Abstract
Early experiences such as prenatal stress significantly influence the development of the brain and the organization of behavior. In particular, prenatal stress impairs memory processes but the mechanism for this effect is not known. Hippocampal granule neurons are generated throughout life and are involved in hippocampal-dependent learning. Here, we report that prenatal stress in rats induced lifespan reduction of neurogenesis in the dentate gyrus and produced impairment in hippocampal-related spatial tasks. Prenatal stress blocked the increase of learning-induced neurogenesis. These data strengthen pathophysiological hypotheses that propose an early neurodevelopmental origin for psychopathological vulnerabilities in aging.
It is well documented from animal studies that during the perinatal period, the development of an organism is subjected to complex environmental influences. Deleterious life events during pregnancy induce neurobiological and behavioral defects in offspring, some of them involving the hippocampal formation (1–5). Indeed, prenatal stress results in an enhanced production of stress hormones by the mother during critical periods of fetal brain development and provokes a definitively longer corticosterone response to stress in the offspring associated with a reduction in the number of hippocampal corticosteroid receptors (1, 3, 5). Behaviorally, the progeny, from adulthood to senescence, exhibit memory deficits in a hippocampal-dependent task (2, 4, 5).
Recently, it has been hypothesized that hippocampal-mediated learning (6) may be related to the generation of new neurons in the adult dentate gyrus (7, 8, 9). These newborn cells migrate in the granule cell layer, and differentiate in granule neurons whose projections, the mossy fibers, extend to the CA3 hippocampal region (10, 11). Furthermore, the size of the mossy fibers' projections correlates with variations in performances in spatial memory tests (12, 13). Finally, glucocorticoid levels regulate de novo cell proliferation in the dentate gyrus. Indeed, adrenalectomy performed in young or aged rats increases neurogenesis, an effect that is prevented by glucocorticoid treatment (14, 15, 16). These results raised the critical question as to whether prenatal stress can impair neurogenesis and, if so, whether it is related to learning ability.
To test this hypothesis, we first examined cell proliferation in the progeny of stressed mothers with 5-bromo-2′-deoxyuridine (BrdUrd), a thymidine analogue incorporated into genetic material during synthetic DNA phase (S phase) of mitotic division. Cell-specific markers were used to phenotype the newly born neurons after longer survival times. We next examined whether the structural hippocampal defects resulting from prenatal stress had functional consequences on learning abilities. Finally, we examined whether the reduction in cell proliferation in the dentate gyrus had an impact on spatial memory, a hypothesis supported by others (17).
Methods
Housing Conditions.
Adult virgin Sprague–Dawley female rats (Iffa Credo) weighing 240 g were housed, 10 days after arrival, in the presence of a sexually experienced male Sprague–Dawley rat weighing 400 g. Pregnant females then were randomly assigned to prenatal stress (PS) or control (C) groups and individually housed in plastic breeding cages. Animals were allowed ad libitum access to food and water and were maintained on a constant light/dark cycle with constant temperature and humidity.
Prenatal Stress.
Stress was performed each day of the last week of pregnancy from day 15 until delivery. Pregnant females were individually restrained for 45 min three times a day during the light phase in plastic transparent cylinders (7-cm diameter, 19-cm long) exposed to bright light. Control pregnant females were left undisturbed in their home cages. The offspring were raised by their biological mothers until weaning (21 days after birth). Only litters of 8–13 pups with similar numbers of males and females were kept for the study, all other litters having been eliminated to rule out additional stressors such as removal of the pups. For each set of experiments, a maximum of two male pups was taken from each litter to remove any “litter effects” (18). After weaning, male rats from each experimental group (PS, n = 39; C, n = 34) were housed in groups of three. One month before each behavioral study, animals were individually housed.
Weight of Adrenal Glands.
To have an index of chronic hypothalamic–pituitary–adrenal (HPA) axis activity (19, 20), adrenal glands were collected on the day of the animals were killed. They were immediately weighed and results are expressed as a ratio of body weight.
Place Navigation Task.
Four-month-old rats (C, n = 10; PS, n = 10) were used in this experiment. The apparatus consisted of a circular swimming pool built of gray plastic (180-cm diameter × 60-cm high), which was filled with water at room temperature and made opaque by the addition of milk. In this task, animals are required to locate a platform submerged (1.5 cm) in the pool by using only the spatial cues available within the testing room. During the habituation phase (no platform), rats (C, n = 5; PS, n = 5) were given one trial per day (of 60 sec) over 3 days. During the testing phase, the platform was hidden in a fixed location in one of four quadrants halfway between the sidewalls and the center of the pool. The animals were given four trials per day (90 sec) over 5 days. As a control of training, rats of both groups (C, n = 5; PS, n = 5) were handled in parallel with the trained rats but were not submitted to the water maze task (manipulated groups).
BrdUrd Injections.
