Abstract
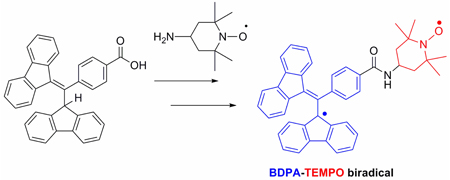
The synthesis and characterization of a biradical containing a 1,3-bisdiphenylene-2-phenylallyl (BDPA) free radical covalently attached to a 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) free radical are described. The synthesis of the biradical is a step towards improved polarizing agents for dynamic nuclear polarization (DNP).
Biradicals are of considerable interest as polarizing agents for microwave driven dynamic nuclear polarization (DNP) NMR experiments.1,2 Irradiation of the biradical’s electron paramagnetic resonance (EPR) drives electron-nuclear transitions that transfer the large polarization of the electrons to the nuclear spins and thereby enhances the signal-to-noise ratio in NMR experiments. The enhancement factors can reach a theoretical maximum of ~660 for electron-1H polarization transfer and ~2600 when 13C is the nuclear spin of interest.3 Thus, optimized biradical polarizing agents can dramatically decrease acquisition times. These signal enhancements are important for a variety of applications involving solid-state NMR, particularly for systems that are not amenable to crystallographic studies, such as amyloid4 and membrane5 proteins.
In previous work, we demonstrated that bis-nitroxide biradicals, which contain two tethered TEMPO moieties, provide 1H/13C-signal enhancements ~200 fold in solid-state magic-angle-spinning (MAS) NMR spectra.2 We are interested in molecules that can produce DNP by a cross effect (CE) mechanism3 that involves three spins, two dipolar coupled electrons and a nuclear spin (usually 1H), denoted by S1 and S2 and I, with electron and nuclear Larmor frequencies, ω0S1, ω0S2, and ω0I, respectively. In a DNP experiment, microwave irradiation of the biradical’s EPR spectrum induces the two electrons to undergo a spin flip-flop process, during which a nuclear spin is polarized if the electron Larmor frequencies are separated by the nuclear Larmor frequency and therefore satisfy the matching condition, ω0S1 –ω0S2 = ω0I. The high 1H polarizaton is then transferred via cross-polarization to 13C or 15N, resulting in an enhanced MAS NMR spectrum.6
The efficiency of the CE mechanism depends on how many pairs of electrons satisfy the matching condition. Thus, the ideal CE polarizing agent would be a biradical with an EPR spectrum consisting of two sharp lines separated by ω0I. However, at the high magnetic fields (>5T) where contemporary NMR experiments are performed, only a few known radicals exhibit narrow spectra. Among them are two stable species – trityl radical derivatives7 and the BDPA radical8 (Scheme 1), which have similar isotropic g-values (giso(trityl)= 2.00307,1 giso(BDPA)= 2.00264). If trityl or BDPA serves as one of the lines in the EPR spectrum of a polarizing agent, then to satisfy the CE matching condition it is necessary to introduce another radical with a line separated from the first by ω0I. There are no known stable radicals that provide a narrow line and meet this condition, however TEMPO derivatives have a broad line with significant spectral density at a frequency separation matching ω0I.
Scheme 1.
BDPA radical syntheses
Recently, one of us showed that the enhancements observed with a trityl-TEMPO mixture are a factor of ~4 larger than those obtained with TEMPO alone.1 The success of this experiment provides the rationale for the synthesis of a biradical that covalently combines a narrow- and broad-line radical, therefore increasing the dipolar coupling. BDPA was chosen as the narrow-line species, because it has greater stability and a narrower linewidth than trityl at high magnetic fields. Currently, DNP can only be effectively performed on biradicals in aqueous solutions that form a rigid glass at T < 90 K. Due to the challenges of making the nonpolar BDPA and TEMPO radicals water-soluble, we first chose to synthesize a hydrophobic BDPA-TEMPO biradical as a model compound. As described in this communication, we have developed synthetic methods to join the two sensitive functionalities and studied the biradical’s EPR properties.
