Abstract
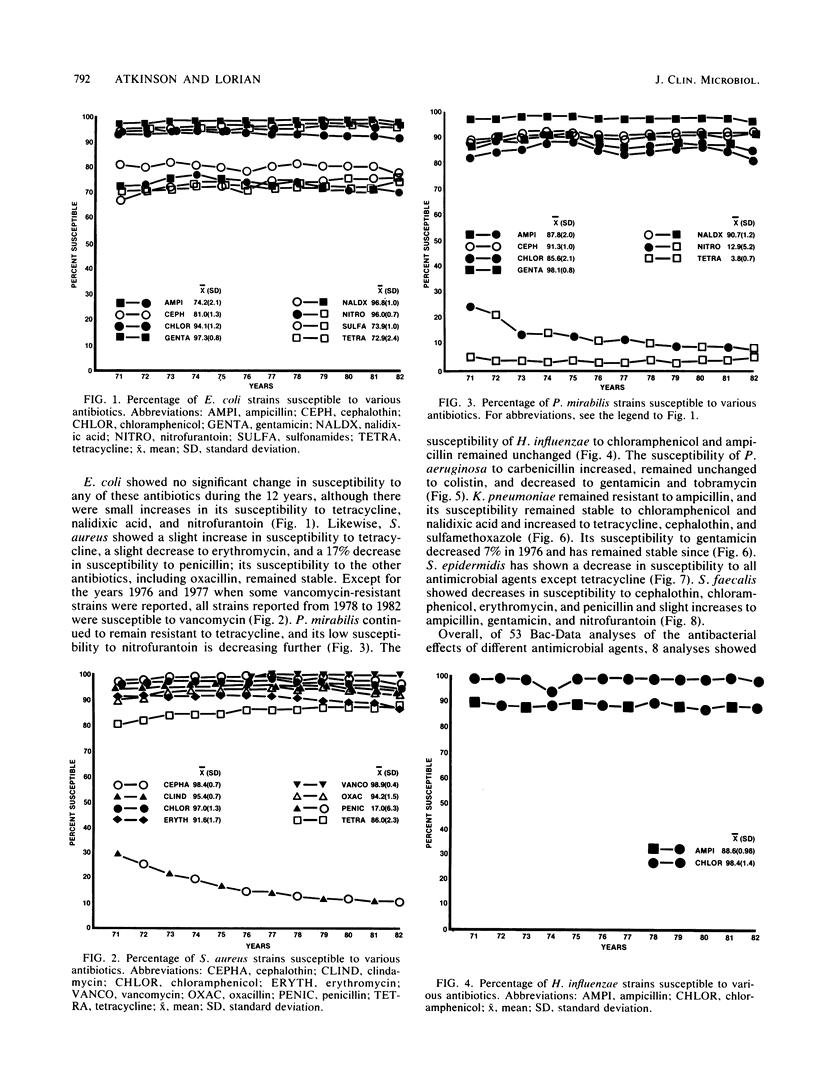
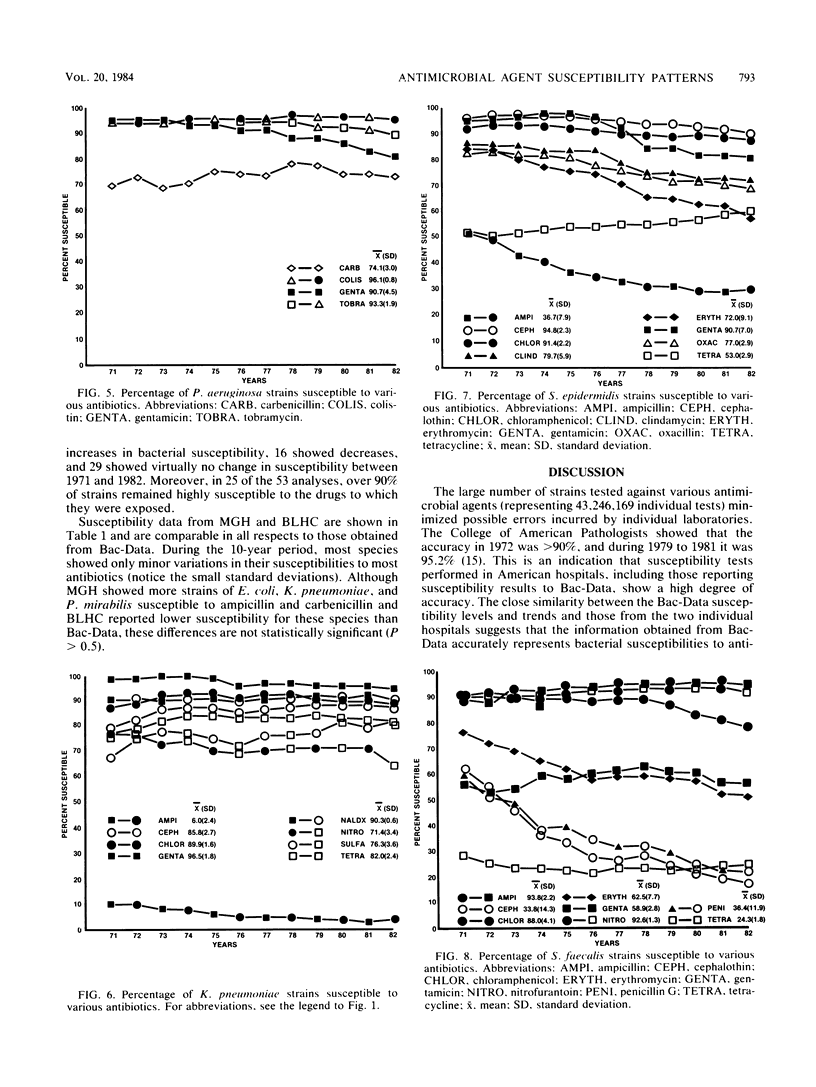
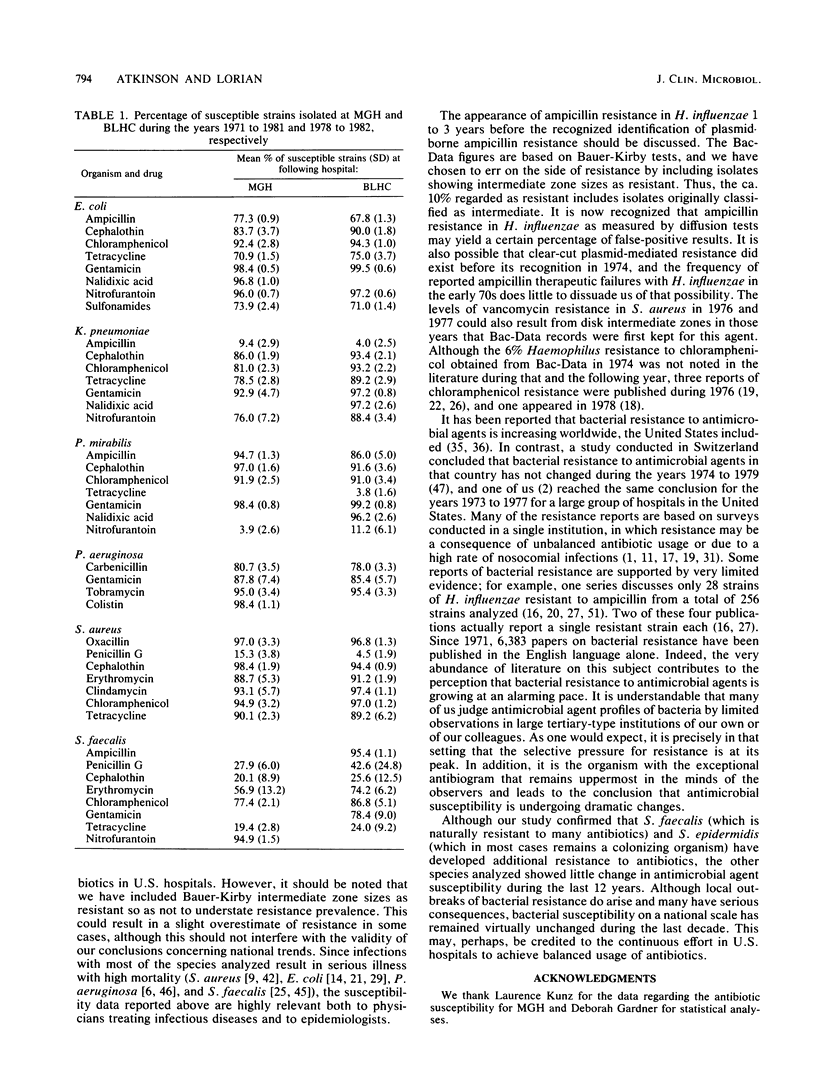
Bacterial susceptibility to 16 commonly used antibiotics was analyzed for a 12-year period (from 1971 to 1982, inclusive). Susceptibilities of 5,828,243 strains isolated from a mean of 242 hospitals nationwide and of 194,575 strains isolated at the Massachusetts General Hospital, Boston, Mass., and the Bronx Lebanon Hospital Center, New York, N.Y., were compared. Strains of Escherichia coli, Staphylococcus aureus, Haemophilus influenzae, and Pseudomonas aeruginosa showed virtually the same susceptibilities to antibiotics throughout the 12-year period, whereas Streptococcus faecalis and Staphylococcus epidermidis showed significant increases in resistance to most antibiotics. The close similarity between antibiotic susceptibilities shown at both the 242 hospitals and the 2 individual hospitals suggests that this analysis accurately reflects trends of bacterial resistance to antibiotics in U.S. hospitals. Since most of the species analyzed produce serious disease and high mortality, their susceptibility to antibiotics is relevant both to physicians treating infectious diseases and to epidemiologists.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeilts G. D., Sapico F. L., Canawati H. N., Malik G. M., Montgomerie J. Z. Methicillin-resistant-Staphylococcus aureus colonization and infection in a rehabilitation facility. J Clin Microbiol. 1982 Aug;16(2):218–223. doi: 10.1128/jcm.16.2.218-223.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Blackwell C. C., Feingold D. S. Frequency and some properties of clinical isolates of methicillin-resistant Staphylococcus aureus. Am J Clin Pathol. 1975 Sep;64(3):372–377. doi: 10.1093/ajcp/64.3.372. [DOI] [PubMed] [Google Scholar]
- Bock B. V., Pasiecznik K., Meyer R. D. Clinical and laboratory studies of nosocomial Staphylococcus aureus resistant to methicillin and aminoglycosides. Infect Control. 1982 May-Jun;3(3):224–228. doi: 10.1017/s0195941700056149. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Boyce J. M., Causey W. A. Increasing occurrence of methicillin-resistant Staphylococcus aureus in the United States. Infect Control. 1982 Sep-Oct;3(5):377–383. doi: 10.1017/s0195941700057337. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Alford R. H. Unsuccessful treatment of staphylococcal endocarditis with cefazolin. JAMA. 1977 Feb 7;237(6):569–570. [PubMed] [Google Scholar]