Rats were injected with BrdUrd dissolved in phosphate buffer (21). Animals of different ages received four injections: once 3 days before death, once 2 days before death, and twice the day before death (15, 16). To determine the phenotype of the newly born cells, 3-month-old control and prenatally stressed rats were killed 2 weeks after the last BrdUrd injection. For the behavioral study, 4-month-old animals trained in the water maze were injected once a day, 2 min before the first trial during the third, fourth, and fifth days of training. The manipulated groups were injected in parallel. Animals were killed the day after the last injection.
Histological Procedure.
Rats were deeply anesthetized with chloral hydrate (400 mg/kg i.p.) and were perfused with 150 ml of phosphate buffer saline (pH, 7.3) containing heparin (5 × 104 units/ml), followed by 300 ml of 4% paraformaldehyde in 0.1 M of phosphate buffer (pH, 7.3). After a 24-h postfixation period of the brains in paraformaldehyde, 50-μm frontal sections were cut on a vibratome and collected in PBS (0.1 M; pH, 7.4). Free-floating sections were processed in a standard immunohistochemical procedure (15, 16, 22). For BrdUrd labeling, sections were treated with 2 M HCl (30 min at 37°C) and then rinsed in borate buffer for 5 min (0.1 M; pH, 8.4). They were extensively washed with PBS, preincubated 45 min with PBS containing 0.3% Triton X-100 and 3% of horse normal serum (blocking solution), and incubated under agitation for 72 h at 4°C in mouse monoclonal anti-BrdUrd antibody (1/200; Dako) diluted in PBS containing 0.3 Triton X-100 and 1% of horse normal serum. The sections were then incubated under agitation for 2 h with a biotin-labeled horse anti-mouse IgG antibody (1/200; Vector Valbiotec, Paris). Sections from all animals were processed in parallel and immunoreactivities (IRs) were visualized by the biotin–streptavidin technique (ABC kit; Dako) with 3,3′-diaminobenzidine as chromogen (10-min incubation).
The phenotype of newly born cells was examined by double-labeled immunohistofluorescence according to a previously described method (22). Briefly, sections were incubated with a rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) antibody (1/10,000; Dako) or with a mouse monoclonal anti-neuronal nuclei (NeuN) antibody (1/1,000; Chemicon). Bound anti-GFAP or -NeuN antibodies were visualized respectively with an Alexa 488 goat anti-rabbit IgG antibody and a Alexa goat anti-mouse IgG antibody (1/1,000; Interchem, Montluçon, France). Then, sections were incubated with a rat monoclonal anti-BrdUrd antibody (1/1,000; Accurate Scientific, Westbury, NY), and bound anti-BrdUrd molecules were revealed by using a Cy3-labeled anti-rat IgG antibody (1/400; Jackson ImmunoResearch).
Quantitative Evaluation of Staining.
For each animal, starting randomly, BrdUrd-IR cells were counted in the left dorsal hippocampus, in one in five sections, 250 μm apart. All BrdUrd-IR cells were counted with a ×100 microscope objective in the subgranular layer and in the granular layer of the dentate gyrus. The sections then were counterstained and the surface of the granule cell layer was measured with a Samba 2640 system (Alcatel system; TITN Answare, Grenoble, France). For each section, the numerical density of BrdUrd-IR was calculated by dividing the number of BrdUrd-IR cells by granule cell layer sectional volume. For each animal, the mean numerical density of BrdUrd-IR was calculated. The total number of BrdUrd-IR cells per dentate gyrus then was calculated by multiplying the numerical density of BrdUrd-IR cells by the reference volume. The volume of the reference space was calculated according to the Cavalieri estimation (23) applying the following formula: Vref = a × t × s, where a is the mean area of the granule cell layer of the dentate gyrus, t is the thickness of the vibratome section (50 μm), and s is the total number of sections through the defined regions of the dorsal hippocampus. The number of pyknotic cells was determined on hematoxylin-counterstained sections by using a similar procedure.
The absolute number of granule cells, as assessed morphologically by hematoxylin staining, was determined by using a Samba 2640 system. For each section, granule cells were counted in 20 to 25 frames (20 μm × 20 μm) at evenly spaced x–y intervals of 110 μm by 110 μm. We disregarded cells that were in sharp focus in the uppermost focal plane (optical disector principle). The numerical density (number of cells/mm3) was calculated according to the following equation:
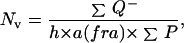 |
1 |
where (Q−) is the sum of cells, (P) the number of frame, (h) the disector height, and a(fra) the area of the counting frame. By using these sampling parameters, the number of optical disectors examined was 53.67 ± 1.10 [CE(Fi), 13.67 ± 0.22%], the number of cells counted per dentate gyrus was 221.32 ± 7.15 [CE(Qi), 13.99 ± 0.25%], and the CE(NV) was 13.69 ± 0.22% (CE is the coefficient of error). The total number of cells then was calculated by multiplying the numerical density by the volume of reference (ref. 23 and see above).
