Abstract
Glutathione (GSH) is a tripeptide composed of glutamate, cysteine, and glycine. The first and rate-limiting step in GSH synthesis is catalyzed by glutamate cysteine ligase (GCL, previously known as γ-glutamylcysteine synthetase). GCL is a heterodimeric protein composed of catalytic (GCLC) and modifier (GCLM) subunits that are expressed from different genes. GCLC catalyzes a unique γ-carboxyl linkage from glutamate to cysteine and requires ATP and Mg++ as cofactors in this reaction. GCLM increases the Vmax and Kcat of GCLC, decreases the Km for glutamate and ATP, and increases the Ki for GSH-mediated feedback inhibition of GCL. While post-translational modifications of GCLC (e.g. phosphorylation, myristoylation, caspase-mediated cleavage) have modest effects on GCL activity, oxidative stress dramatically affects GCL holoenzyme formation and activity. Pyridine nucleotides can also modulate GCL activity in some species. Variability in GCL expression is associated with several disease phenotypes and transgenic mouse and rat models promise to be highly useful for investigating the relationships between GCL activity, GSH synthesis, and disease in humans.
Keywords: Glutamate cysteine ligase, GCL, GCLC, GCLM, Glutathione, GSH, Post-translational
1. Introduction
Glutathione (GSH) is an endogenously synthesized tripeptide thiol (γ-glutamylcysteinylglycine) with important biochemical and antioxidant properties. GSH is part of numerous basic cellular processes including protein synthesis, DNA synthesis and repair, cell proliferation, and redox signaling (Meister, 1983; Wu et al., 2004; Townsend, 2007). GSH is utilized in phase-II metabolism of many drugs and xenobiotics via glutathione S-transferase (GST)-mediated GSH conjugation reactions (Hayes and Pulford, 1995). As an antioxidant, GSH scavenges ROS, RNS, and other free radicals produced in association with electron transport, xenobiotic metabolism, and inflammatory responses (Rahman et al., 2004; Haddad and Harb, 2005; Rahman et al., 2005). GSH appears to be especially important in protecting mitochondria from xenobiotic- and ROS-induced toxicity (Reed, 2004; Fernandez-Checa and Kaplowitz, 2005). Importantly, although most cells have the capacity for de novo GSH biosynthesis, GSH is transported from the liver into the blood for use by other organs in the body, and into the bile for use by the gastrointestinal system (Griffith and Meister, 1979a; Meister, 1983; Martensson et al., 1990).
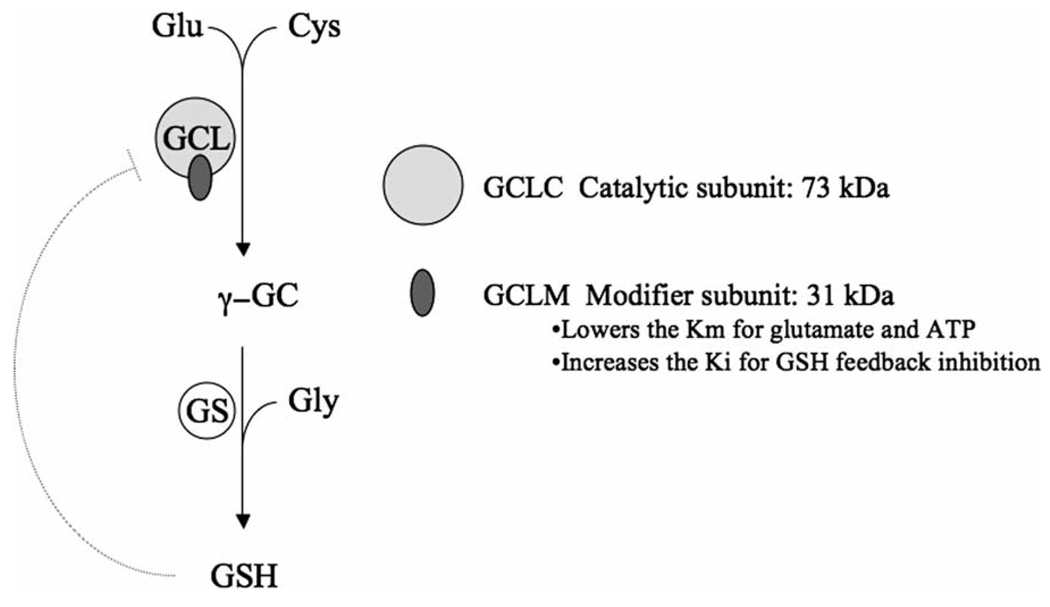
Cellular GSH homeostasis is regulated by the rate of GSH synthesis, utilization, and export from the cell (Griffith, 1999; Griffith and Mulcahy, 1999). The GSH biosynthetic capacity of various cells and tissues throughout the body is also controlled by multiple factors, including substrate availability (especially cysteine) and the activity of glutamate cysteine ligase (GCL), the rate-limiting enzyme in GSH synthesis (Griffith, 1999; Griffith and Mulcahy, 1999). GCL carries out the first of two ATP-dependent steps in GSH synthesis, forming γ-glutamylcysteine (γ-GC) from glutamate and cysteine (Fig. 1). The second step is catalyzed by glutathione synthetase (GSS), which ligates glycine to γ-GC, thus forming GSH.
Fig. 1.
Glutathione synthesis. The first step in glutathione (GSH) synthesis is carried out by glutamate cysteine ligase (GCL), which is composed of catalytic (GCLC) and modifier (GCLM) subunits. In this rate-limiting step, glutamate and cysteine are ligated to form γ-glutamylcysteine (γ-GC). The second step is carried out by glutathione synthetase (GS), which ligates glycine to γ-GC. Both steps are ATP dependent.
Because GCL is a major determinant of cellular GSH levels, many laboratories have investigated the factors that regulate GCL expression and activity (for a review, see Lu, 2008, this issue). In many lower organisms the GCL enzyme is a single polypeptide, but most eukaryotic GCL enzymes are heterodimeric complexes consisting of two distinct gene products. The catalytic subunit (GCLC) is the larger of the two subunits (637 amino acids, approximately 73 kDa) and contains the active site responsible for the ATP-dependent bond formation between the amino group of cysteine and the γ-carboxyl group of glutamate. The modifier subunit (GCLM) is smaller (274 amino acids, approximately 31 kDa) and through direct interaction with GCLC acts to increase the catalytic efficiency of GCLC. GCLM lowers the Km for glutamate and ATP, and increases the Ki for GSH feedback inhibition (Meister, 1983; Griffith, 1999; Yang et al., 2007). GSH competitively inhibits GCL in a non-allosteric fashion by competing with glutamate at the active site of GCLC. Interestingly, under some circumstances alternative forms of GCL can be observed that migrate at a higher molecular weight than a GCLC/GCLM heterodimer upon SDS–PAGE. Furthermore, gel filtration analysis of Drosophila GCL suggests a putative heterotrimeric structure (Fraser et al., 2002). Whether these more highly complexed forms of GCL have unique biochemical properties is not known.
Some interesting differences in GCL composition and function exist among non-mammalian organisms. For instance, while there is apparently no GCLM counterpart in plants, GCL can still dimerize (i.e. two GCLC subunits) in a redox-dependent manner, which dramatically affects enzymatic activity (Hicks et al., 2007). Yeast also have a second related enzyme (YdbK) that can carry out this first step in GSH synthesis, albeit with much lower efficiency (approximately 500-fold) than yeast GCL (Lehmann et al., 2004). Finally, in some bacteria, GCL and GS activities are present in a single polypeptide, allowing for the complete synthesis of GSH to be carried out by a single gene product (GCL-GS) (Janowiak and Griffith, 2005; Janowiak et al., 2006; Vergauwen et al., 2006; Kino et al., 2007).
2. GCLC and GCLC gene structure and polymorphisms
GCLC and GCLM are expressed from separate genes on distinct chromosomes (6p12, and 1 p22.1 in humans; and 9 D–E and 3 H1–3 in mice, for GCLC and GCLM, respectively) (Sierra-Rivera et al., 1995; Tsuchiya et al., 1995; Walsh et al., 1996). In humans, the GCLC gene is composed of 16 exons and spans approximately 48 kb of DNA sequence, whereas GCLM is composed of 7 exons stretching over 22 kb. The coding regions of these genes are highly conserved among eukaryotes, showing only small differences in amino acid sequence among mammalian species (e.g. the human GCLC amino acid sequence is 97.6% similar to the mouse GCLC sequence; and the human GCLM amino acid sequence is 98.2% similar to the mouse GCLM sequence).
Trans-acting regulatory transcription factors that are known to interact with the promoters of these genes include members of the Nrf2 family of transcription factors, and AP1, AP3, NFκB, Maf family proteins, JunD, Fra, CREB, and others (for a review of this literature see Lu, 2008, this issue).
3. GCL subunit protein structures
Limited data exist on the three-dimensional structure of mammalian GCL. However, the crystal structures for bacterial (Escherichia coli) and plant (Brassica juncea) GCL have been solved (Hibi et al., 2004; Hothorn et al., 2006). Such studies have revealed similarities between the active site of bacterial GCL and that of glutamine synthase, which has a similar catalytic mechanism and cofactor requirements (ATP and Mg++). The similarity in active sites between GCL and glutamine synthase underlies the observation that l-methionine sulfoximine, a potent inhibitor of glutamine synthase also inhibited rat GCL (Richman et al., 1973). This ultimately led to the discovery by Griffith and Meister that l-buthionine-(SR)-sulfoximine (BSO) is a highly selective mechanism-based inhibitor of GCL (Griffith and Meister, 1979b). BSO is widely used to investigate the roles of GSH and its synthesis in many biological processes.
3.1. GCLC protein structure
In eukaryotes, the GCLC amino acid sequence appears to be rather unique to this class of proteins, with little homology to other proteins. However, as with the bacterial GCL enzyme, there are apparent similarities between the active site of trypanosome GCLC and that of glutamine synthase (Abbott et al., 2001).
Using an intensive search of structures present in the protein database, Hamilton and colleagues used computer-based modeling to predict the structure of human GCLC and its catalytically active site (Hamilton et al., 2003). This permitted a structure-based explanation for the markedly attenuated activity of a mutant form of the human GCL enzyme. A subsequent in silico screen of the NCI compound library identified 10 novel inhibitors of GCL, some of which were capable of depleting cellular GSH levels and sensitizing cells to the chemotherapeutic agent melphalan (Hamilton et al., 2007). Using computer modeling software and the yeast YbdK protein as a reference structure, Mañú Pereira et al. have also proposed a three-dimensional structure for human GCLC (Mañú Pereira et al., 2007). In a similar approach, using the B. juncea GCLC crystal structure as a reference, Pieper and colleagues have also proposed two structures for human GCLC (Pieper et al., 2007a).