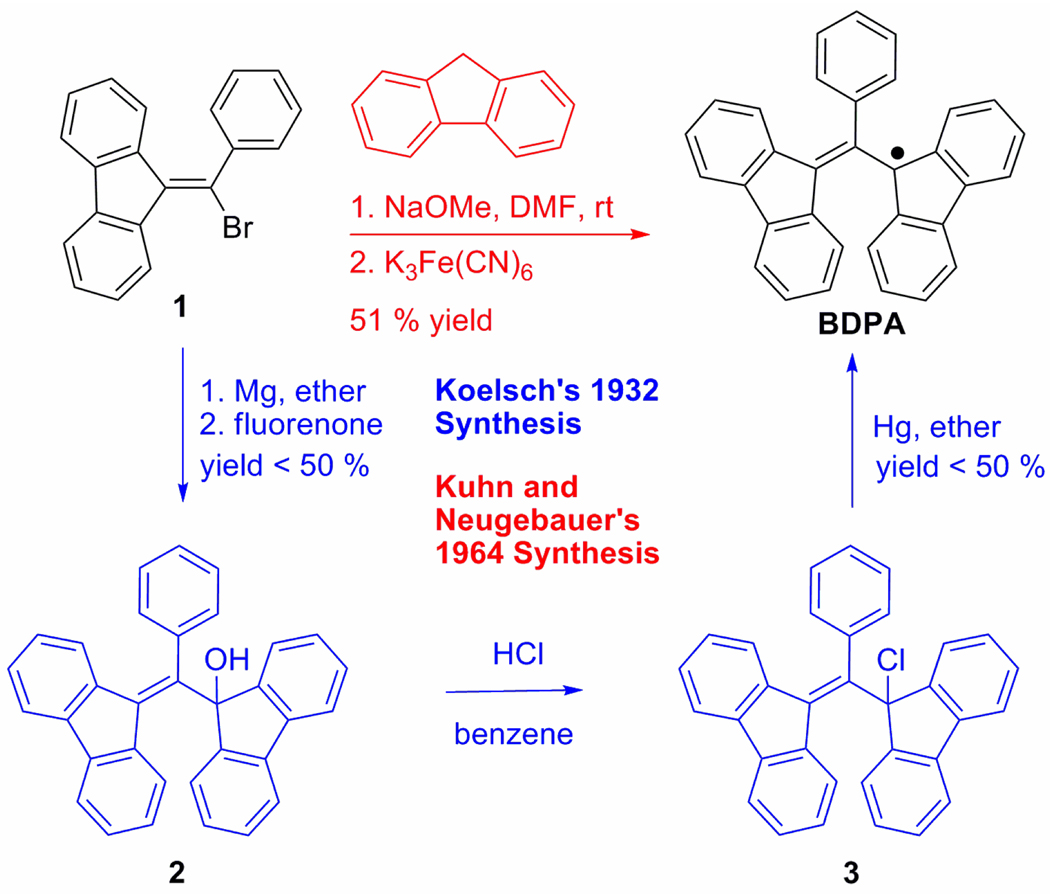
In attempting to synthesize a BDPA-TEMPO biradical (9), our work began by investiging how BDPA was previously made. Koelsch’s original method9 (Scheme 1, blue) involves the formation of alcohol 2, the displacement of the hydroxyl-group with a chloride to form 3, and the removal of a chlorine radical with mercury. The method developed by Kuhn and Neugebauer10 (Scheme 1, red) involves a conjugate addition of the fluorene anion to 1, followed by a one-electron oxidation of the stable carbanion intermediate. The latter method was pursued to synthesize a functionalized BDPA derivative because it requires fewer steps and provides higher yields.