General Procedures.
First experiment: Prenatal stress and cell proliferation.
Juvenile, adult, middle-aged, and old rats (C, n = 20; PS, n = 25) were killed the day after the last BrdUrd injection. These rats were considered “naive” as they were not manipulated except for injections and were not behaviorally tested. The total number of BrdUrd-IR cells per dentate gyrus was counted on diaminobenzidine-immunostained sections. The absolute number of granule neurons was determined on the same sections after counterstaining. The total number of pyknotic cells was determined on alternate counterstained sections.
Second experiment: Phenotype of the newly born cells.
Adult naive rats (C, n = 4; PS, n = 4) were injected four times with BrdUrd and were killed 2 weeks after the last BrdUrd injection. The total number of BrdUrd-IR cells per dentate gyrus was counted on diaminobenzidine-immunostained sections. Double immunofluorescence was performed and the identity of newly born cells was examined with cell-specific markers.
Third experiment: Training in the water maze and cell proliferation.
Four-month-old rats were either trained in the water maze (trained groups: C, n = 5; PS, n = 5) or handled in parallel with the trained rats but not submitted to the water maze task (manipulated groups: C, n = 5; PS, n = 5). Rats were injected four times with BrdUrd (as described above) and were killed the day after the last injection. The total number of BrdUrd-IR cells per dentate gyrus was counted on diaminobenzidine-immunostained sections. The absolute number of granule neurons was determined on the same sections after counterstaining.
Results
Effect of Prenatal Stress on Cell Proliferation.
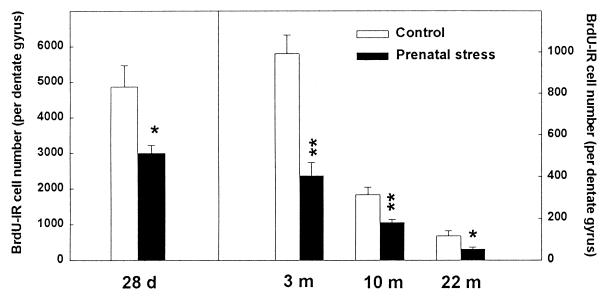
We first examined, in the progeny of stressed mothers, neurogenesis in the dentate gyrus, a process known to be regulated by corticosterone levels (14, 15, 16). We also examined whether cell proliferation was affected at specific times during the lifespan, including in senescent rats. Cell proliferation was evaluated in rats injected with BrdUrd; cells that incorporated BrdUrd (BrdUrd-IR cells) were revealed by immunohistochemistry. BrdUrd-IR cell number was determined in juvenile (28-day-old), adult (3-month-old), middle-aged (10-month-old), and old (22-month-old) naive rats (Figs. 1 and 2). There was a significant decline of proliferation as age increased in both control [H(3, 19) = 16.75, P < 0.001; 28 days, >3 months, >10 months, >22 months at P < 0.05 with a nonparametric Kruskal–Wallis ANOVA] and prenatally stressed rats [H(3, 25) = 22.09, P < 0.001; 28 days, >3 months, >10 months, >22 months at P < 0.01]. Furthermore, for the four ages investigated, prenatal stress consistently decreased cell proliferation {−38.4% at 28 days [H(1, 11) = 4.03, P < 0.05]; −59.3% at 3 months [H(1, 11) = 7.51, P < 0.01]; −42.3% at 10 months [H(1, 12) = 6.56, P = 0.01]; and −55.2% at 22 months of age [H(1, 10) = 4.69; P < 0.05]}. These differences were not related to differences in the volume of the granule cell layer [28 days: H(1, 11) = 0.33, not significant (n.s.); 3 months: H(1, 11) = 2.13, n.s.; 10 months: H(1, 12) = 0.10, n.s.; 22 months: H(1, 10) = 0.64, n.s.].
Figure 1.
Effect of prenatal stress on cell proliferation in 28-day-, 3-month-, 10-month-, and 22-month-old male rats. There was a significant decline of cell proliferation with increasing age in both control and prenatally stressed rats. Furthermore, for the four ages investigated, prenatal stress consistently decreased cell proliferation as compared with control. *, P < 0.05 in comparison to age-matched control group; **, P < 0.01. Notice the different ordinal scale between data for 28-day-old rats and the other ages studied.
Figure 2.