3.2. GCLM protein structure
Mammalian GCLM appears to possess a highly conserved domain homologous to aldo-keto reductases (AKR) (Soltanin-assab et al., 2000). This is interesting because NADPH is a cofactor for AKRs and Toroser et al. have shown a putative role for NADPH in regulating the activity of Drosophila and mouse GCL (see Section 4.3) (Toroser et al., 2006). Pieper et al. have also proposed several structures for human GCLM using aldo-keto reductases as reference structures (Pieper et al., 2007b). It remains to be determined whether any of the proposed structures for human GCLC or GCLM are indeed correct.
4. Post-translational regulation of GCL activity
4.1. Oxidative stress and redox-dependent changes in GCL activity
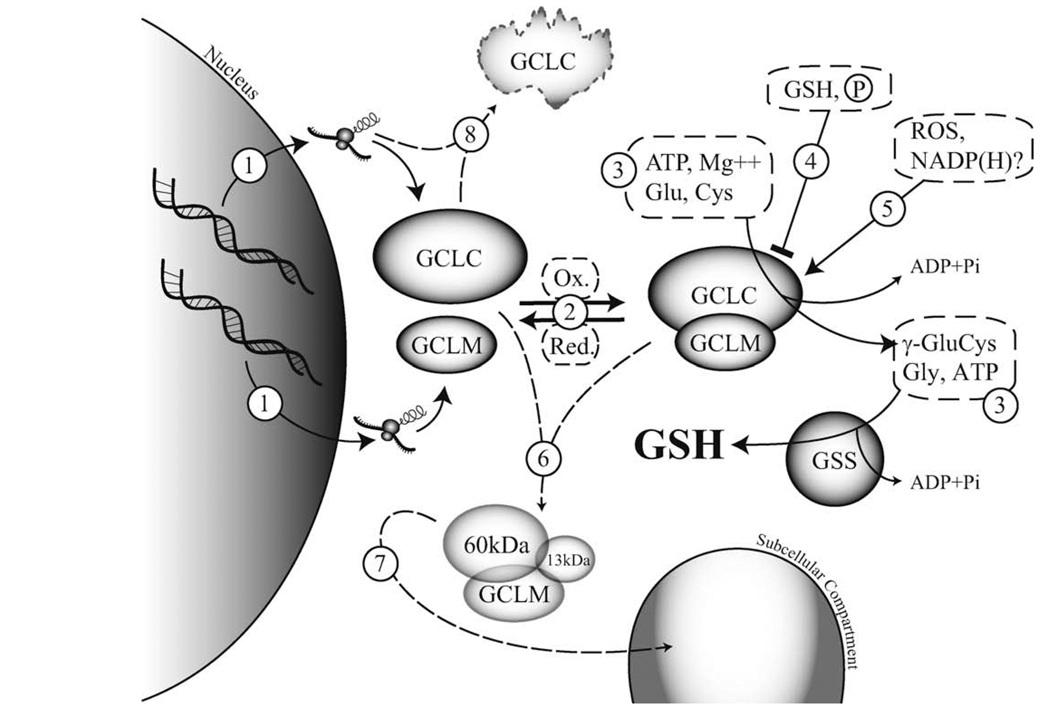
The relative levels of the GCL subunits are a major determinant of cellular GCL activity and are highly regulated at the transcriptional and post-transcriptional level in response to oxidative stress (Griffith, 1999; Griffith and Mulcahy, 1999; Rahman and MacNee, 2000; Wild and Mulcahy, 2000). The GCL subunits are often coordinately induced in response to oxidative stress, but distinct transcriptional and post-transcriptional mechanisms mediate their differential rates and levels of induction (Yao et al., 1995; Cai et al., 1997; Galloway et al., 1997; Tian et al., 1997; Liu et al., 1998). While these transcriptional events invariably lead to increased protein translation, only recently have direct and functional quantification of the GCL subunit protein levels demonstrated that GCLM is limiting for GCL holoenzyme formation in most cell types and tissues (Krzywanski et al., 2004; Chen et al., 2005; Lee et al., 2006). Thus, while GCLC levels are clearly important in dictating cellular GCL activity, increased expression of GCLM alone is likely an effective mechanism for enhancing cellular GCL activity (Tipnis et al., 1999; Neurohr et al., 2003; Lee et al., 2006; Dasgupta et al., 2007). GCL activity can also be rapidly regulated by post-translational modification of pre-existing GCLC and/or GCLM protein (see Fig. 2 for a summary of the major post-translational modifications and their effects). In this regard, oxidative stress has been shown to stimulate GCL activity prior to, or in the absence of, an increase in GCL subunit protein expression. Sub-toxic concentrations of hydrogen peroxide (H2O2), menadione, phorone, or other oxidative agents, lead to the transient stimulation of GCL activity without detectable increases in GCL subunit protein levels (Ochi, 1995, 1996; Krejsa C.M., et al., unpublished observations). The rapid time-course of GCL activation (10 min – 1 h) and the inability of de novo protein synthesis inhibition to prevent menadione- or H2O2-induced GCL activity also precludes the involvement of increased GCL subunit protein expression in these responses (Ochi, 1995, 1996). Thus, in addition to transcriptional regulation of GCL subunit expression, oxidative stress appears to enhance cellular GCL activity via direct post-translational modification of one or both GCL subunits (Fig. 2, #5).
Fig. 2.
Post-translational regulation of glutamate cysteine ligase activity. Glutathione (GSH) is synthesized by a two-step ATP-dependent process. The first and rate-limiting step is the formation of γ-glutamylcysteine (γ-GluCys) by glutamate cysteine ligase (GCL), which is composed of catalytic (GCLC) and modifier (GCLM) subunits. The second step is the ligation of glycine (Gly) to γ-GluCys, which is catalyzed by glutathione synthetase (GSS), to form GSH. Cellular regulation of GCL activity can occur by a number of mechanisms. (1) Transcriptional or post-transcriptional: activation or suppression of gene transcription [Sections 2, 4.1 and 4.6] and/or mRNA stability, and intra- and 5′UTR GCLC polymorphisms [Sections 4.1 and 5]. (2) Reversible GCL holoenzyme formation: redox-sensitive [Section 4.1], dependent on molar ratios of GCL subunits (GCLM is usually limiting) [Section 4.1], subunit stability [Section 4.6]. (3) Substrate (Glu, Cys, Gly) and co-factor (ATP, Mg++) availability [Section 1]. (4) Inhibition of GCLC activity: negative feedback regulation by GSH [Sections 1 and 4.1], autophosphorylation and kinase-mediated phosphorylation of GCLC [Section 4.2]. (5) Activation of GCL: oxidative stress [Section 4.1], NADPH (activation or inhibition) [Section 4.3]. (6) Caspase-mediated cleavage of GCLC [Sections 4.4 and 4.6]. (7) Myristoylation of the 13 kDa C-terminal fragment of caspase-cleaved GCLC and/or altered subcellular localization [Section 4.5]. (8) Inducible or mutational regulation of GCLC protein stability [Section 4.6].
Reducing agents reversibly dissociate a large portion of GCL holoenzyme, highlighting the importance of intermolecular disulfide bonds in GCL holoenzyme formation (Seelig et al., 1984; Tu and Anders, 1998; Fraser et al., 2002, 2003). This finding has given rise to the concept that cellular GCL activity may be regulated through a redox switch mechanism whereby oxidizing conditions stimulate GCL activity by enhancing GCL holoenzyme formation, while reducing conditions inhibit GCL activity via dissociation of the GCL subunits (Fig. 2, #2) (Huang et al., 1993). Studies employing sulfhydryl modifying agents and mutational analysis of human GCLC (Tu and Anders, 1998) and Drosophila melanogaster GCLM (Fraser et al., 2003) demonstrate that cysteine residues in both GCL subunits play a role in regulating GCL holoenzyme formation and activity. The cysteine modifier cystamine inactivates GCL from most species presumably via interaction with a critical cysteine residue within the GCL active site (Seelig and Meister, 1982; Huang et al., 1988; Lueder and Phillips, 1996; Fraser et al., 2002; Jez et al., 2004). However, E. coli GCL is insensitive to cystamine (Huang et al., 1988) and mutation of an invariant cysteine within Trypanasoma brucei (Cys319), while preventing cystamine inactivation, had no effect on GCL activity or enzyme kinetics (Brekken and Phillips, 1998). In contrast, the cysteine modifier N-ethylmaleimide abolishes the enzymatic activity of both monomeric murine GCLC and GCL holoenzyme (Backos et al., unpublished observation).
Mutational analysis has begun to identify the specific cysteine residues mediating these responses. While mutation of several conserved cysteine residues within human GCLC decreased GCLC enzymatic activity, only mutation of Cys553 significantly reduced GCL holoenzyme activity (Tu and Anders, 1998). However, mutation of Cys553 did not prevent GCL holoenzyme formation, suggesting the existence of additional intersubunit covalent interactions (Tu and Anders, 1998). Three cysteine residues within Drosophila GCLM (Cys213, Cys214, Cys267), two of which are conserved within human GCLM (Cys193, Cys194), have also been determined to be important for intersubunit disulfide bond formation (Fraser et al., 2003). Mutation of these residues inhibited intersubunit disulfide bond formation under non-reducing conditions, but did not prevent subunit association. In fact, this mutant GCLM was still capable of enhancing GCLC activity, albeit at a reduced level, and exhibited enhanced sensitivity to GSH feedback inhibition.
GSH feedback inhibition of GCL (Fig. 2, #4) is competitive with glutamate and the reduction of an intersubunit disulfide bond is thought to result in a conformational change that affects accessibility to the substrate binding site (Huang et al., 1993; Fraser et al., 2002, 2003). However, GSH does not induce subunit dissociation even at high concentrations (5–10 mM) (Tu and Anders, 1998; Yang et al., 2007). Furthermore, while GCL holoenzyme is largely dissociated in the presence of 50 mM dithiothreitol, 10–25% of the enzyme is resistant to dissociation (Seelig et al., 1984; Tu and Anders, 1998). A fraction of GCL holoenzyme formed in response to cysteine deprivation is also resistant to reduction and heat denaturation (Lee et al., 2006). In aggregate, these findings suggest that multiple covalent and non-covalent intermolecular interactions are important in the formation, stability, and activity of the GCL holoenzyme complex.