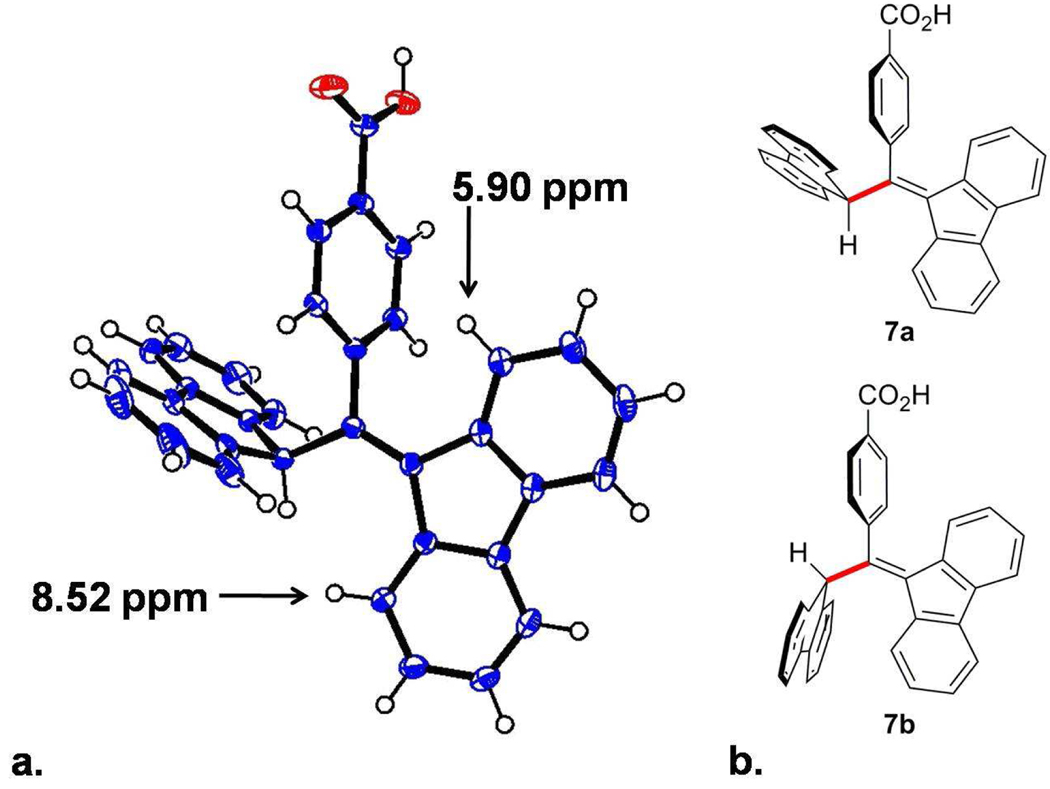
Figure 1.
(a) X-ray ORTEP representation of 7 (50% probability level). (b) Rotamers of 7.
Efforts to synthesize BDPA derivatives have been limited.11 Kuhn10 reported halogenated BDPA derivatives, and Fox12 reported the synthesis of BDPA derivatives with methoxy-, cyano-, and nitro-groups at the 4-position of the phenyl ring. Previously synthesized biradicals containing BDPA have been limited to molecules containing two BDPA radicals linked through the phenyl ring.10
The reactivity of the BDPA radical complicates its incorporation in biradicals. Although the radical is remarkably stable to oxygen in the solid state, and has been reported to be indefinitely stable to oxygen in solution with the exclusion of light, its photoreactivity produces a variety of oxidation products in solution. 12 Additionally, solutions of the radical are reduced to give the corresponding carbanion when exposed to strong bases, such as hydroxide or alkoxide, and BDPA also reacts with strong acids. 9
The unpaired electron of BDPA is delocalized throughout the fluorenyl blades, but it is not appreciably delocalized into the phenyl blade.13 Based on this fact, we chose to connect TEMPO through an amide linkage14 at the para-position of the phenyl ring to minimize the disruption of the radical’s stability and its propeller-like geometry.