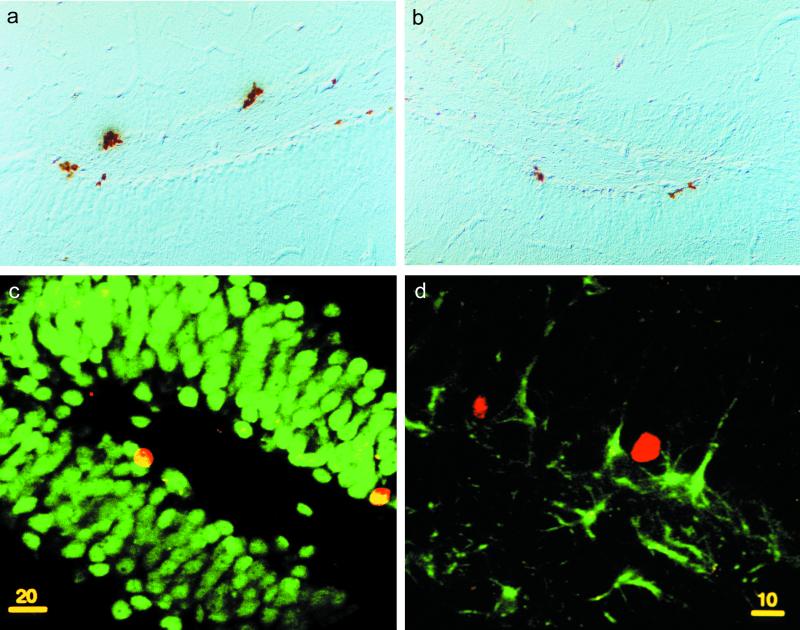
Illustration of BrdUrd-labeled cells in the dentate gyrus. Photomicrographs of BrdUrd immunoreactivity in frontal section of the dentate gyrus in a control (a) and a prenatally stressed (b) rat . (Scale = 1.5 cm for 500 μm.) (c) Confocal illustration of BrdUrd-labeled cells in the dentate gyrus showing that newborn cells were mainly neurons. Indeed, BrdUrd-IR cells (red nuclear stain, Cy3) colocalized with the neuronal marker NeuN (green stain) as indicated by the yellow nuclear staining. (d) Newly born cells are rarely astrocytes because BrdUrd-IR cells (red nuclear stain) is not stained for the astroglial marker GFAP (green stain).
Identity of Newly Born Cells.
To examine the phenotype of BrdUrd-IR cells born in the dentate gyrus of control (Fig. 2a) or prenatally stressed (Fig. 2b) rats, animals were allowed to survive for 2 weeks after the last BrdUrd injection. Newborn cells, phenotyped by analysis of double-labeled immunofluorescence sections, were mainly neurons and rarely astrocytes (Fig. 2 c and d). Indeed, 72 ± 2% and 70 ± 2% of BrdUrd-IR cells were immunoreactive for NeuN in control and prenatally stressed rats, respectively. These ratios were not significantly different among groups [H(1, 8) = 0.08; n.s.]. On the other hand, 20 ± 1.6% and 20 ± 1.6% of BrdUrd-IR cells were immunoreactive for GFAP in control and prenatally stressed rats, respectively. These ratios were not significantly different among groups [H(1, 8) = 2.08, n.s.]. The double labeling was verified with confocal microscopy. Exploration in the Z plane revealed that bound anti-GFAP antibodies were present throughout the section thickness, but bound anti-NeuN antibodies penetrate only a portion of the slice. Thus, the quantification of BrdUrd-NeuN double-labeled cells was underestimated.
Effect of Prenatal Stress on the Total Number of Granule Neurons.
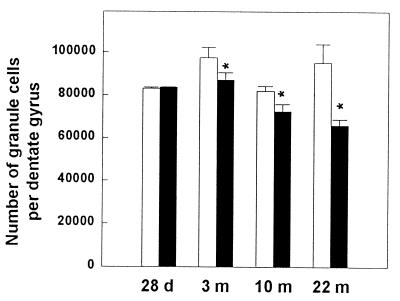
Because prenatal stress reduces cell proliferation in the dentate gyrus early in life and in aged rats, it was of interest to evaluate the consequence of this reduction on the total granule cell number. Although the total number of granule neurons was not different among different ages in control rats [H(3, 20) = 6.61, n.s.] (Fig. 3), there was a progressive decline of total granule cell number with increasing age after prenatal stress [H(3, 25) = 14.24, P < 0.01; 28 days, 3 months, >10 months, >22 months at P < 0.01]. Prenatal stress decreased the total number of granule neurons beginning at 3 months of age [H(1, 11) = 4.80, P < 0.05] and continued until 10 [H(1, 12) = 4.33, P < 0.05] and 22 [H(1, 10) = 4.69, P < 0.05] months of age.
Figure 3.
Effect of prenatal stress on total number of granule cells in 28-day-, 3-month-, 10-month-, and 22-month-old male rats. Although the total number of granule cells was not different among different ages in control rats, there was a progressive decline of total granule cell number with increasing age after prenatal stress. Interestingly, this decline in prenatally stressed rats was observable from 3 months of age continuing until 22 months of age. *, P < 0.05 in comparison to age-matched control group.
The proportion of newborn cells in the dentate gyrus was computed in percentage of total granule cells and represented 5.8% ± 0.7 in 28-day-old control rats (the largest percentage) to 0.08% ± 0.02 in 22-month-old prenatally stressed rats (the lowest percentage). Thus, the low proportion of newborn cells could explain why the deficit in cell proliferation is detectable before the decrease in the total number of granule cells in prenatally stressed rats.