In contrast to mammalian GCL, plant GCL activity is regulated by a unique post-translational redox switch mechanism involving the reversible oxidation/reduction of specific intramolecular sulfhydryl bonds (Hothorn et al., 2006). While mammalian GCL is a heterodimeric holoenzyme, plant GCL is composed of a single gene product that is regulated and functions as a monomeric protein, though it exists as a homodimer (Jez et al., 2004; Hothorn et al., 2006). The crystal structure of B. juncea GCL and mass spectrometry data from Arabidopsis thaliana GCL revealed two redox-sensitive intramolecular disulfide bonds (CC1 and CC2) located at the homodimer interface that regulate plant GCL activity (Hicks et al., 2007; Gromes et al., 2008). Reduction of CC1 causes a conformational change that shields the active site and impairs substrate binding, while reduction of CC2 causes a reversible dimer to monomer transition that nearly abolishes GCL activity. In contrast to mammalian GCL (Ochi, 1995, 1996), H2O2- and menadione-induced Arabidopsis GCL activity is associated with the oxidation of GCL and increased homodimer formation (Hicks et al., 2007). While determination of the crystal structures of monomeric GCL from B. juncea (Hothorn et al., 2006) and E. coli (Hibi et al., 2004) have greatly enhanced our knowledge of the molecular mechanisms regulating GCL activity, the lack of sequence homology and structural similarity with mammalian GCL subunits limits the application of these findings to higher species. Elucidation of the tertiary/quaternary structure of mammalian GCL will improve our understanding of this crucial enzyme.
4.2. Phosphorylation
γ-Glutamylcysteine synthesis involves the GCL-mediated phosphorylation of l-glutamate creating the activated enzyme-bound γ-glutamylphosphate intermediate (Orlowski and Meister, 1971). GCL also mediates the phosphorylation of BSO, which is required for its tight and irreversible binding to the active site of GCL (Griffith and Meister, 1979b). In addition to this substrate/inhibitor kinase activity, purified rat kidney GCL holoenzyme is capable of undergoing autophosphorylation (Sekhar and Freeman, 1999). This phosphorylation is specific for the GCLC subunit as no phosphorylation of the GCLM subunit was detected. GCLC autophosphorylation decreased the Vmax of GCL activity by 50%, but had no effect on the Km for glutamate (Fig. 2, #4). While the phosphorylation site(s) and target residue(s) (Ser, Thr, or Tyr) were not identified, the ability of GCL substrates (l-glutamate and l-α-aminobutyrate) to competitively inhibit autophosphorylation suggests that phosphorylation occurs within or near the enzyme active site. The physiological relevance and potential regulation of GCLC autophosphorylation remain to be determined.
The first indication that phosphorylation may play an important role in regulating GCL activity in vivo was the ability of various hormones and agents that activate protein kinase C (PKC), cAMP-dependent protein kinase (PKA), or Ca2+-calmodulin-dependent protein kinase II (CMKII) to inhibit GCL activity in cultured rat hepatocytes (Lu et al., 1991). GCLC was subsequently found to be inducibly phosphorylated in response to dibutyryl cAMP or phenylephrine in this cultured cell system (Sun et al., 1996). In vitro studies confirmed that PKC, PKA, and CMKII were capable of directly phosphorylating GCLC and inhibiting GCL activity (Fig. 2, #4). Phosphoamino acid analysis revealed that phosphorylation of GCLC occurred on serine and threonine residues in vitro and the phosphorylation site(s) were likely identical for all three kinases (Sun et al., 1996). Similar to GCLC autophosphorylation, kinase-mediated phosphorylation of rat kidney GCLC decreased the Vmax of GCL activity, but had no effect on the Km for cysteine or glutamate. GCLC phosphorylation did not cause dissociation of the GCL holoenzyme into its monomeric subunits, suggesting a molecular mechanism involving a conformational change that suppresses specific activity, but does not affect holoenzyme formation or substrate affinity. While the degree of phosphorylation correlated with the extent of GCL inhibition, only a modest reduction in activity was observed in rat hepatocytes. However, cell type- and species-specific effects may account for this, as in vitro manipulation of conditions purported to affect GCLC phosphorylation/dephosphorylation had a much greater effect on GCL activity in extracts from Drosophila and mouse liver, brain, and heart (Toroser et al., 2006). In these studies, preincubation of cell or tissue extracts in the presence of MgATP (phosphorylating conditions) nearly abolished GCL activity, while dephosphorylating conditions (absence of ATP and phosphatase inhibitors) resulted in the “spontaneous activation” of GCL. While the phosphorylation status of GCLC was not assessed, these findings suggest that GCL activity may be constitutively suppressed by GCLC phosphorylation in some species and tissues. This would be consistent with the basal level of GCLC phosphorylation detected in cultured rat hepatocytes and may explain the minimal effects of subsequent kinase-mediated phosphorylation on rat kidney GCL activity in vitro (Sun et al., 1996). Further work is required to identify and functionally characterize the relevant protein kinases and/or phosphatases that reversibly regulate GCL activity in vivo
4.3. Pyridine dinucleotide phosphates (NAD(P)H)
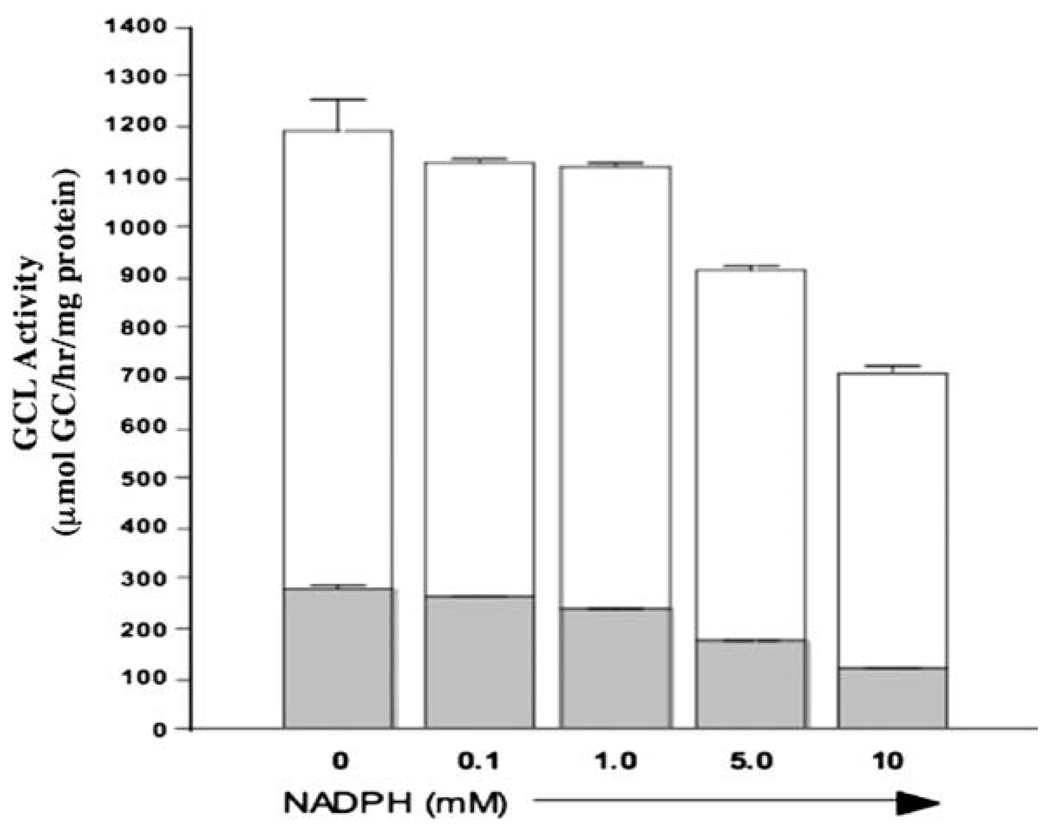
A screen of various small molecular weight compounds related to redox signaling revealed that nicotinamide adenine dinucleotide phosphates (NADP+ and NADPH) are capable of regulating Drosophila GCL activity in vitro (Toroser et al., 2006). Direct addition of reduced NADPH to a GCL assay mixture containing Drosophila extract increased GCL activity ~two-fold, while oxidized NADP+ suppressed GCL activity (Fig. 2, #5). There was specificity amongst nicotinamide adenine dinucleotides as NAD+ and NADH had no effect on GCL activity. The stimulatory effects of NADPH were due to an increase in Vmax with no detectable change in substrate affinity. While there are no clear NADPH-binding sites in GCLC, a BLAST search using the human or mouse GCLM sequences revealed some homology to several members of the aldo-keto reductase (AKR) family (Soltaninassab et al., 2000). AKRs contain a highly conserved NAD(P)H-binding pocket (Jez and Penning, 2001), which is partially conserved in GCLM. If NADP+ and NADPH directly affect GCL activity, it seems likely that these effects are mediated via interaction with the GCLM subunit. To test this hypothesis, we included NADPH in a GCL assay mixture containing recombinant murine GCLC or GCL holoenzyme purified as reported previously (Krzywanski et al., 2004). In contrast to the stimulatory effects observed in Drosophila extracts (Toroser et al., 2006), NADPH did not increase monomeric GCLC or GCL holoenzyme activity and even suppressed GCL activity at higher concentrations (Fig. 3). While the functional effects of NADP+ were not examined, these findings suggest that either the effects of NADPH are species-specific or NADPH-induced activation of GCL in Drosophila extracts is due to indirect effects on a factor(s) that influences GCL activity (Toroser et al., 2006). Should NADPH prove to be an authentic activator of GCL, this would provide an additional NADPH-dependent means of regulating cellular levels of reduced GSH in addition to that mediated by the GSH reductase salvage pathway (Griffith, 1999).
Fig. 3.
NADPH does not directly enhance the activity of recombinant GCLC or GCL holoenzyme in vitro. The enzymatic activity of purified recombinant murine GCLC and GCL holoenzyme was assessed in the absence or presence of increasing concentrations of NADPH. Open bars indicate recombinant GCL holoenzyme, and closed bars are the GCLC subunit alone. For GCL holoenzyme, GCLC-His and GCLM-His fusion proteins (Krzywanski et al., 2004) were mixed at a molar ratio of 1:4 and preincubated for 10 min at 37 °C prior to addition of NADPH and initiation of the GCL assay (White et al., 2003).