- Buckwold F. J., Albritton W. L., Ronald A. R., Lertzman J., Henriksen R. Investigations of the occurrence of gentamicin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1979 Feb;15(2):152–156. doi: 10.1128/aac.15.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley K., Loesch D., Landesman B., Mead K., Chern M., Strate R. An outbreak of infections caused by strains of Staphylococcus aureus resistant to methicillin and aminoglycosides. I. Clinical studies. J Infect Dis. 1979 Mar;139(3):273–279. doi: 10.1093/infdis/139.3.273. [DOI] [PubMed] [Google Scholar]
- Finland M. Emergence of antibiotic resistance in hospitals, 1935-1975. Rev Infect Dis. 1979 Jan-Feb;1(1):4–22. doi: 10.1093/clinids/1.1.4. [DOI] [PubMed] [Google Scholar]
- Hardy D. J., Legeai R. J., O'Callaghan R. J. Klebsiella neonatal injections: mechanism of broadening aminoglycoside resistance. Antimicrob Agents Chemother. 1980 Oct;18(4):542–548. doi: 10.1128/aac.18.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway W. J., Scott E. G. Gram-negative rod septicemia. A review. Del Med J. 1968 Jun;40(6):181–185. [PubMed] [Google Scholar]
- Jones R. N., Edson D. C. Interlaboratory performance of disk agar diffusion and dilution antimicrobial susceptibility tests, 1979-1981. A summary of the microbiology portion of the College of American Pathologists (CAP) surveys. Am J Clin Pathol. 1982 Oct;78(4 Suppl):651–658. [PubMed] [Google Scholar]
- Jones R. N., Slepack J., Bigelow J. Ampicillin-resistant Haemophilus paraphrophilus laryngo-epiglottitis. J Clin Microbiol. 1976 Nov;4(5):405–407. doi: 10.1128/jcm.4.5.405-407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman C. A., Ramundo N. C., Williams S. G., Dey C. R., Phair J. P., Watanakunakorn C. Surveillance of gentamicin-resistant gram-negative bacilli in a general hospital. Antimicrob Agents Chemother. 1978 Jun;13(6):918–923. doi: 10.1128/aac.13.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinmonth A. L., Storrs C. N., Mitchell R. G. Meningitis due to chloramphenicol-resistant Haemophilus influenzae type b. Br Med J. 1978 Mar 18;1(6114):694–694. doi: 10.1136/bmj.1.6114.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek J. J., Marsik F. J., Bartlett R. C., Weir B., Shea P., Quintiliani R. Clinical, epidemiologic and bacteriologic observations of an outbreak of methicillin-resistant Staphylococcus aureus at a large community hospital. Am J Med. 1976 Sep;61(3):340–345. doi: 10.1016/0002-9343(76)90370-3. [DOI] [PubMed] [Google Scholar]