Effect of Prenatal Stress on the Total Number of Pyknotic Cells.
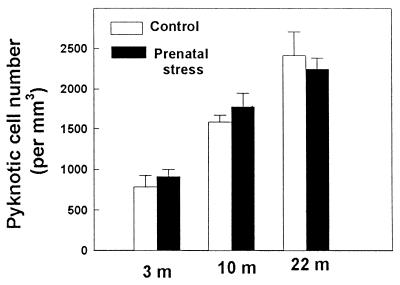
To determine whether the observed alteration of granule neurons number was related specifically to differences in cell proliferation, we evaluated the consequence of prenatal stress on pyknotic cell number (Fig. 4). The degenerating profiles were characterized by a condensed chromatin and a light or absent cytoplasm. Prenatal stress did not influence the total number of pyknotic cells at all ages tested [3 months, H(1, 11) = 0.80, n.s.; 10 months, H(1, 11) = 0.38, n.s.; 22 months, H(1, 10) = 0.11, n.s.]. In contrast, there was an age-related increase in degenerating profile in both control [H(2, 12) = 9.9, P < 0.01; 3 months, >10 months, >22 months at P < 0.01] and prenatally stressed [H(2, 19) = 22.09, P < 0.01; 3 months, >10 months, >22 months at P < 0.01] rats.
Figure 4.
Effect of prenatal stress on number of pyknotic cells in 3-month-, 10-month-, and 22-month-old male rats. With increasing age, there was a progressive increase in the total number of pyknotic cells. There was no effect of prenatal stress.
HPA Axis Activity.
The weight of adrenal glands (expressed as a ratio of the body weight) previously has been demonstrated to be a reliable index of the chronic activity of the HPA axis because a hypertrophy of the adrenal glands is observed in conditions of chronic HPA axis hyperactivity (19, 20). As shown on Table 1, this ratio was higher in prenatally stressed rats when compared with control rats [H(1, 11) = 4.03, P < 0.05; H(1, 11) = 7.50, P < 0.01; H(1, 12) = 3.69, P < 0.05)] indicating a chronic up-regulation of the HPA axis.
Table 1.
Effect of prenatal stress on adrenal weight
| Group | 28 Days | 3 Months | 10 Months |
|---|---|---|---|
| Control | 260.49 ± 7.74 | 105.41 ± 8.51 | 64.66 ± 1.49 |
| Prenatal stress | 297.88 ± 12.91* | 159.36 ± 6.10** | 101.00 ± 14.24* |
Data are expressed as a ratio of body weight (mg/kg of body weight). *, P < 0.05 and **, P < 0.01 as compared to controls.
Effect of Prenatal Stress on Spatial Learning.
We next examined whether such structural hippocampal defects resulting from prenatal stress had functional correlates on spatial memory and whether cell proliferation was influenced by training. We tested 4-month-old rats for spatial memory in a water maze.
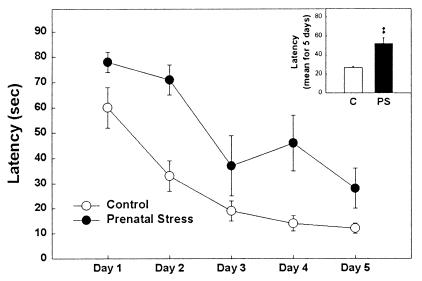
Rats were injected with BrdUrd before the testing session on days 3, 4, and 5 of learning. At 24 h after the last BrdUrd injection, all animals were perfused and cell proliferation was determined. There were significant differences in the rate of acquisition between control and prenatally stressed rats (Fig. 5). The latency to find the platform by the control group was significantly less than that observed for the prenatally stressed group [H(1, 10) = 6.82, P < 0.001]. The distance covered by control animals was shorter than that covered by prenatally stressed rats (data not shown, P < 0.05).
Figure 5.
Spatial learning in a water maze. The latency to find the platform by the control group was significantly less than that observed for the prenatally stressed group. (Inset) The means over the 5 days of testing. **, P < 0.01 in comparison to control group.
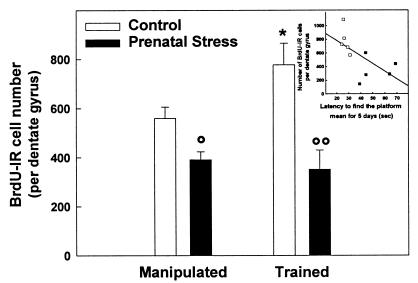
As shown in Fig. 6, acquisition of this spatial task increased hippocampal neurogenesis in control rats [H(1, 10) = 4.81, P < 0.05] but not in prenatally stressed rats [H(1, 10) = 0.88, n.s.]. As previously shown, prenatal stress decreased the total number of BrdUrd-IR cells. The number of BrdUrd-IR cells in prenatally stressed rats was identical for manipulated and trained animals. Furthermore, training did not influence the total number of cells in control [H(1, 10) = 1.84, n.s.] and prenatally stressed [H(1, 10) = 0.53, n.s., data not shown] rats.