4.4. Caspase-mediated cleavage of GCLC
Intracellular GSH levels are rapidly depleted in many models of apoptotic cell death and this is often accompanied by the efflux of reduced GSH and the post-translational cleavage of GCLC protein (Fig. 2, #6) (Ghibelli et al., 1995; Coppola and Ghibelli, 2000; Franklin et al., 2002). Both GCLC cleavage and GSH depletion are caspase-dependent (z-VAD-fmk-inhibitable), yet they are functionally and mechanistically distinct as GSH depletion/efflux can occur in the absence of GCLC cleavage in caspase-3-deficient cells (Franklin et al., 2002). While members of both the multidrug resistance protein (ABCC/MRP) and organic anion transporter protein (SLCO/OATP) families have been implicated in the efflux of reduced GSH during apoptotic cell death (van den Dobbelsteen et al., 1996; Oda et al., 1999; Ballatori et al., 2005; Franco and Cidlowski, 2006), the functional effects of GCLC cleavage are not clear. It is has been postulated that GCLC cleavage inhibits GCL activity and prevents the repletion of depleted cellular GSH pools during apoptosis, but little direct evidence supports this contention. Caspase-dependent GCLC cleavage has been reported in response to multiple apoptotic stimuli, including chemotherapeutics (Siitonen et al., 1999; Franklin et al., 2002) death receptor agonists TNF-α and anti-Fas antibody (Franklin et al., 2002), TGFβ1 (Franklin et al., 2003), UV irradiation (Zhu and Bowden, 2004; Franklin et al., 2002, p. 45), and arsenicals (Sumi et al., 2007, and our unpublished results). GCLC cleavage generates N- and C-terminal fragments of 60 and 13 kDa, respectively (Fig. 2, #6) (Franklin et al., 2002). The GCLC cleavage site (Asp499) is located N-terminal to a cysteine residue (Cys553) reportedly involved in disulfide bond formation with GCLM (Tu and Anders, 1998) and removal of this interaction domain would likely suppress GCL enzymatic activity either directly or by affecting GCL holoenzyme formation (Griffith, 1999; Griffith and Mulcahy, 1999). However, GCLC cleavage did not result in a significant reduction in GCL activity during apoptotic cell death (Siitonen et al., 1999; Zhu and Bowden, 2004).
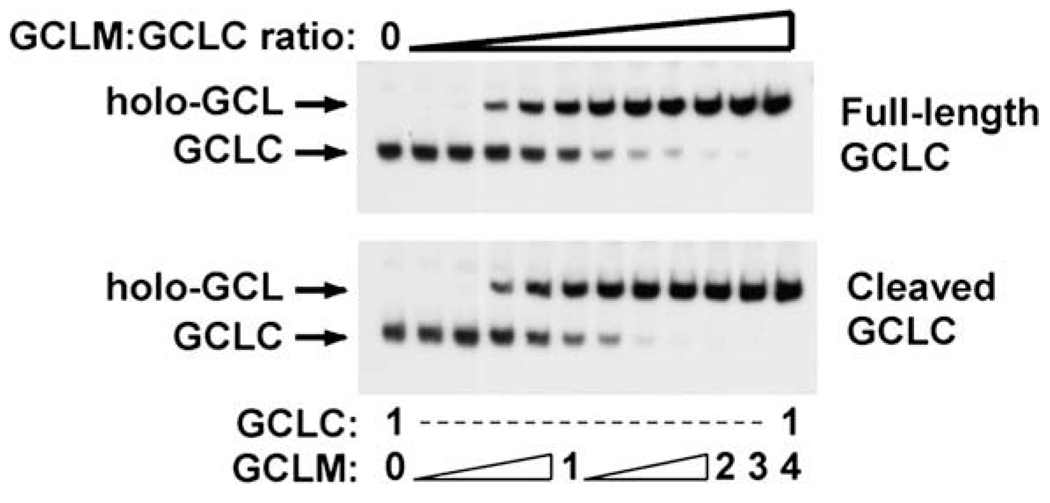
Interestingly, in vitro studies utilizing purified recombinant full-length and caspase-cleaved GCLC indicate that cleavage does not result in the dissociation of the small 13 kDa fragment containing the putative GCLM-binding domain from the larger 60 kDa cleavage fragment (unpublished results) (Fig. 2, #6). This likely provides the molecular basis by which cleaved GCLC retains full enzymatic activity since cleavage of GCLC does not affect its ability to associate with GCLM and form fully functional GCL holoenzyme (Fig. 4). In this regard, GCLC cleavage has no effect on the Vmax or the Km for glutamate, cysteine or ATP, of either monomeric GCLC or GCL holoenzyme (unpublished results). The failure to detect any clear effect of GCLC cleavage on GCL enzyme kinetics suggests that any functional effects of this post-translational modification are likely to be subtle and/or complex in nature.
Fig. 4.
Caspase-mediated cleavage of GCLC does not alter GCL holoenzyme formation in vitro. Murine GCLC-His or GCLM-His fusion proteins were synthesized in BL21(DE3) E. coli in the absence or presence of pGEX2T-caspase-3 (Deak et al., 1998) and purified as previously described (Krzywanski et al., 2004). Purified full-length and caspase-cleaved GCLC-His were incubated in the absence or presence of increasing molar equivalents of purified recombinant GCLM-His as indicated and GCL holoenzyme formation was assessed by native gel electrophoresis and immunoblotting for GCLC.
4.5. Myristoylation and regulation of GCL subunit subcellular localization
Caspase-mediated subcellular redistribution can also alter protein function during apoptosis. A classic example of this is the translocation of the caspase-8-cleaved 15 kDa truncated BID (tBID) fragment to the outer mitochondrial membrane leading to mitochondrial dysfunction and the release of cytochrome C (Li et al., 1998; Luo et al., 1998). Mitochondrial targeting of tBID results from the post-translational N-myristoylation of the tBID fragment (Zha et al., 2000). While co-translational N-myristoylation occurs at penultimate N-terminal glycine residues after removal of the initiator methionine, post-translational myristoylation can occur at internal glycine residues exposed by caspase-mediated cleavage (de Jonge et al., 2000). Caspase-mediated cleavage of GCLC occurs at AVVD499G creating a 13 kDa C-terminal fragment with an N-terminal glycine residue (Franklin et al., 2002). Analysis of the C-terminal sequences downstream of the cleavage site utilizing The MYR Predictor indicates that this exposed N-terminal glycine residue is a “reliable” myristoylation site (Eisenhaber et al., 2003). To date, 48 caspase targets have been identified that contain a glycine residue immediately C-terminal to the cleavage site and 9 are predicted to be candidates for myristoylation, including GCLC (Fig. 2, #7) (Fischer et al., 2003; Martin et al., 2008). To examine GCLC post-translational myristoylation, Martin et al. developed a novel nonradioactive approach that is six orders of magnitude more sensitive than radioactive labeling techniques (Martin et al., 2008). By employing this methodology, an EGFP fusion protein containing the first 10 amino acids downstream of the GCLC cleavage site (AVVD499GCGLAQNSTE) was found to be myristoylated in transiently transfected COS7 cells. Furthermore, this myristoylation was sufficient to confer partial nuclear exclusion of the GCLC–EGFP chimera and association with intracellular membranes as judged by confocal microscopy. These findings imply that myristoylation of caspase-cleaved GCLC may result in its subcellular redistribution during apoptosis (Fig. 2, #6 and #7). However, only the first ten amino acids downstream of the GCLC cleavage site were utilized in this study and it is not known whether cleavage of bona fide full-length endogenous GCLC undergoes post-translational myristoylation during apoptosis. Furthermore, the small 13 kDa and large 60 kDa GCLC fragments remain associated after caspase-mediated cleavage (see above and Fig. 2, #6) and it is not clear whether the N-terminus of the small C-terminal fragment would be accessible for post-translational myristoylation. This approach also measures co-translational myristoylation of the GCLC–EGFP chimera as a surrogate marker for post-translational myristoylation and this may not accurately reflect myristoylation of cleaved GCLC protein in the cytosol of apoptotic cells. While myristoylation alone is sufficient for targeting to intracellular membranes, additional lipid modification or a vicinal polybasic domain is required for localization to the plasma membrane (McCabe and Berthiaume, 1999). The lack of basic amino acids downstream of the GCLC caspase cleavage site suggests that GCLC is either not redistributed to the plasma membrane or undergoes an additional covalent lipid modification. While it remains to be determined how GCLC cleavage might affect GCL activity, the ability of caspase cleavage to target other caspase substrates for post-translational myristoylation and altered subcellular redistribution (Zha et al., 2000; Utsumi et al., 2003; Vilas et al., 2006) makes this an intriguing and previously unrecognized mechanism for regulating GCL activity during apoptotic cell death.
While GCLC and GCLM are generally considered to be cytosolic proteins there is evidence that they may exhibit altered subcellular localization in certain circumstances (Fig. 2, #7). In vivo immunohistochemical analysis indicates that hepatocyte-specific deletion of GCLC leads to the nuclear accumulation of GCLM (Chen et al., 2007). This is consistent with in silico analysis of the GCL subunits using PSORT II, which predicts a nuclear localization for GCLM and a cytoplasmic localization for GCLC. Deletion of GCLC ultimately leads to punctate cytoplasmic GCLM staining (Chen et al., 2007), suggesting either the altered compartmentalization and/or aggregation of GCLM in the absence of GCLC for extended periods of time (post-natal day 21/28). It cannot be delineated from this study whether GCLC per se dictates the subcellular localization of GCLM or the subsequent change in cellular redox homeostasis resulting from deletion of GCLC mediates these responses, although there is in vitro evidence supporting both contentions. We and others have found that GCLM is quite sensitive to aggregation in vitro in the absence of GCLC (Fraser et al., 2003; Yang et al., 2007; unpublished observations). Recombinant monomeric GCLM also undergoes a dose-dependent, H2O2-induced multimerization in vitro (Yang et al., 2007). However, it should be noted that all cultured cell lines and tissues examined to date (except kidney) contain a molar excess of GCLC and only under extreme conditions of GCLC down-regulation would GCLM exist as a monomer in vivo (Krzywanski et al., 2004; Chen et al., 2005; Lee et al., 2006). Additional research will reveal how the subcellular distribution and biophysical properties of the GCL subunits are regulated in response to various cellular conditions (i.e. apoptosis, GSH depletion, and oxidative stress).
4.6. Regulation of GCL subunit protein stability
Caspase-dependent apoptosis is usually associated with the cleavage of GCLC to a stable 60 kDa fragment (Siitonen et al., 1999; Franklin et al., 2002; Botta et al., 2004; Zhu and Bowden, 2004). However, TGFβ1- and diphenylarsinic acid (DPAsV)-induced apoptosis in murine hepatocytes is associated with the cleavage and loss of GCLC protein expression and GCL activity (Franklin et al., 2003; Sumi et al., 2007). TGFβ1 also suppresses Gclc gene transcription (Arsalane et al., 1997; De Bleser et al., 1999; Jardine et al., 2002; Franklin et al., 2003; Bakin et al., 2005; Hosler et al., 2006; Fu et al., 2008), although it is unlikely that transcriptional repression alone accounts for the observed decrease in GCLC protein and GCL activity as the GCL subunits are extremely stable proteins in unstimulated conditions. In fact, little or no change in steady-state GCLC and/or GCLM protein expression is observed upon inhibition of de novo protein synthesis with cycloheximide for up to 72 h (Franklin et al., 2002, 2003; Sumi et al., 2007). Given the stability of GCLC, it is intriguing to speculate that cleavage targets GCLC for ubiquitination and proteasomal degradation during TGFβ1- and DPAsV-induced apoptosis (Fig. 2, #8). Cleavage-dependent degradation has been reported for the caspase targets Bid (Breitschopf et al., 2000) and the atypical protein kinase C ζ(Smith et al., 2000), whereby caspase-mediated cleavage is necessary and sufficient for their degradation by the ubiquitin–proteasome system. However, while proteasome inhibitors have been shown to affect Gclc gene expression (Sekhar et al., 2000; Hosler et al., 2006), there is no evidence that GCLC protein undergoes ubiquitination and/or proteasome-mediated degradation. The GCLC protein also contains a putative PEST sequence between residues 210–229, however, it is not clear whether this sequence is functional as there was no degradation of GCLC detected within 6 h in an in vitro cell-free system (Sekhar et al., 1997; Soltaninassab et al., 2000).