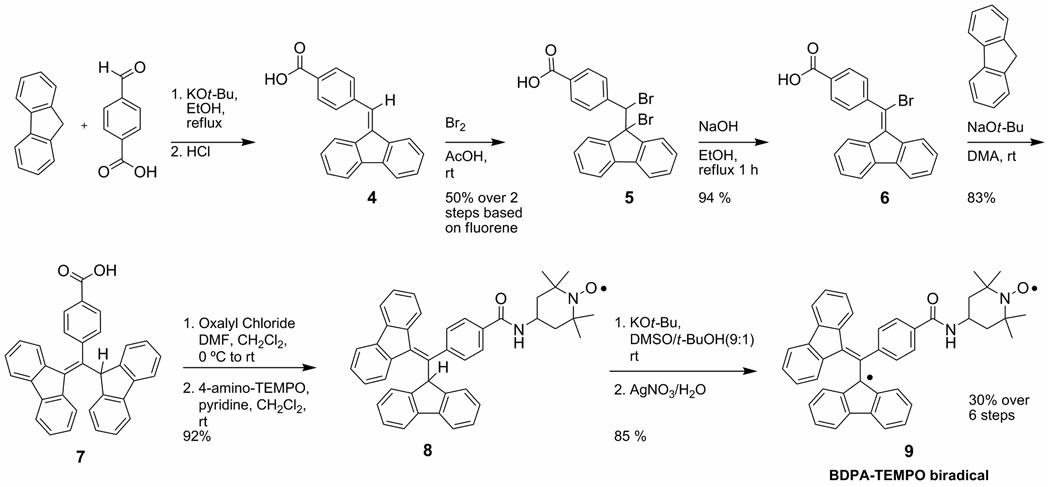
The synthesis of the BDPA-TEMPO biradical is shown in Scheme 2. Compound 4 was prepared by a condensation of fluorene and 4-carboxybenzaldehyde. Purification of 4 was inefficient as a result of the presence of 4-methylbenzoic acid, which is difficult to remove from commercial 4-carboxybenzaldehyde.15 Nevertheless, pure acid 4 was obtained by recrystallization tetrahydrofuran/acetic acid), but with a significant loss of material. Carrying forward the impure material proved a better option, because bromination of acid 4 gives compound 5, which has lower solubility and allows isolation of the pure solid by simple filtration. Dibromide 5 was refluxed in ethanol with sodium hydroxide to eliminate hydrogen bromide and produce the conjugate acceptor 6. We found this reaction to be concentration sensitive. At higher concentrations, bromine (Br2) instead of hydrogen bromide was eliminated. Subsequent reaction of 6 with the fluorene anion, which is generated by deprotonation with sodium t-butoxide in dimethylacetamide (DMA), yields a deep blue solution of the stabilized carbanion. This intermediate is quenched with 2M HCl and purified to give the functionalized BDPA precursor 7, which is isolated as a white, air-stable powder.
Scheme 2.
Synthesis of BDPA-TEMPO biradical.
The X-ray crystal structure of 7 (Figure 1) shows its preferred geometry, which explains its unique 1H-NMR spectrum. In solution, one 1H-nucleus resonates at 5.90 ppm. The upfield shift is caused by the nearby phenyl group’s local magnetic field. In contrast, the 1H-nucleus on the opposite side of the fluorene moiety resonates at 8.52 ppm, and appears to interact with the proximate proton on the sp3-hybridized carbon. This interaction has been confirmed by the observation of a 20% NOE enhancement in solution. In the process of characterizing 7 by NMR, we observed a second rotamer, 7b, that appears stable in the solid state, but which slowly converts to the more stable form, 7a, in solution (Figure 1).
Rotamer 7b is observed in the proton NMR of nonrecrystallized 7. The conversion of 7b to 7a was monitored by NMR and was complete within a week in d8-tetrahydrofuran at room temperature. Additionally, 7b can be regenerated from samples of pure 7a by deprotonation followed by an acid quench.16 To synthesize 9, carboxylic acid 7 was converted to the acid chloride with oxalyl chloride and catalytic DMF, and then reacted with 4-amino TEMPO. The proton on the sp3-hybridized carbon of 7 is presumed to be slighly more acidic than that of the protonated BDPA precursor (pKa = 14, DMSO).17 To avoid side reactions, the weak base pyridine is used to accelerate amide formation and scavenge protons. A portion of 8 was reduced with ascorbic acid, converting the TEMPO radical to the hydroxylamine, for characterization using 1H- and 13C-NMR. The biradical 9 was generated by deprotonating 8, followed by one-electron oxidation.