- Lerman S. J., Brunken J. M., Bollinger M. Prevalence of ampicillin-resistant strains of Haemophilus influenzae causing systemic infection. Antimicrob Agents Chemother. 1980 Sep;18(3):474–475. doi: 10.1128/aac.18.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A. M., Federman M. J. Gram-negative bacillary pneumonia. J Infect Dis. 1971 Oct;124(4):425–427. doi: 10.1093/infdis/124.4.425. [DOI] [PubMed] [Google Scholar]
- Ma M. Y., Goldstein E. J., Friedman M. H., Anderson M. S., Mulligan M. E. Resistance of gram-negative bacilli as related to hospital use of antimicrobial agents. Antimicrob Agents Chemother. 1983 Sep;24(3):347–352. doi: 10.1128/aac.24.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliwan N., Grieble H. G., Bird T. J. Hospital Pseudomonas aeruginosa: surveillance of resistance to gentamicin and transfer of aminoglycoside R factor. Antimicrob Agents Chemother. 1975 Oct;8(4):415–420. doi: 10.1128/aac.8.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Kaye D., Levison M. E., Hook E. W. Enterococcal endocarditis. An analysis of 38 patients observed at the New York Hospital-Cornell Medical Center. Arch Intern Med. 1970 Feb;125(2):258–264. doi: 10.1001/archinte.125.2.258. [DOI] [PubMed] [Google Scholar]
- Manten A., van Klingeren B., Dessens-Kroon M. Chloramphenicol resistance in Haemophilus influenzae. Lancet. 1976 Mar 27;1(7961):702–702. doi: 10.1016/s0140-6736(76)92832-4. [DOI] [PubMed] [Google Scholar]
- Markowitz S. M. Isolation of an ampicillin-resistant, non-beta-lactamase-producing strain of Haemophilus influenzae. Antimicrob Agents Chemother. 1980 Jan;17(1):80–83. doi: 10.1128/aac.17.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. E., Jr Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983 Nov-Dec;5(6):1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Barnes M. W., Finland M. Bacteremia at Boston City Hospital: Occurrence and mortality during 12 selected years (1935-1972), with special reference to hospital-acquired cases. J Infect Dis. 1975 Sep;132(3):316–335. doi: 10.1093/infdis/132.3.316. [DOI] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Minshew B. H., Pollock H. M., Schoenknecht F. D., Sherris J. C. Emergence in a burn center of populations of bacteria resistant to gentamicin, tobramycin, and amikacin: evidence for the need for changes in zone diameter interpretative standards. Antimicrob Agents Chemother. 