Several rare GCLC missense mutations have also been identified that affect GCL activity and/or protein stability leading to low erythrocyte GSH levels and hemolytic anemia (Fig. 2, #8) (Beutler et al.,1990, 1999; Ristoff et al., 2000; Hamilton et al., 2003). In one case, a patient homozygous for an A-T transversion at nucleotide 1109, which produced a His370Leu substitution, expressed extremely low erythrocyte GCLC protein levels presumably due to the instability of the mutant GCLC protein (Beutler et al., 1999). GCLM levels were also compromised in this patient, suggesting that GCLC may be required for GCLM stabilization which is reminiscent of the altered biophysical characteristics of GCLM upon targeted deletion of GCLC (Chen et al., 2007).
5. GCLC and GCLM polymorphisms and disease susceptibility in humans
There exists variability among humans with respect to induction of GCL after exposure to GSH-depleting drugs (O’Dwyer et al., 1996). These differences in GCL activity and expression among individual humans are not likely to be due to common polymorphisms that affect the amino acid sequence of either subunit, although rare mutations do exist that are associated with disease (Beutler et al., 1990, 1999; Ristoff et al., 2000; Hamilton et al., 2003; Mañú Pereira et al., 2007). In those cases where such changes have resulted in significantly diminished GCL expression and activity, the disease phenotype can be quite severe, including hemolytic anemia, aminoaciduria and spinocerebellar degeneration (Ristoff et al., 2000; Mañú Pereira et al., 2007, and references therein). Walsh and colleagues have shown that a common trinucleotide repeat (GAG) polymorphism in the 5′-untranslated region (5′-UTR) of the human GCLC mRNA is functionally important, with the number of repeats affecting GSH levels and drug sensitivities in multiple human tumor cell lines (Walsh et al., 2001). We have found that the number of GAG repeats in GCLC is directly correlated with lung function (FEV1) in patients with cystic fibrosis (CF) having weak CF mutations (McKone et al., 2006). We have also demonstrated that GCLC and GCLM polymorphisms are associated with chronic beryllium disease pathogenesis (Bekris et al., 2006). In addition, a number of studies indicate that polymorphisms in both GCLC and GCLM gene promoters influence the risk of cardiovascular disease (Nakamura et al., 2002, 2003; Koide et al., 2003; Campolo et al., 2007); however see (Muehlhause et al., 2007). These studies report lower plasma GSH levels or impaired GCL induction by oxidants, as well as compromised vasodilation in response to acetylcholine administration in patients having GCLM -588 C/T or GCLC -129 C/T or T/T genotypes. Other diseases in which polymorphisms in the promoters of these genes are likely to play a role in disease risk include schizophrenia (Tosic et al., 2006; Gysin et al., 2007), stroke (Baum et al., 2007), drug abuse (Hashimoto et al., 2008), carbon disulfide metabolism (Jönsson et al., 2007), mercury burden (Custodio et al., 2005), diabetes mellitus (Bekris et al., 2007), and asthma (Polonikov et al., 2007). In several of these latter studies, the effects of these polymorphisms on GCLM and GCLC mRNA expression, GCL activity and/or GSH synthesis have been investigated in various cell types and tissues from study patients, including cultured fibroblasts (Tosic et al., 2006; Gysin et al., 2007), blood monocytes (Nakamura et al, 2002), plasma and blood (Nakamura et al, 2002, 2003; Nichenametla et al, 2008). Although there is some variability depending on the cell and tissue types examined, the GCLC 7 GAG repeat allele is generally associated with higher GCLC expression and GSH levels than the 8 and 9 GAG repeat alleles, and the GCLM -588T and GCLC -129T alleles are associated with lower expression or inducibility and GSH levels. Interestingly, the GCLC C-129T polymorphism is apparently in linkage disequilibrium with the GCLC GAG repeat polymorphism (Bekris et al., 2007). In light of the functional significance of these polymorphisms, it is clear that transgenic animals having variable GCL subunit expression are needed to model GCL expression in humans and to predict what effects such variability might have on disease susceptibility.
6. Transgenic and knock-out models with altered GCL subunit expression
We and others have developed animal models of GCL over expression and insufficiency to begin to address the role of GSH synthesis in xenobiotic toxicity and in various diseases characterized by oxidative stress (Dalton et al., 2000; Shi et al., 2000; Yang et al., 2002; Dalton et al., 2004; Botta et al., 2006; Chen et al., 2007; McConnachie et al., 2007). We have developed Gclc and Gclm transgenic mice designed to conditionally over express GCL in the liver. We recently showed that conditional Gcl transgene expression in these mice promotes resistance to acetaminophen (APAP)-induced liver injury (Botta et al., 2006). While Gclc null mice do not survive beyond embryonic day 9, heterozygotes express only 50% of normal GCLC protein levels and appear to have a normal phenotype (Dalton et al., 2000; Shi et al., 2000). Using Cre recombinase technology, Dalton and co-workers developed transgenic mice having a liver specific, albumin promoter directed and Cre mediated deletion of the Gclc gene (Chen et al., 2007). These mice survive for approximately one month postnatally after the Gclc gene is deleted and die of extensive liver failure secondary to hepatic steatosis, necrosis and inflammation. In an interesting and related series of studies, Akai et al. found that transgenic rats carrying an inducible short hair-pin RNA directed against Gclc showed decreased GSH levels and susceptibility to APAP-induced liver injury (Akai et al., 2007).
While deletion of Gclc is an embryonic lethal phenotype in mice, deletion of Gclm has little effect on survival or development (Yang et al., 2002; McConnachie et al., 2007). As expected Gclm null mice do have the ability to synthesize GSH in limited amounts (about 10–20% of normal levels). We have found that Gclm null mice are sensitive to APAP-induced liver injury and confirmed that N-acetylcysteine mediated protection against APAP is dependent upon its being metabolized to GSH (McConnachie et al., 2007). These various GCL transgenic and knock-out mice and rats will be highly useful for modeling the role of GSH synthesis in human disease susceptibility.
7. Conclusions
GSH has many critical functions in the cell including maintaining redox status, scavenging free radicals and electrophilic intermediates, conjugation/detoxification reactions, apoptosis, and cell signaling. Because of its important role in regulating the synthesis and intracellular concentration of GSH, GCL has received a great deal of attention by those working in the areas of biochemistry, toxicology, free radical biology, embryonic/fetal development, reproduction, and cancer research. The many factors that regulate GCL expression and activity make it a fascinating enzyme to study. The molecular cloning and crystal structures of GCL from various species have dramatically advanced our understanding of this enzyme. Additional structural information on the mammalian enzymes and the availability of GCL transgenic and knock-out models will substantially advance this field, and the molecular mechanisms by which GCL and its subunits are regulated will continue to be actively investigated in the years to come. While much work has been done to elucidate mechanisms regulating GCL activity, our appreciation of its many roles in human physiology and disease is just beginning.
Acknowledgements
We wish to acknowledge the many investigators who have contributed to our understanding of GCL expression and regulation, but whose work we were unable to cite due to space limitations. We also would like to thank members of the Franklin, Kavanagh and Forman laboratories for their support and participation in the research we have done together, as well as the many collaborators we have had the pleasure to work with over the years. This work was supported by NIH Grants R01ES10849, P01AG01751, P42ES04696, P30ES07033, T32ES07032, R01CA90473, and RO1ES05511. This manuscript is dedicated to the memory of my father Dean Franklin, a bluegrass bass guitar player at heart and biophysicist by trade whose insatiable curiosity led to numerous significant advancements in cardiovascular research including his pioneering work in the development of biomedical instrumentation to measure cardiac function in conscious animals and his invention of the ultrasonic Doppler flowmeter. He is missed immensely by his family, friends, and colleagues.
