92:4066–4068. doi: 10.1073/pnas.92.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein JE, et al. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis. 2007;196:145–154. doi: 10.1086/518510. [DOI] [PubMed] [Google Scholar]
- 37.Trager W, Jenson JB. Cultivation of malarial parasites. Nature. 1978;273:621–622. doi: 10.1038/273621a0. [DOI] [PubMed] [Google Scholar]
- 38.Crabb BS, et al. Transfection of the human malaria parasite Plasmodium falciparum. Methods Mol Biol. 2004;270:263–276. doi: 10.1385/1-59259-793-9:263. [DOI] [PubMed] [Google Scholar]
- 39.Hollingdale MR, Leland P, Leef JL, Schwartz AL. Entry of Plasmodium berghei sporozoites into cultured cells, and their transformation into trophozoites. Am J Trop Med Hyg. 1983;32:685–690. doi: 10.4269/ajtmh.1983.32.685. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol Res. 1994;80:16–21. doi: 10.1007/BF00932618. [DOI] [PubMed] [Google Scholar]
- 41.Nardin EH, et al. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982;156:20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam JP, Zavala F. Multiple antigen peptide. A novel approach to increase detection sensitivity of synthetic peptides in solid-phase immunoassays. J Immunol Methods. 1989;124:53–61. doi: 10.1016/0022-1759(89)90185-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.