1977 Dec;12(6):688–696. doi: 10.1128/aac.12.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Kunz L. J., Poitras J. W. Resistance to gentamicin, tobramycin and amikacin among clinical isolates of bacteria. Am J Med. 1977 Jun;62(6):873–881. doi: 10.1016/0002-9343(77)90655-6. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Tsao J., Panida J. Enterococci from Bangkok, Thailand, with high-level resistance to currently available aminoglycosides. Antimicrob Agents Chemother. 1983 Jun;23(6):799–802. doi: 10.1128/aac.23.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The emergence of bacterial resistance and its influence on empiric therapy. Rev Infect Dis. 1983 Mar-Apr;5 (Suppl 1):S9–20. doi: 10.1093/clinids/5.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Acar J. F., Medeiros A. A., Norton R. A., Goldstein F., Kent R. L. International comparison of prevalence of resistance to antibiotics. JAMA. 1978 Apr 14;239(15):1518–1523. [PubMed] [Google Scholar]
- O'Brien T. F., Norton R. A., Kent R. L., Medeiros A. A. International surveillance of prevalence of antibiotic resistance. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):59–66. doi: 10.1093/jac/3.suppl_c.59. [DOI] [PubMed] [Google Scholar]
- Overturf G. D., Zawacki B. E., Wilkins J. Emergence of resistance to amikacin during treatment of burn wounds: the role of antimicrobial susceptibility testing. Surgery. 1976 Feb;79(02):224–228. [PubMed] [Google Scholar]
- Preheim L. C., Penn R. G., Sanders C. C., Goering R. V., Giger D. K. Emergence of resistance to beta-lactam and aminoglycoside antibiotics during moxalactam therapy of Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 1982 Dec;22(6):1037–1041. doi: 10.1128/aac.22.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. E., Kresel P. A., Farchione L. A., Siskin S. B., Karpow S. A. Epidemiological studies of aminoglycoside resistance in the U.S.A. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):89–105. doi: 10.1093/jac/8.suppl_a.89. [DOI] [PubMed] [Google Scholar]
- Richardson J. F. Frequency of resistance to trimethoprim among isolates of Staphylococcus epidermidis and Staphylococcus saprophyticus. J Antimicrob Chemother. 1983 Feb;11(2):163–167. doi: 10.1093/jac/11.2.163. [DOI] [PubMed] [Google Scholar]