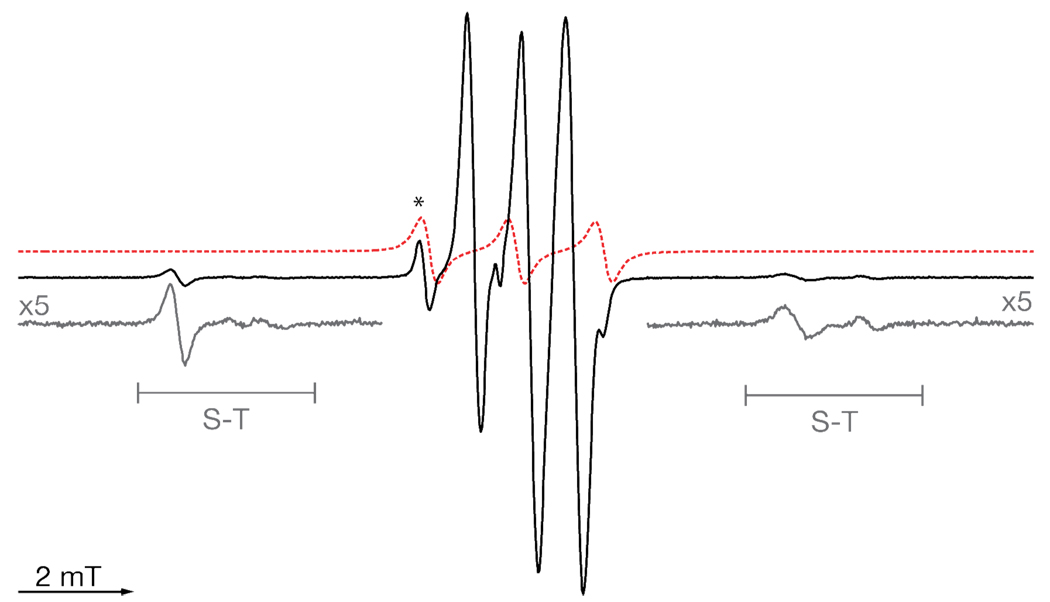
The 9 GHz liquid-state EPR spectrum of 9 and of precursor 8 are shown in Figure 2. A typical spectrum of a nitroxide radical consists of three sharp lines. The spectrum of the biradical differs due to the presence of the BDPA radical and the interaction between the two paramagnetic centers. Additionally, several new resonances are observed well separated from the central region of the spectrum. These features arise from forbidden singlet-triplet transitions (S–T) and are common in spectra of biradicals that feature an intermediate J-coupling. A J-coupling of 140 MHz was measured based on the separation of the peaks from the central region.18
Figure 2.
9 GHz liquid-state EPR of 9 (black) and 8 (red) in toluene. The regions with enlarged vertical scales (grey) show the forbidden singlet-triplet transitions. Experimental parameters: rt, microwave power 2 mW, sweep width 20 mT, modulation amplitude 10 mT.
Present in the spectrum is an impurity with an EPR signal characteristic of a TEMPO radical attached to a diamagnetic fragment (Figure 2, indicated by asterisk), which we attribute to unreacted 8. We have evaluated the efficiency of the conversion of 8 to 9 using several pieces of evidence. First, we obtain nearly quantitative mass recovery (95%), and see no indication of amide-bond cleavage under the reaction conditions in TLC. Additionally, the TEMPO moiety is unaffected by the reaction conditions. Integration of the EPR signal indicates that on a per molecule basis there is less than 10% of the monoradical impurity present (see Supporting Information). Based on this evidence, we estimate the efficiency of the reaction to be 85%.