References
- Abbott JJ, Pei J, Ford JL, Qi Y, Grishin VN, Pitcher LA, Phillips MA, Grishin NV. Structure prediction and active site analysis of the metal binding determinants in γ-glutamylcysteine synthetase. J. Biol. Chem. 2001;276(45):42099–42107. doi: 10.1074/jbc.M104672200. [DOI] [PubMed] [Google Scholar]
- Akai S, Hosomi H, Minami K, Tsuneyama K, Katoh M, Nakajim M, Yokoi T. Knock down of γ-glutamylcysteine synthetase in rat causes acetaminophen induced hepatotoxicity. J. Biol. Chem. 2007;282(33):23996–24003. doi: 10.1074/jbc.M702819200. [DOI] [PubMed] [Google Scholar]
- Arsalane K, Dubois CM, Muanza T, Begin R, Boudreau F, Asselin C, Cantin AM. Transforming growth factor-β1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme γ-glutamylcysteine synthetase. Am. J. Respir. Cell Mol. Biol. 1997;17:599–607. doi: 10.1165/ajrcmb.17.5.2833. [DOI] [PubMed] [Google Scholar]
- Bakin AV, Stourman NV, Sekhar KR, Rinehart C, Yan X, Meredith MJ, Arteaga CL, Freeman ML. Smad3-ATF3 signaling mediates TGF-β suppression of genes encoding Phase II detoxifying proteins. Free Radic. Biol. Med. 2005;38(3):375–387. doi: 10.1016/j.freeradbiomed.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol. Appl. Pharmacol. 2005;204(3):238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Baum L, Chen X, Cheung WS, Cheung CK, Cheung LW, Chiu KF, Wen HM, Poon P, Woo KS, Ng HK, Wong KS. Polymorphisms and vascular cognitive impairment after ischemic stroke. J. Geriatr. Psychiatry Neurol. 2007;20(2):93–99. doi: 10.1177/0891988706298627. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Shephard C, Janer M, Graham J, McNeney B, Shin J, Zarghami M, Griffith W, Farin F, Kavanagh TJ, Lernmark A. Glutamate cysteine ligase catalytic subunit promoter polymorphisms and associations with type 1 diabetes age-at-onset and GAD65 autoantibody levels. Exp. Clin. Endocrinol. Diabetes. 2007;115(4):221–228. doi: 10.1055/s-2007-970574. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Viernes HM, Farin FM, Maier LA, Kavanagh TJ, Takaro TK. Chronic beryllium disease and glutathione biosynthesis genes. J. Occup. Environ. Med. 2006;48(6):599–606. doi: 10.1097/01.jom.0000201845.02369.ba. [DOI] [PubMed] [Google Scholar]
- Beutler E, Gelbart T, Kondo T, Matsunaga AT. The molecular basis of a case of γ-glutamylcysteine synthetase deficiency. Blood. 1999;94(8):2890–2894. [PubMed] [Google Scholar]
- Beutler E, Moroose R, Kramer L, Gelbart T, Forman L. γ-Glutamylcysteine synthetase deficiency and hemolytic anemia. Blood. 1990;75(1):271–273. [PubMed] [Google Scholar]
- Botta D, Franklin CC, White CC, Krejsa CM, Dabrowski MJ, Pierce RH, Fausto N, Kavanagh TJ. Glutamate-cysteine ligase attenuates TNF-induced mitochondrial injury and apoptosis. Free Radic. Biol. Med. 2004;37(5):632–642. doi: 10.1016/j.freeradbiomed.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Botta D, Shi S, White CC, Dabrowski MJ, Keener CL, Srinouanprachanh SL, Farin FM, Ware CB, Ladiges WC, Pierce RH, Fausto N, Kavanagh TJ. Acetaminophen-induced liver injury is attenuated in male glutamate-cysteine ligase transgenic mice. J. Biol. Chem. 2006;281(39):28865–28875. doi: 10.1074/jbc.M605143200. [DOI] [PubMed] [Google Scholar]
- Breitschopf K, Zeiher AM, Dimmeler S. Ubiquitin-mediated degradation of the proapoptotic active form of Bid. A functional consequence on apoptosis induction. J. Biol. Chem. 2000;275(28):21648–21652. doi: 10.1074/jbc.M001083200. [DOI] [PubMed] [Google Scholar]
- Brekken DL, Phillips MA. Trypanosoma brucei γ-glutamylcysteine synthetase. J. Biol. Chem. 1998;273(41):26317–26322. doi: 10.1074/jbc.273.41.26317. [DOI] [PubMed] [Google Scholar]
- Cai J, Huang ZZ, Lu SC. Differential regulation of γ-glutamylcysteine synthetase heavy and light subunit gene expression. Biochem. J. 1997;326(Pt 1):167–172. doi: 10.1042/bj3260167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolo J, Penco S, Bianchi E, Colombo L, Parolini M, Caruso R, Sedda V, Patrosso MC, Cighetti G, Marocchi A, Parodi O. Glutamate-cysteine ligase polymorphism, hypertension, and male sex are associated with cardiovascular events. Biochemical and genetic characterization of Italian subpopulation. Am. Heart J. 2007;154(6):1123–1129. doi: 10.1016/j.ahj.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J. Biol. Chem. 2005;280(40):33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Y, Miller ML, Shen D, Shertzer HG, Stringer KF, Wang B, Schneider SN, Nebert DW, Dalton TP. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology. 2007;45(5):1118–1128. doi: 10.1002/hep.21635. [DOI] [PubMed] [Google Scholar]
- Coppola S, Ghibelli L. GSH extrusion and the mitochondrial pathway of apoptotic signalling. Biochem. Soc. Trans. 2000;28(2):56–61. doi: 10.1042/bst0280056. [DOI] [PubMed] [Google Scholar]
- Custodio HM, Harari R, Gerhardsson L, Skerfving S, Broberg K. Genetic influences on the retention of inorganic mercury. Arch. Environ. Occup. Health. 2005;60(1):17–23. doi: 10.3200/AEOH.60.1.17-23. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic. Biol. Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Yang Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem. Biophys. Res. Commun. 2000;279(2):324–329. doi: 10.1006/bbrc.2000.3930. [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Das S, Sarkar PK. Thyroid hormone promotes glutathione synthesis in astrocytes by up regulation of glutamate cysteine ligase through differential stimulation of its catalytic and modulator subunit mRNAs. Free Radic. Biol. Med. 2007;42(5):617–626. doi: 10.1016/j.freeradbiomed.2006.11.030. [DOI] [PubMed] [Google Scholar]
- De Bleser PJ, Xu G, Rombouts K, Rogiers V, Geerts A. Glutathione levels discriminate between oxidative stress and transforming growth factor-β signaling in activated rat hepatic stellate cells. J. Biol. Chem. 1999;274(48):33881–33887. doi: 10.1074/jbc.274.48.33881. [DOI] [PubMed] [Google Scholar]
- de Jonge HR, Hogema B, Tilly BC. Protein N-myristoylation: critical role in apoptosis and salt tolerance. Sci. STKE 2000. 2000;63 doi: 10.1126/stke.2000.63.pe1. PE1. [DOI] [PubMed] [Google Scholar]
- Deak JC, Cross JV, Lewis M, Qian Y, Parrott LA, Distelhorst CW, Templeton DJ. Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc. Natl. Acad. Sci. USA. 1998;95(10):5595–5600. doi: 10.1073/pnas.95.10.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhaber F, Eisenhaber B, Kubina W, Maurer-Stroh S, Neuberger G, Schneider G, Wildpaner M. Prediction of lipid posttranslational modifications and localization signals from protein sequences: big-Pi, NMT and PTS1. Nucleic Acids Res. 2003;31(13):3631–3634. doi: 10.1093/nar/gkg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol. Appl. Pharmacol. 2005;204(3):263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10(1):76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J. Biol. Chem. 2006;281(40):29542–29557. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Krejsa CM, Pierce RH, White CC, Fausto N, Kavanagh TJ. Caspase-3-dependent cleavage of the glutamate-l-cysteine ligase catalytic subunit during apoptotic cell death. Am. J. Pathol. 2002;160(5):1887–1894. doi: 10.1016/S0002-9440(10)61135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Rosenfeld-Franklin ME, White C, Kavanagh TJ, Fausto N. TGFβ1-induced suppression of glutathione antioxidant defenses in hepatocytes: caspase-dependent posttranslational and caspase-independent transcriptional regulatory mechanisms. FASEB J. 2003;17(11):1535–1537. doi: 10.1096/fj.02-0867fje. [DOI] [PubMed] [Google Scholar]
- Fraser JA, Kansagra P, Kotecki C, Saunders RD, McLellan LI. The modifier subunit of Drosophila glutamate-cysteine ligase regulates catalytic activity by covalent and noncovalent interactions and influences glutathione homeostasis in vivo. J. Biol. Chem. 2003;278(47):46369–46377. doi: 10.1074/jbc.M308035200. [DOI] [PubMed] [Google Scholar]
- Fraser JA, Saunders RD, McLellan LI. Drosophila melanogaster glutamate-cysteine ligase activity is regulated by a modifier subunit with a mechanism of action similar to that of the mammalian form. J. Biol. Chem. 2002;277(2):1158–1165. doi: 10.1074/jbc.M106683200. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zheng S, Lu SC, Chen A. Epigallocatechin-3-gallate inhibits growth of activated hepatic stellate cells by enhancing the capacity of glutathione synthesis. Mol. Pharmacol. 2008;73(5):1465–1473. doi: 10.1124/mol.107.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway DC, Blake DG, Shepherd AG, McLellan LI. Regulation of human γ-glutamylcysteine synthetase: co-ordinate induction of the catalytic and regulatory subunits in HepG2 cells. Biochem. J. 1997;328(Pt 1):99–104. doi: 10.1042/bj3280099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibelli L, Coppola S, Rotilio G, Lafavia E, Maresca V, Ciriolo MR. Non-oxidative loss of glutathione in apoptosis via GSH extrusion. Biochem. Biophys. Res. Commun. 1995;216(1):313–320. doi: 10.1006/bbrc.1995.2626. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999;27(9–10):922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc. Natl. Acad. Sci. USA. 1979a;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J. Biol. Chem. 1979b;254(16):7558–7560. [PubMed] [Google Scholar]
- Griffith OW, Mulcahy RT. The enzymes of glutathione synthesis: γ-glutamylcysteine synthetase. Adv. Enzymol. Relat. Areas Mol. Biol. 1999;73:209–267. doi: 10.1002/9780470123195.ch7. [DOI] [PubMed] [Google Scholar]
- Gromes R, Hothorn M, Lenherr ED, Rybin V, Scheffzek K, Rausch T. The redox switch of γ-glutamylcysteine ligase via a reversible monomerdimer transition is a mechanism unique to plants. Plant J. 2008;54(6):1063–1075. doi: 10.1111/j.1365-313X.2008.03477.x. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P, Tosic M, Werge T, Cuénod M, Do KQ. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc. Natl. Acad. Sci. USA. 2007;104(42):16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJ, Harb HL. l-γ-Glutamyl-l-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol. Immunol. 2005;42(9):987–1014. doi: 10.1016/j.molimm.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Hamilton D, Wu JH, Alaoui-Jamali M, Batist G. A novel missense mutation in the γ-glutamylcysteine synthetase catalytic subunit gene causes both decreased enzymatic activity and glutathione production. Blood. 2003;102(2):725–730. doi: 10.1182/blood-2002-11-3622. [DOI] [PubMed] [Google Scholar]