- Richmond A. S., Simberkoff M. S., Schaefler S., Rahal J. J., Jr Resistance of Staphylococcus aureus to semisynthetic penicillins and cephalothin. J Infect Dis. 1977 Jan;135(1):108–112. doi: 10.1093/infdis/135.1.108. [DOI] [PubMed] [Google Scholar]
- Sande M. A., Johnson M. L. Antimicrobial therapy of experimental endocarditis caused by Staphylococcus aureus. J Infect Dis. 1975 Apr;131(4):367–375. doi: 10.1093/infdis/131.4.367. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance during therapy with the newer beta-lactam antibiotics: role of inducible beta-lactamases and implications for the future. Rev Infect Dis. 1983 Jul-Aug;5(4):639–648. doi: 10.1093/clinids/5.4.639. [DOI] [PubMed] [Google Scholar]
- Schaefler S., Jones D., Perry W., Ruvinskaya L., Baradet T., Mayr E., Wilson M. E. Emergence of gentamicin- and methicillin-resistant Staphylococcus aureus strains in New York City hospitals. J Clin Microbiol. 1981 Apr;13(4):754–759. doi: 10.1128/jcm.13.4.754-759.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler R. F., Greenough 3d W. B., Stolley P. D. A descriptive study of nosocomial bacteremias at The Johns Hopkins Hospital, 1968--1974. Johns Hopkins Med J. 1978 Mar;142(3):77–84. [PubMed] [Google Scholar]
- Vogel L., Nathan C., Sweeney H. M., Kabins S. A., Cohen S. Infections due to gentamicin-resistant Staphylococcus aureus strain in a nursery for neonatal infants. Antimicrob Agents Chemother. 1978 Mar;13(3):466–472. doi: 10.1128/aac.13.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward T. T., Winn R. E., Hartstein A. I., Sewell D. L. Observations relating to an inter-hospital outbreak of methicillin-resistant Staphylococcus aureus: role of antimicrobial therapy in infection control. Infect Control. 1981 Nov-Dec;2(6):453–459. doi: 10.1017/s0195941700055715. [DOI] [PubMed] [Google Scholar]
- Wormser G. P., Tatz J., Donath J. Endemic resistance to amikacin among hospital isolates of gram-negative bacilli: implications for therapy. Infect Control. 1983 Mar-Apr;4(2):93–99. doi: 10.1017/s0195941700057829. [DOI] [PubMed] [Google Scholar]
- Yogev R., Melick C., Glogowski W. In vitro development of rifampin resistance in clinical isolates of Haemophilus influenzae type b. Antimicrob Agents Chemother. 1982 Mar;21(3):387–389. doi: 10.1128/aac.21.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