Figure 6.
Effect of training in the water maze on cell proliferation in control and prenatally stressed rats. Acquisition of a spatial task increased the rate of hippocampal neurogenesis in control rats but not in prenatally stressed rats. Prenatal stress decreased the total number of BrdUrd-IR cells and, in this group, the number of newly born cells was identical for manipulated as well as for trained animals. (Inset) Correlation between cell proliferation and mean latency to reach the platform. *, P < 0.05 as compared with manipulated control group; °, P < 0.05 and °°, P < 0.01 as compared with control group.
Discussion
The aims of the present study were to investigate (i) whether prenatal events alter hippocampal plasticity, (ii) to which extent these possible alterations may undermine behavioral performances in spatial memory tasks depending on the hippocampal formation, and (iii) whether neurogenesis in the dentate gyrus and learning process can be related. The results show that aging is accompanied by a progressive decline in cell proliferation. In addition, prenatally stressed offspring exhibited a greater reduction in hippocampal cell proliferation by approximately 45% in all age groups tested. These animals also displayed delayed learning in a spatial memory task. Furthermore, learning a spatial memory task stimulated cell proliferation in the dentate gyrus of control rats whereas prenatal stress altered the ability of progenitor cells to divide in response to a learning situation.
Prenatal stress induced structural abnormalities in the hippocampal formation. Our results show for the first time that prenatal stress affects cell proliferation from adolescence until aging. With increasing age, there was a decline in cell proliferation in the dentate gyrus of control male rats, an effect consistent with previous studies (24, 25). Prenatally stressed animals displayed a greater reduction in cell proliferation during aging, suggesting that the early stressful experience accelerated the age-related decline in hippocampal plasticity. It remains to be determined whether the age-related decline in neurogenesis and the effects of prenatal stress are caused by an alteration in total number of stem cells (or progenitors cells) or differences in cell cycle length. Interestingly, these effects were specific to neurogenesis because pyknosis was not modified by prenatal stress at all ages tested. Because glucocorticoids inhibit hippocampal cell proliferation (14–16), the increased HPA axis activity of prenatally stressed animals (1, 3, 5), which is confirmed here by increased adrenal mass, could explain their reduced neurogenesis. The toxic effects of prolonged secretion of corticosterone have been extensively studied on hippocampal pyramidal cells (26, 27) but it is not known whether the age-related increase in pyknosis within the dentate gyrus is related to the activity of the HPA axis. The lack of effect of prenatal stress on pyknosis suggests the existence of a complex relationship between corticosterone and cell death. The maintenance of the effect of prenatal stress on neurogenesis during aging can thus be related to the “glucocorticoid cascade” hypothesis, which proposes that stressful experiences, through an excessive release of corticosterone, are responsible for alterations in the structure and function of the hippocampal formation in senescent animals (28).
The effect of aging on total cell number was complex. Indeed, a decline in cell number was observed between 3 and 10 months of age but this tendency was reversed later in life. The lack of an overall effect of aging on granule neuron number was not consistent with the fact that a net decline in neurogenesis and an increase in cell death was observed in the same animals. There is conflicting evidence on the effects of aging on the number of granule neurons, and the reasons for which variations in this number may or may not have occurred remain unknown (29–32). In prenatally stressed rats, despite the early impact of the manipulation on neurogenesis, an alteration of the total granule cell number could be observed beginning only at 3 months of age. This delayed decline in total granule cell number could be related to the fact that the percentage of granule neurons produced in adulthood is relatively small as compared with the 1 or 2 millions mature granule neurons. Thus, it is reasonable to suppose that a relatively long period of neurogenesis reduction is necessary to affect the total granule cell number and that the deficits in newborn cells between the beginning of life and 28 postnatal days is negligible as compared with the total pool of granule neurons. Later in life, deficits in total granule cell number appear in prenatally stressed rats; this result may be a consequence of an alteration in cell proliferation, a hypothesis reinforced by the lack of influence of prenatal stress on pyknosis. It has been shown that prenatal stress decreased synaptic density in the CA3 area, the projection site of the granule neurons (33) and hippocampal density of nitric oxide-producing neurons (34). Taken together, these data indicate that early experiences alter the structure of the hippocampal formation. This finding is of particular interest because the dentate gyrus is associated with spatial learning and memory (35, 36), which clearly are affected in prenatally stressed rats (present data and refs. 2, 4, 5).