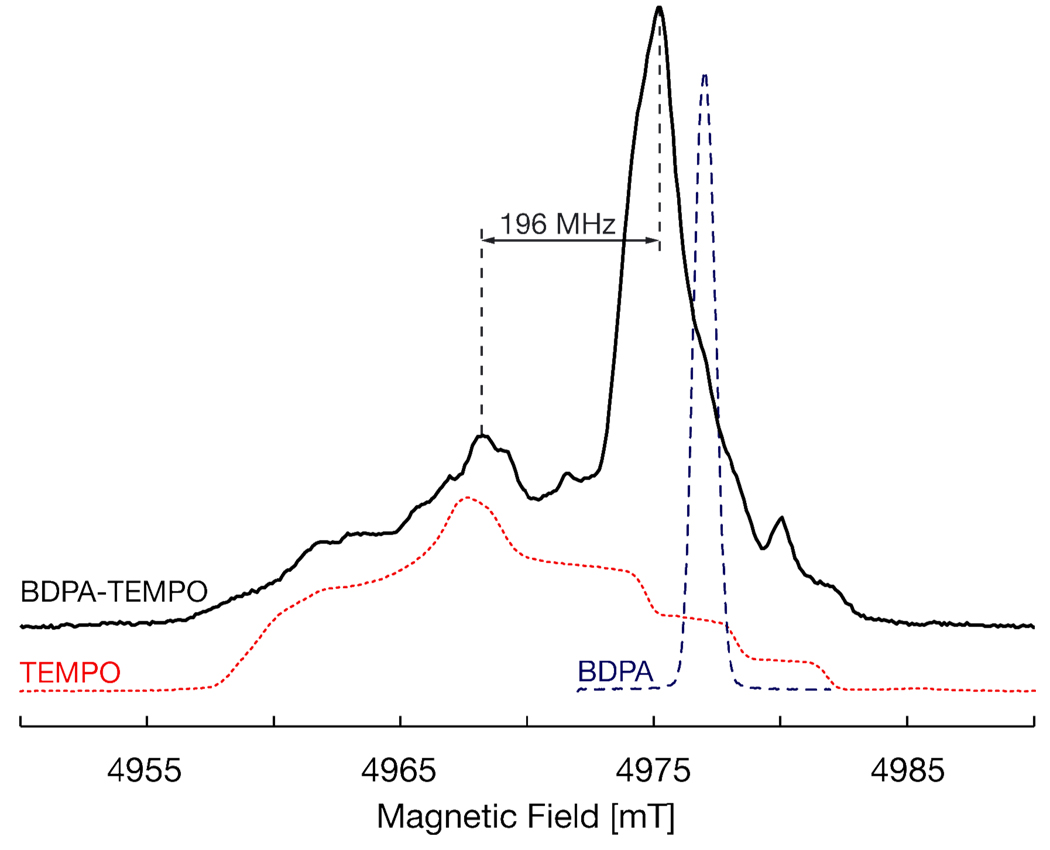
For high-field (HF) DNP experiments, the HF-EPR spectrum in the solid-state is of particular interest (Figure 3). The spectrum of the biradical shows features characteristic of both BDPA and TEMPO, as well as new features that indicate the presence of an electron-electron interaction. Most notable is the shift of the BDPA line to lower field.
Figure 3.
140 GHz echo-detected solid-state EPR spectra of 9 (black), TEMPO (red), and BDPA (blue) in toluene. Experiments are performed at 20 K, with tp(π/2) = 44 ns, and typically 100 scans are averaged for each point.
In summary, we have developed a general method to functionalize BDPA by synthesizing a BDPA precursor (7) with a carboxylic acid functional group. We have used this precursor to synthesize a BDPA-TEMPO biradical (9) in good yield and studied the resulting EPR spectrum. The HF-EPR spectrum, with two peaks, one narrow and one broad, separated by a value close to the1H Larmor frequency, has the desired characteristics for DNP. Future work will focus on adding water-solubilizing groups so that the biradical can be tested for DNP-enhancements in aqueous solutions.
Supplementary Material
Supporting Information Available
Experimental and spectral data for all compounds is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We thank Dr. Peter Mueller (MIT) for providing X-ray crystal structures. This work was funded in part by the National Science Foundation DMR-0706408. RGG acknowledges the support of the National Institute of Bio-medical Imaging and Bioengineering (EB-002804 and EB-002026)
References
- 1.Hu K-N, Bajaj VS, Rosay MM, Griffin RG. J. Chem. Phys. 2007;126:044512. doi: 10.1063/1.2429658. [DOI] [PubMed] [Google Scholar]
- 2.(a) Hu K-N, Yu H-h, Swager TM, Griffin RG. J. Am. Chem. Soc. 2004;126:10844. doi: 10.1021/ja039749a. [DOI] [PubMed] [Google Scholar]; (b) Song C, Hu K-N, Swager TM, Griffin RG. J. Amer. Chem. Soc. 2006;128:11385. doi: 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]
- 3.(a) Maly T, Debelouchina GT, Bajaj VS, Hu K-N, Joo C-G, Mak-Jurkauskas ML, Sigirhi JR, van der Wel PCA, Herzfeld J, Temkin RJ, Griffin RG. J. Chem. Phys. 2008;128:052211. doi: 10.1063/1.2833582. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barnes AB, Paëpe GD, Wel PCAvd, Hu K-N, Joo C-G, Bajaj VS, Mak-Jurkauskas ML, Sirigiri JR, Herzfeld J, Temkin RJ, Griffin RG. Appl. Magn. Reson. 2008;34:237. doi: 10.1007/s00723-008-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Jaroniec CP, MacPhee CE, Bajaj VS, McMahon MT, Dobson CM, Griffin RG. Proc. Natl. Acad. Sci. U.S.A. 2004;101:711. doi: 10.1073/pnas.0304849101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) van der Wel PCA, Hu K-N, Lewandowski JR, Griffin RG. J. Amer. Chem. Soc. 2006;128:10840. doi: 10.1021/ja0626685. [DOI] [PubMed] [Google Scholar]; (c) van der Wel PCA, Lewandowski JR, Griffin RG. J. Amer. Chem. Soc. 2007;129:5117. doi: 10.1021/ja068633m. [DOI] [PubMed] [Google Scholar]