- Hamilton D, Wu JH, Batist G. Structure based identification of novel human γ-glutamylcysteine synthetase inhibitors. Mol. Pharmacol. 2007;71(4):1140–1147. doi: 10.1124/mol.106.024778. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hashimoto K, Miyatake R, Matsuzawa D, Sekine Y, Inada T, Ozaki N, Iwata N, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Iyo M. Association study between polymorphisms in glutathione-related genes and methamphetamine use disorder in a Japanese population. Am. J. Med. Genet. B: Neuropsychiatr. Genet. 2008;147B(7):1040–1046. doi: 10.1002/ajmg.b.30703. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995;30(6):445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hibi T, Nii H, Nakatsu T, Kimura A, Kato H, Hiratake J, Oda J. Crystal structure of γ-glutamylcysteine synthetase: insights into the mechanism of catalysis by a key enzyme for glutathione homeostasis. Proc. Natl. Acad. Sci. USA. 2004;101(42):15052–15057. doi: 10.1073/pnas.0403277101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks LM, Cahoon RE, Bonner ER, Rivard RS, Sheffield J, Jez JM. Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana. Plant Cell. 2007;19(8):2653–2661. doi: 10.1105/tpc.107.052597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler MR, Wang-Su ST, Wagner BJ. Role of the proteasome in TGF-β signaling in lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 2006;47(5):2045–2052. doi: 10.1167/iovs.05-0650. [DOI] [PubMed] [Google Scholar]
- Hothorn M, Wachter A, Gromes R, Stuwe T, Rausch T, Scheffzek K. Structural basis for the redox control of plant glutamate cysteine ligase. J. Biol. Chem. 2006;281(37):27557–27565. doi: 10.1074/jbc.M602770200. [DOI] [PubMed] [Google Scholar]
- Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney γ-glutamylcysteine synthetase. J. Biol. Chem. 1993;268(26):19675–19680. [PubMed] [Google Scholar]
- Huang CS, Moore WR, Meister A. On the active site thiol of γ-glutamylcysteine synthetase: relationships to catalysis, inhibition, and regulation. Proc. Natl. Acad. Sci. USA. 1988;85(8):2464–2468. doi: 10.1073/pnas.85.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowiak BE, Griffith OW. Glutathione synthesis in Streptococcus agalactiae. One protein accounts for γ-glutamylcysteine synthetase and glutathione synthetase activities. J. Biol. Chem. 2005;280:11829–11839. doi: 10.1074/jbc.M414326200. [DOI] [PubMed] [Google Scholar]
- Janowiak BE, Hayward MA, Peterson FC, Volkman BF, Griffith OW. γ-Glutamylcysteine synthetase-glutathione synthetase: domain structure and identification of residues important in substrate and glutathione binding. Biochemistry. 2006;45(35):10461–10473. doi: 10.1021/bi052483v. [DOI] [PubMed] [Google Scholar]
- Jardine H, MacNee W, Donaldson K, Rahman I. Molecular mechanism of transforming growth factor (TGF)-β 1-induced glutathione depletion in alveolar epithelial cells. Involvement of AP-1/ARE and Fra-1. J. Biol. Chem. 2002;277(24):21158–21166. doi: 10.1074/jbc.M112145200. [DOI] [PubMed] [Google Scholar]
- Jez JM, Cahoon RE, Chen S. Arabidopsis thaliana glutamate-cysteine ligase: functional properties, kinetic mechanism, and regulation of activity. J. Biol. Chem. 2004;279(32):33463–33470. doi: 10.1074/jbc.M405127200. [DOI] [PubMed] [Google Scholar]
- Jez JM, Penning TM. The aldo-keto reductase (AKR) superfamily: an update. Chem. Biol. Interact. 2001;130–132(1–3):499–525. doi: 10.1016/s0009-2797(00)00295-7. [DOI] [PubMed] [Google Scholar]
- Jönsson LS, Broberg K, Bergendorf U, Axmon A, Littorin M, Jönsson BA. Levels of 2-thiothiazolidine-4-carboxylic acid (TTCA) and effect modification of polymorphisms of glutathione-related genes in vulcanization workers in the southern Sweden rubber industries. Int. Arch. Occup. Environ. Health. 2007;80(7):589–598. doi: 10.1007/s00420-007-0171-6. [DOI] [PubMed] [Google Scholar]
- Kino K, Kuratsu S, Noguchi A, Kokubo M, Nakazawa Y, Arai T, Yagasaki M, Kirimura K. Novel substrate specificity of glutathione synthesis enzymes from Streptococcus agalactiae and Clostridium acetobutylicum. Biochem. Biophys. Res. Commun. 2007;352:351–359. doi: 10.1016/j.bbrc.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Koide S, Kugiyama K, Sugiyama S, Nakamura S, Fukushima H, Honda O, Yoshimura M, Ogawa H. Association of polymorphism in glutamate-cysteine ligase catalytic subunit gene with coronary vasomotor dysfunction and myocardial infarction. J. Am. Coll. Cardiol. 2003;41(4):539–545. doi: 10.1016/s0735-1097(02)02866-8. [DOI] [PubMed] [Google Scholar]
- Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu RM, Kavanagh TJ, Forman HJ. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch. Biochem. Biophys. 2004;423(1):116–125. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Lee JI, Kang J, Stipanuk MH. Differential regulation of glutamate-cysteine ligase subunit expression and increased holoenzyme formation in response to cysteine deprivation. Biochem. J. 2006;393(Pt 1):181–190. doi: 10.1042/BJ20051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C, Doseeva V, Pullalarevu S, Krajewski W, Howard A, Herzberg O. YbdK is a carboxylate-amine ligase with a γ-glutamyl:cysteine ligase activity: crystal structure and enzymatic assays. Proteins. 2004;56(2):376–383. doi: 10.1002/prot.20103. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Liu RM, Gao L, Choi J, Forman HJ. γ-Glutamylcysteine synthetase: mRNA stabilization and independent subunit transcription by 4-hydroxy-2-nonenal. Am. J. Physiol. 1998;275(5 Pt 1):L861–L869. doi: 10.1152/ajplung.1998.275.5.L861. [DOI] [PubMed] [Google Scholar]
- Lu SC, Kuhlenkamp J, Garcia-Ruiz C, Kaplowitz N. Hormone-mediated down-regulation of hepatic glutathione synthesis in the rat. J. Clin. Invest. 1991;88(1):260–269. doi: 10.1172/JCI115286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Mol. Aspects Med. 2008 doi: 10.1016/j.mam.2008.05.005. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueder DV, Phillips MA. Characterization of Trypanosoma brucei γ-glutamylcysteine synthetase, an essential enzyme in the biosynthesis of trypanothione (diglutathionylspermidine) J. Biol. Chem. 1996;271(29):17485–17490. doi: 10.1074/jbc.271.29.17485. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94(4):481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Mañú Pereira M, Gelbart T, Ristoff E, Crain KC, Bergua JM, López Lafuente A, Kalko SG, García Mateos E, Beutler E, Vives Corrons JL. Chronic non-spherocytic hemolytic anemia associated with severe neurological disease due to γ-glutamylcysteine synthetase deficiency in a patient of Moroccan origin. Haematologica. 2007;92(11):e102–e105. doi: 10.3324/haematol.11238. (online) [DOI] [PubMed] [Google Scholar]
- Martensson J, Jain A, Meister A. Glutathione is required for intestinal function. Proc. Natl. Acad. Sci. USA. 1990;87(5):1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DD, Vilas GL, Prescher JA, Rajaiah G, Falck JR, Bertozzi CR, Berthiaume LG. Rapid detection, discovery, and identification of post-translationally myristoylated proteins during apoptosis using a bio-orthogonal azidomyristate analog. FASEB J. 2008;22(3):797–806. doi: 10.1096/fj.07-9198com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe JB, Berthiaume LG. Functional roles for fatty acylated amino-terminal domains in subcellular localization. Mol. Biol. Cell. 1999;10(11):3771–3786. doi: 10.1091/mbc.10.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnachie LA, Mohar I, Hudson FN, Ware CB, Ladiges WC, Fernandez C, Chatterton-Kirchmeier S, White CC, Pierce RH, Kavanagh TJ. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol. Sci. 2007;99(2):628–636. doi: 10.1093/toxsci/kfm165. [DOI] [PubMed] [Google Scholar]
- McKone EF, Shao J, Frangolias DD, Keener CL, Shephard CA, Farin FM, Tonelli MR, Pare PD, Sandford AJ, Aitken ML, Kavanagh TJ. Variants in the glutamate-cysteine-ligase gene are associated with cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 2006;174(4):415–419. doi: 10.1164/rccm.200508-1281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Muehlhause A, Kropf S, Gardemann A. C-588T polymorphism of the human glutamate-cysteine ligase modifier subunit gene is not associated with the risk and extent of ischemic heart disease in a German cohort. Clin. Chem. Lab. Med. 2007;45(10):1416–1418. doi: 10.1515/CCLM.2007.295. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kugiyama K, Sugiyama S, Miyamoto S, Koide S, Fukushima H, Honda O, Yoshimura M, Ogawa H. Polymorphism in the 5′-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation. 2002;105(25):2968–2973. doi: 10.1161/01.cir.0000019739.66514.1e. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Sugiyama S, Fujioka D, Kawabata K, Ogawa H, Kugiyama K. Polymorphism in glutamate-cysteine ligase modifier subunit gene is associated with impairment of nitric oxide-mediated coronary vasomotor function. Circulation. 2003;108(12):1425–1427. doi: 10.1161/01.CIR.0000091255.63645.98. [DOI] [PubMed] [Google Scholar]
- Neurohr C, Lenz AG, Ding I, Leuchte H, Kolbe T, Behr J. Glutamate-cysteine ligase modulatory subunit in BAL alveolar macrophages of healthy smokers. Eur. Respir. J. 2003;22(1):82–87. doi: 10.1183/09031936.03.00080403. [DOI] [PubMed] [Google Scholar]
- Nichenametla SN, Ellison I, Calcagnotto A, Lazarus P, Muscat JE, Richie JP. Functional significance of the GAG trinucleotide-repeat polymorphism in the gene for the catalytic subunit of gamma-glutamylcysteine ligase. Free Radic. Biol. Med. 2008;45(5):645–650. doi: 10.1016/j.freeradbiomed.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dwyer PJ, Szarka CE, Yao KS, Halbherr TC, Pfeiffer GR, Green F, Gallo JM, Brennan J, Frucht H, Goosenberg EB, Hamilton TC, Litwin S, Balshem AM, Engstrom PF, Clapper ML. Modulation of gene expression in subjects at risk for colorectal cancer by the chemopreventive dithiolethione oltipraz. J. Clin. Invest. 1996;98(5):1210–1217. doi: 10.1172/JCI118904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi T. Hydrogen peroxide increases the activity of γ-glutamylcysteine synthetase in cultured Chinese hamster V79 cells. Arch. Toxicol. 1995;70(2):96–103. doi: 10.1007/BF02733669. [DOI] [PubMed] [Google Scholar]
- Ochi T. Menadione causes increases in the level of glutathione and in the activity of γ-glutamylcysteine synthetase in cultured Chinese hamster V79 cells. Toxicology. 1996;112(1):45–55. doi: 10.1016/0300-483x(96)03348-3. [DOI] [PubMed] [Google Scholar]
- Oda T, Sadakata N, Komatsu N, Muramatsu T. Specific efflux of glutathione from the basolateral membrane domain in polarized MDCK cells during ricin-induced apoptosis. J. Biochem. (Tokyo) 1999;126(4):715–721. doi: 10.1093/oxfordjournals.jbchem.a022508. [DOI] [PubMed] [Google Scholar]
- Orlowski M, Meister A. Partial reactions catalyzed by γ-glutamylcysteine synthetase and evidence for an activated glutamate intermediate. J. Biol. Chem. 1971;246(23):7095–7105. [PubMed] [Google Scholar]
- Pieper U, Narayanan E, Sali A. Models of human glutamate-cysteine ligase catalytic subunit ( P48506) 2007a < http://modbase.compbio.ucsf.edu>.