We show that training in the water maze enhances cell proliferation in control rats. Previous studies have reported that survival of newly born cells is enhanced by exposure to an enriched environment, which also increased spatial learning in the water maze (37), and by exposure to training in the water maze (8). So far, the only condition that seems to increase cell proliferation is voluntary exercise on a running wheel (38). We show here that training in the water maze enhances cell proliferation in control rats; however, this effect was not seen in a previous study (38). This discrepancy could be related to the timing of BrdUrd injections. In this experiment, BrdUrd was injected at the end of the learning phase, when control rats begin to reach asymptotic level of performance, instead of throughout testing. These days were chosen because they correspond to a period of learning consolidation in which the hippocampal formation is activated (39). The stimulatory effect of training on cell proliferation observed in control rats is not caused by an increased motor activity because these animals cover a shorter distance than prenatally stressed rats do. Training by itself is not a sufficient condition to increase cell proliferation because no such stimulatory effect of training was observed in prenatally stressed rats. Thus, low cell proliferation is associated with poor behavioral performance whereas high cell proliferation is associated with good learning capabilities. Taken together with the decreased cell proliferation observed after prenatal stress, the behavioral deficits induced by prenatal stress suggest an enabling role for granular cell proliferation in the dentate gyrus to facilitate spatial memory performances. These results confirm the importance of the network into which these cells are inserted, because newborn neurons in the granule cell layer establish connections with the CA3 pyramidal neurons (10), which are known to be implicated in spatial memory (35, 36). Reciprocally, information processing influences brain structures, and spatial-learning-induced hippocampal plasticity takes place in the GCL-CA3 network.
The effect of prenatal stress on spatial learning may be the consequence of a deficit in neurogenesis, which can itself result from the dysfunction of the HPA axis. Indeed, cognition is modulated by corticosterone in a complex way (40), and high levels of corticosterone impair learning and memory (27, 41). Exposure to the water maze increases corticosterone secretion (42) and prenatally stressed animals show a delayed habituation of the corticosterone response to repeated exposure to stress (43). Thus, it seems reasonable to hypothesize that the prenatal stress-induced cognitive impairments may result from a prolonged corticosterone secretion that inhibits cell proliferation.
Taken together, the results of this study allow us to propose a pathophysiological path where the effect of prenatal stress on spatial learning may be the consequence of a deficit in neurogenesis that could results from HPA axis dysfunction. Although a direct effect of corticosterone on neurogenesis is possible, another hypothesis is that corticosterone acts through serotonin to reduce neurogenesis. Indeed, serotonin stimulates neurogenesis (44), but serotonin levels are reduced by corticosterone (45). Thus, the decreased serotonin levels observed in prenatally stressed rats (33) provide further evidence that corticosterone could be at the origin of the effect of prenatal stress that we report here. It can be hypothesized that the present results obtained following maternal restraint stress could be generalized to other procedures. Indeed, other types of maternal gestational stress were shown to have similar effects on endocrine and behavioral parameters (1).
In conclusion, the major impact of our data lies in the demonstration that deleterious environmental conditions occurring early in life have profound effects on neurogenesis in the dentate gyrus, an index of hippocampal plasticity, and that these alterations are associated with impaired performances in a spatial memory task. Neurogenesis, cell number, and cognitive capabilities are altered from adulthood to senescence in prenatally stressed rats. The fact that stressful experiences during development could have a long-term effect on hippocampal function by directly altering its structure recently has been suggested by Gould and Tanapat (17). Thus, it may be hypothesized that elevated corticosterone levels throughout the lifespan of these animals could lead to cognitive deficits through developmental inhibition of neurogenesis (27). Our results reinforce the hypothesis that many psychopathological affections have their origin in early developmental influences. More generally, they show the heuristic value of accurate animal models to better understand the mechanisms by which early stress and epigenetic risk factors promote learning disabilities in children and in age-related disorders, such as Alzheimer's disease (46).
Acknowledgments
We thank Drs. G. Rougon, W. Mayo, and P. V. Piazza for helpful comments, and Dr. M.-F. Montaron and Mrs. C. Brechenmacher for their help with the confocal analysis. The technical help of Mrs. M. C. Donat, Mrs. J. M. Claustrat, and O. George is acknowledged. Supported by Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique (98.72.017), and University of Bordeaux II.
Abbreviations
- PS group
prenatal stress group
- C group
control group
- HPA
hypothalamic–pituitary–adrenal
- IR
immunoreactivity
- GFAP
glial fibrillary acidic protein
- NeuN
neuronal nuclei
- n.s.