- 5.(a) Bajaj VS, Hornstein MK, Kreischer KE, Sirigiri JR, Woskov PP, Mak-Jurkauskas ML, Herzfeld J, Temkin RJ, Griffin RG. J. Magn. Reson. 2007;189:251. doi: 10.1016/j.jmr.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mak-Jurkauskas ML, Bajaj VS, Hornstein MK, Belenky M, Griffin RG. Proc. Natl. Acad. Sci. U.S.A. 2008;105:883. doi: 10.1073/pnas.0706156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Pine A, Gibby MG, Waugh JS. J. Chem. Physics. 1972;56:1776. [Google Scholar]; (b) Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. J. Chem. Physics. 1995;103:6951. [Google Scholar]
- 7.(a) Reddy TJ, Iwama T, Halpern HJ, Rawal VH. J. Org. Chem. 2002;67:4635. doi: 10.1021/jo011068f. [DOI] [PubMed] [Google Scholar]; (b) Bowman MK, Mailer C, Halpern HJ. J. Magn. Reson. 2005;172:254. doi: 10.1016/j.jmr.2004.10.010. [DOI] [PubMed] [Google Scholar]; (c) Liu Y, Villamena FA, Sun J, Xu Y, Dhimitruka I, Zweier JL. J. Org. Chem. 2008;73:1490. doi: 10.1021/jo7022747. [DOI] [PubMed] [Google Scholar]
- 8.1,3-bisdiphenylene-2-phenylallyl (BDPA) free radical and 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) free radical will be referred to as simply BDPA and TEMPO throughout the text.
- 9.Koelsch CF. J. Amer. Chem. Soc. 1957;79:4439. [Google Scholar]
- 10.Kuhn R, Neugebauer FA. Monatsh. Chem. 1964;95:3. [Google Scholar]
- 11.(a) Plater MJ, Kemp S, Lattmann E. J. Chem. Soc., Perkin Trans. 2000;1:971. [Google Scholar]; (b) Nishide H, Yoshioka N, Saitoh Y, Gotoh R, Miyakawa T, Tsuchida E. J. Macromol. Sci. Pure Appl. Chem. 1992;A29:775. [Google Scholar]
- 12.Breslin DT, Fox MA. J. Phys. Chem. 1993;97:13341. [Google Scholar]
- 13.Azuma N, Ozawa T, Yamauchi J. Bull. Chem. Soc. Jpn. 1994;67:31. [Google Scholar]
- 14.Sosnovsky G, Lukszo J, Brasch RC, Eriksson UG, Tozer TN. Eur. J. Med. Chem. 1989;24:241. [Google Scholar]
- 15.The impurity was identified by 1H-NMR and in our hands could not be removed from the commercial material through recrystallization, extraction, or vacuum sublimation.
- 16.See Supporting Information figure 1 and figure 2.
- 17.Kuhn R, Rewicki D. Liebigs Ann. Chem. 1967;706:250. [Google Scholar]
- 18.(a) Eaton SS, DuBois DL, Eaton GR. J. Magn. Reson. 1978;32:251. [Google Scholar]; (b) Eaton GR, Eaton SS. In: Spin Labelling, Theory and Applications. Berliner LJ, Reuben J, editors. New York, NY: Plenum Press; 1989. pp. 339–397. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available
Experimental and spectral data for all compounds is available free of charge via the Internet at http://pubs.acs.org.