- Pieper U, Narayanan E, Sali A. Models of human glutamate-cysteine ligase modifier subunit ( P48507) 2007b < http://modbase.compbio.ucsf.edu>.
- Polonikov AV, Ivanov VP, Solodilova MA, Khoroshaya IV, Kozhuhov MA, Panfilov VI. The relationship between polymorphisms in the glutamate cysteine ligase gene and asthma susceptibility. Respir. Med. 2007;101(11):2422–2424. doi: 10.1016/j.rmed.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid. Redox Signal. 2005;7(1–2):42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000;16(3):534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem. Pharmacol. 2004;68(6):1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Reed DJ. Mitochondrial glutathione and chemically induced stress including ethanol. Drug Metab. Rev. 2004;36(3–4):569–582. doi: 10.1081/dmr-200033449. [DOI] [PubMed] [Google Scholar]
- Richman PG, Orlowski M, Meister A. Inhibition of γ-glutamylcysteine synthetase by l-methionine-S-sulfoximine. J. Biol. Chem. 1973;248(19):6684–6690. [PubMed] [Google Scholar]
- Ristoff E, Augustson C, Geissler J, de Rijk T, Carlsson K, Luo JL, Andersson K, Weening RS, van Zwieten R, Larsson A, Roos D. A missense mutation in the heavy subunit of γ-glutamylcysteine synthetase gene causes hemolytic anemia. Blood. 2000;95(7):2193–2196. [PubMed] [Google Scholar]
- Seelig GF, Meister A. Cystamine-sepharose. A probe for the active site of γ-glutamylcysteine synthetase. J. Biol. Chem. 1982;257(9):5092–5096. [PubMed] [Google Scholar]
- Seelig GF, Simondsen RP, Meister A. Reversible dissociation of γ-glutamylcysteine synthetase into two subunits. J. Biol. Chem. 1984;259(15):9345–9347. [PubMed] [Google Scholar]
- Sekhar KR, Freeman ML. Autophosphorylation inhibits the activity of γ-glutamylcysteine synthetase. J. Enzyme Inhib. 1999;14(4):323–330. doi: 10.3109/14756369909030325. [DOI] [PubMed] [Google Scholar]
- Sekhar KR, Long M, Long J, Xu ZQ, Summar ML, Freeman ML. Alteration of transcriptional and post-transcriptional expression of γ-glutamylcysteine synthetase by diethyl maleate. Radiat. Res. 1997;147(5):592–597. [PubMed] [Google Scholar]
- Sekhar KR, Soltaninassab SR, Borrelli MJ, Xu ZQ, Meredith MJ, Domann FE, Freeman ML. Inhibition of the 26S proteasome induces expression of GLCLC, the catalytic subunit for γ-glutamylcysteine synthetase. Biochem. Biophys. Res. Commun. 2000;270(1):311–317. doi: 10.1006/bbrc.2000.2419. [DOI] [PubMed] [Google Scholar]
- Shi ZZ, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, Lukin DJ, Danney CM, Matzuk MM, Lieberman MW. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc. Natl. Acad. Sci. USA. 2000;97(10):5101–5106. doi: 10.1073/pnas.97.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Rivera E, Summar ML, Dasouki M, Krishnamani MRS, Phillips JA, Freeman ML. Assignment of the gene (GLCLC) that encodes the heavy subunit of γ-glutamylcysteine synthetase to human chromosome 6. Cytogenet. Cell Genet. 1995;70(3–4):278–279. doi: 10.1159/000134051. [DOI] [PubMed] [Google Scholar]
- Siitonen T, Alaruikka P, Mantymaa P, Savolainen ER, Kavanagh TJ, Krejsa CM, Franklin CC, Kinnula V, Koistinen P. Protection of acute myeloblastic leukemia cells against apoptotic cell death by high glutathione and γ-glutamylcysteine synthetase levels during etoposide-induced oxidative stress. Ann. Oncol. 1999;10(11):1361–1367. doi: 10.1023/a:1008382912096. [DOI] [PubMed] [Google Scholar]
- Smith L, Chen L, Reyland ME, DeVries TA, Talanian RV, Omura S, Smith JB. Activation of atypical protein kinase C zeta by caspase processing and degradation by the ubiquitin-proteasome system. J. Biol. Chem. 2000;275(51):40620–40627. doi: 10.1074/jbc.M908517199. [DOI] [PubMed] [Google Scholar]
- Soltaninassab SR, Sekhar KR, Meredith MJ, Freeman ML. Multi-faceted regulation of γ-glutamylcysteine synthetase. J. Cell Physiol. 2000;182(2):163–170. doi: 10.1002/(SICI)1097-4652(200002)182:2<163::AID-JCP4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Sumi D, Manji A, Shinkai Y, Toyama T, Kumagai Y. Activation of the Nrf2 pathway, but decreased γ-glutamylcysteine synthetase heavy subunit chain levels and caspase-3-dependent apoptosis during exposure of primary mouse hepatocytes to diphenylarsinic acid. Toxicol. Appl. Pharmacol. 2007;223(3):218–224. doi: 10.1016/j.taap.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Sun W-M, Huang Z-Z, Lu SC. Regulation of γ-glutamylcysteine synthetase by protein phosphorylation. Biochem. J. 1996;320:321–328. doi: 10.1042/bj3200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Shi MM, Forman HJ. Increased transcription of the regulatory subunit of γ-glutamylcysteine synthetase in rat lung epithelial L2 cells exposed to oxidative stress or glutathione depletion. Arch. Biochem. Biophys. 1997;342(1):126–133. doi: 10.1006/abbi.1997.9997. [DOI] [PubMed] [Google Scholar]
- Tipnis SR, Blake DG, Shepherd AG, McLellan LI. Overexpression of the regulatory subunit of γ-glutamylcysteine synthetase in HeLa cells increases γ-glutamylcysteine synthetase activity and confers drug resistance. Biochem. J. 1999;337:559–566. [PMC free article] [PubMed] [Google Scholar]
- Toroser D, Yarian CS, Orr WC, Sohal RS. Mechanisms of γ-glutamylcysteine ligase regulation. Biochim. Biophys. Acta. 2006;1760(2):233–244. doi: 10.1016/j.bbagen.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F, Matthey ML, Parnas J, Preisig M, Saraga M, Solida A, Timm S, Wang AG, Werge T, Cuenod M, Do KQ. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. Am. J. Hum. Genet. 2006;79(3):586–592. doi: 10.1086/507566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM. S-Glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol. Interv. 2007;7(6):313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K, Mulcahy RT, Reid LL, Disteche CM, Kavanagh TJ. Mapping of the glutamate-cysteine ligase catalytic subunit gene (GLCLC) to human chromosome 6p12 and mouse chromosome 9D-E and of the regulatory subunit gene (GLCLR) to human chromosome 1p21-p22 and mouse chromosome 3H1-3. Genomics. 1995;30(3):630–632. doi: 10.1006/geno.1995.1293. [DOI] [PubMed] [Google Scholar]
- Tu Z, Anders MW. Identification of an important cysteine residue in human glutamate-cysteine ligase catalytic subunit by site-directed mutagenesis. Biochem. J. 1998;336(Pt 3):675–680. doi: 10.1042/bj3360675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi T, Sakurai N, Nakano K, Ishisaka R. C-terminal 15 kDa fragment of cytoskeletal actin is posttranslationally N-myristoylated upon caspase-mediated cleavage and targeted to mitochondria. FEBS Lett. 2003;539(1–3):37–44. doi: 10.1016/s0014-5793(03)00180-7. [DOI] [PubMed] [Google Scholar]
- van den Dobbelsteen DJ, Nobel CSI, Schlegel J, Cotgreave IA, Orrenius S, Slater AFG. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J. Biol. Chem. 1996;271(26):15420–15427. doi: 10.1074/jbc.271.26.15420. [DOI] [PubMed] [Google Scholar]
- Vergauwen B, Vos DD, Van Beeumen JJ. Characterization of the bifunctional γ-glutamate-cysteine ligase/glutathione synthetase (GshF) of Pasteurella multocida. J. Biol. Chem. 2006;281:4380–4394. doi: 10.1074/jbc.M509517200. [DOI] [PubMed] [Google Scholar]
- Vilas GL, Corvi MM, Plummer GJ, Seime AM, Lambkin GR, Berthiaume LG. Posttranslational myristoylation of caspase-activated p21-activated protein kinase 2 (PAK2) potentiates late apoptotic events. Proc. Natl. Acad. Sci. USA. 2006;103(17):6542–6547. doi: 10.1073/pnas.0600824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh AC, Feulner JA, Reilly A. Evidence for functionally significant polymorphism of human glutamate cysteine ligase catalytic subunit: association with glutathione levels and drug resistance in the National Cancer Institute tumor cell line panel. Toxicol. Sci. 2001;61(2):218–223. doi: 10.1093/toxsci/61.2.218. [DOI] [PubMed] [Google Scholar]
- Walsh AC, Li W, Rosen DR, Lawrence DA. Genetic mapping of GLCLC, the human gene encoding the catalytic subunit of γ-glutamyl-cysteine synthetase, to chromosome band 6p12 and characterization of a polymorphic trinucleotide repeat within its 5′ untranslated region. Cytogenet. Cell Genet. 1996;75(1):14–16. doi: 10.1159/000134447. [DOI] [PubMed] [Google Scholar]
- White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ. Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal. Biochem. 2003;318(2):175–180. doi: 10.1016/s0003-2697(03)00143-x. [DOI] [PubMed] [Google Scholar]
- Wild AC, Mulcahy RT. Regulation of γ-glutamylcysteine synthetase subunit gene expression: insights into transcriptional control of antioxidant defenses. Free Radic. Res. 2000;32(4):281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J. Nutr. 2004;134(3):489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen Y, Johansson E, Schneider SN, Shertzer HG, Nebert DW, Dalton TP. Interaction between the catalytic and modifier subunits of glutamate-cysteine ligase. Biochem. Pharmacol. 2007;74(2):372–381. doi: 10.1016/j.bcp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J. Biol. Chem. 2002;277(51):49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- Yao KS, Godwin AK, Johnson SW, Ozols RF, Odwyer PJ, Hamilton TC. Evidence for altered regulation of γ-glutamylcysteine synthetase gene expression among cisplatin-sensitive and cisplatin-resistant human ovarian cancer cell lines. Cancer Res. 1995;55(19):4367–4374. [PubMed] [Google Scholar]
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290(5497):1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- Zhu M, Bowden GT. Molecular mechanism(s) for UV-B irradiation-induced glutathione depletion in cultured human keratinocytes. Photochem. Photobiol. 2004;80(2):191–196. doi: 10.1562/2004-02-26-RA-091. [DOI] [PubMed] [Google Scholar]