not significant
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Weinstok M. Neurosci Biobehav Rev. 1997;21:1–10. doi: 10.1016/s0149-7634(96)00014-0. [DOI] [PubMed] [Google Scholar]
- 2.Szuran T, Zimmerman E, Welzl H. Behav Brain Res. 1994;65:153–155. doi: 10.1016/0166-4328(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 3.Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 4.Lordi B, Poroais P, Mellier D, Caston J. Physiol Behav. 1997;62:1087–1092. doi: 10.1016/s0031-9384(97)00261-8. [DOI] [PubMed] [Google Scholar]
- 5.Vallée M, Maccari S, Dellu F, Le Moal M, Simon H, Mayo W. Eur J Neurosci. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 6.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 7.Altman J, Das G D. J Comp Neurol. 1965;124:319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 8.Gould E, Beylin A, Tanapat P, Reeves A, Shors T J. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 9.Gould E, Tanapat P, Hastings N B, Shors J T. Trends Cognit Sci. 1999;3:186–191. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 10.Stanfield B B, Trice J E. Exp Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- 11.Cameron H A, Wooley C S, McEwen B S, Gould E. Neuroscience. 1993;61:203–209. [Google Scholar]
- 12.Schwegler H, Crusio W E. Behav Brain Res. 1995;67:29–41. doi: 10.1016/0166-4328(95)91998-3. [DOI] [PubMed] [Google Scholar]
- 13.Schöpke R, Wolfer D P, Lipp H P, Leisinger-Trigona M C. Hippocampus. 1991;1:315–328. doi: 10.1002/hipo.450010322. [DOI] [PubMed] [Google Scholar]
- 14.Gould E, Cameron H A, Daniels D C, Woolley C S, McEwen B S. J Neuroci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez J J, Montaron M F, Petry K G, Aurousseau C, Marinelli M, Premier S, Rougon G, Le Moal M, Abrous D N. Eur J Neurosci. 1998;10:2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 16.Montaron M F, Petry K G, Rodriguez J J, Marinelli M, Aurousseau C, Rougon G, Le Moal M, Abrous D N. Eur J Neurosci. 1999;11:1479–1485. doi: 10.1046/j.1460-9568.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 17.Gould E, Tanapat P. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 18.Chapman R H, Stern J. J Comp Physiol Psychol. 1978;92:1074–1083. doi: 10.1037/h0077509. [DOI] [PubMed] [Google Scholar]
- 19.Akana S F, Shinsako J, Dallman M F. Endocrinology. 1983;113:2232–2237. doi: 10.1210/endo-113-6-2232. [DOI] [PubMed] [Google Scholar]
- 20.Lemaire V, Taylor G T, Mormède P. Psychoneuroendocrinology. 1997;22:563–573. doi: 10.1016/s0306-4530(97)00051-6. [DOI] [PubMed] [Google Scholar]
- 21.Nowakowsky R S, Lewin S B, Miller M W. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 22.Lemaire V, Aurousseau C, Le Moal M, Abrous D N. Eur J Neurosci. 1999;11:4006–4014. doi: 10.1046/j.1460-9568.1999.00833.x. [DOI] [PubMed] [Google Scholar]
- 23.Howard C V, Reed M G. Unbiased Stereology. Three-Dimensional Measurement in Microscopy. New York: Springer; 1998. [Google Scholar]
- 24.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki T, Arai Y. NeuroReport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 26.Woolley C, Gould E, McEwen B S. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 27.Sapolsky R M, Krey L C, McEwen B S. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sapolsky R M. Stress, the Aging Brain, and the Mechanisms of Neuron Death. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- 29.Rapp P R, Gallagher M. Proc Natl Acad Sci USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen T, Schliemann T, Sorensen J C, Zimmer J, West M J. Neurobiol Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 31.Landfield P W, Baskin R, Pitler T. Science. 1981;214:5881–5884. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- 32.Boss B D, Peterson G M, Cowan W M. Brain Res. 1985;338:144–150. doi: 10.1016/0006-8993(85)90257-4. [DOI] [PubMed] [Google Scholar]
- 33.Yahashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Int J Dev Neurosci. 1998;16:209–216. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 34.Vaid R R, Yee B K, Shalev U, Rawlins J N, Weiner I, Feldon J, Totterdell S. J Neurosci. 1997;17:5599–5609. doi: 10.1523/JNEUROSCI.17-14-05599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung M W, McNaughton B L. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 36.McNaughton B L, Barnes C A, Meltzer J, Sutherland R J. Exp Brain Res. 1989;76:485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- 37.Kempermann G, Kuhn G F, Gage F. Nature (London) 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 38.Van Praag H, Kempermann G, Gage F H. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 39.Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Nature (London) 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- 40.De kloet E R, Oitzl M S, Joëls M. Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 41.De Quervain D J-F, Roozendaal B, McGaugh J L. Nature (London) 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 42.Sandi C, Loscertales M, Guaza C. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 43.Fride E, Dan Y, Feldon J, Halevy G, Weinstock M. Physiol Behav. 1986;37:681–687. doi: 10.1016/0031-9384(86)90172-1. [DOI] [PubMed] [Google Scholar]
- 44.Brezun J M, Daszuta A. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- 45.Chaouloff F. Fundam Clin Pharmacol. 1955;9:219–233. doi: 10.1111/j.1472-8206.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 46.Leckman J F. Am J Psychiatry. 1999;156:1495–1448. doi: 10.1176/ajp.156.10.1495. [DOI] [PubMed] [Google Scholar]